Abstract
Depth of invasion in early invasive colorectal cancer is considered an important predictive factor for lymph node metastasis. However, no large‐scale reports have established the relationship between invasion depth of pedunculated type early invasive colorectal cancers and risk of lymph node metastasis. The aim of this retrospective cohort study was to clarify the risk of lymph node metastasis in pedunculated type early invasive colorectal cancers in a large series. Patients with pedunculated type early invasive colorectal cancer who underwent endoscopic or surgical resection at seven referral hospitals in Japan were enrolled. Haggitt’s line was used as baseline and the invasion depth was classified into two groups, head invasion and stalk invasion. The incidence of lymph node metastasis was investigated between patients with head and stalk invasion. We analyzed 384 pedunculated type early invasive colorectal cancers in 384 patients. There were 154, 156, and 74 endoscopic resection cases, endoscopic resection followed by surgical operation, and surgical resection cases, respectively. There were 240 head invasion and 144 stalk invasion lesions. Among the lesions treated surgically, the overall incidence of lymph node metastasis was 3.5% (8/230). The incidence of lymph node metastasis was 0.0% (0/101) in patients with head invasion, as compared with 6.2% (8/129) in patients with stalk invasion. Pedunculated type early invasive colorectal cancers pathologically diagnosed as head invasion can be managed by endoscopic treatment alone. (Cancer Sci 2011; 102: 1693–1697)
It has been reported that intramucosal colorectal cancers show no lymph node metastasis and are good candidates for endoscopic resection.( 1 , 2 ) In contrast, 6–12% of early invasive colorectal cancers (i.e. cancer cells invade through the muscularis mucosae into the submucosal layer but do not extend into the muscularis propria) are associated with lymph node metastasis requiring surgical resection including lymph node dissection for curative treatment.( 3 , 4 , 5 , 6 , 7 ) Recently, increasing evidence suggests that lesions with submucosal invasion limited to <1000 μm without lymphovascular invasion and/or poorly differentiated components do not metastasize to lymph nodes.( 8 ) Endoscopic resection is an appropriate treatment for early stage colorectal cancers, however, the resected specimen must be examined to determine whether there is a clinically significant risk of lymph node metastasis that would warrant additional surgery. Colorectal lesions can be subdivided according to endoscopic appearance using the Paris classification (Fig. S1), whereas Haggitt’s classification is frequently used to define the depth of invasion of pedunculated lesions.( 9 ) Haggitt and colleagues stratified the level of cancer invasion according to the following criteria: level 0, carcinoma in situ (i.e. has not extended below the muscularis mucosae); level 1, carcinoma invading through the muscularis mucosae but limited to the head of the polyp (i.e. above the junction between the adenoma and its stalk); level 2, carcinoma invading the level of the neck (i.e. the junction between adenoma and its stalk); level 3, carcinoma invading any part of the stalk; and level 4, carcinoma invading into the submucosa of the bowel wall below the stalk (Fig. S2). The authors concluded a low risk of metastasis or local recurrence when the level is <4. Pedunculated lesions can easily be treated endoscopically, however, there are no large‐scale reports establishing the risk of lymph node metastasis in this lesion type stratified by depth of invasion. We report the incidence of lymph node metastasis in pedunculated type early invasive colorectal cancers in a large series.
Materials and Methods
Patients. Patients with pedunculated type early invasive colorectal cancers that had been treated by endoscopic resection or surgical resection at seven institutions in Japan (National Cancer Center Hospitals [Tokyo, Kashiwa], Tokyo Medical University Hospital, Okayama University Hospital, Shizuoka Cancer Center, Tochigi Cancer Center, and Okayama Saisei‐kai General Hospital) between January 1992 and December 2007 were examined retrospectively. Patients eligible for this study had pathologically proven adenocarcinoma invading through the muscularis mucosae into the submucosal layer but not extending deeply into the muscularis propria. Eligibility also required the lesion to be endoscopically diagnosed as pedunculated type suitable for one‐piece resection. Patients with synchronous advanced colorectal cancer, multiple early invasive colorectal cancers, inflammatory bowel disease, hereditary non‐polyposis colorectal cancer, and familial adenomatous polyposis were excluded from this study. This study was carried out with the approval of each institution’s ethics review board.
Treatment strategy. Endoscopic resection: All lesions diagnosed as intramucosal or superficial submucosal invasive cancers at colonoscopy were removed by polypectomy or endoscopic mucosal resection. If the histopathological result did not meet the criteria for complete endoscopic resection, additional surgery was recommended. Surgical operation: Patients with endoscopic features suggestive of submucosal invasion into the stalk were referred directly for surgical operation (i.e. colectomy with lymph node dissection). Among the lesions treated surgically, the incidence of lymph node metastasis was analyzed. Recurrence was recorded as local, distant, and overall. Recurrent lesions were identified by endoscopic examinations, CT scan, or abdominal ultrasound.
Histopathologic evaluation. Resected specimens were immediately fixed in a 10% buffered formalin solution. Paraffin‐embedded samples were then sliced into 3‐μm sections and were stained by H&E. Experienced gastrointestinal pathologists blinded to each endoscopic diagnosis evaluated all pathological specimens. The histopathological type and lymphovascular (lymphatic and venous) invasion, poor differentiation, and depth of invasion were examined. Histopathological diagnosis was based on the World Health Organization criteria.( 10 ) The upper limit of level 2 according to Haggitt’s classification was used as baseline for all lesions and the invasion depth was classified into two groups (head invasion and stalk invasion).
Definition of terms. Haggitt’s line: The baseline to distinguish between head invasion and stalk invasion. This imaginary line is drawn according to an upper limit of level 2 invasion by Haggitt et al. (Fig. 1). Head invasion: The deepest portion of cancer invasion is limited to above the baseline (Haggitt’s line), as shown in Figure 1(A). Stalk invasion: The cancer has invaded into the submucosal layer deeply beyond Haggitt’s line (Fig. 1B).
Figure 1.
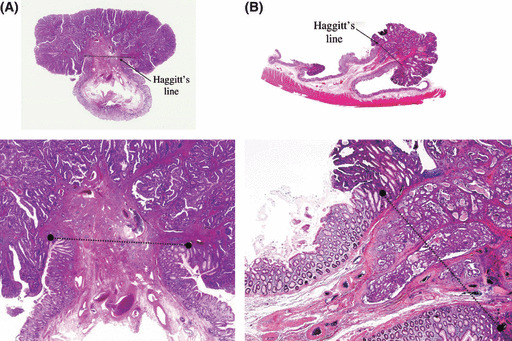
Definition of head invasion (A) and stalk invasion (B) in pedunculated type early invasive colorectal cancer.
Statistical analysis. Patients’ characteristics were summarized using mean and standard deviation for continuous variables, and percentage for discrete variables. Both the chi squared test and Fisher’s exact tests were used to examine the difference in incidence (lymph node metastasis and recurrence) between head invasion and stalk invasion. Risk factors for lymph node metastasis were also examined by chi squared or Fisher’s exact tests. All statistical tests were two‐sided and the significance level was set at 5%. All statistical analysis was carried out using spss statistical software (version 16.0J for Windows; SPSS, Tokyo, Japan).
Results
A total of 384 patients with pedunculated type early invasive colorectal cancer (male, 286 [74%]; female, 98 [26%]; mean age, 62.7 years [range, 29–89 years]; follow‐up period [median], 44 months) were enrolled in this study. There were 154 (40%), 156 (41%), and 74 (19%) endoscopic resection cases, endoscopic resection followed by surgical operation, and surgical resection cases, respectively. The mean tumor size was 18.2 ± 8.0 mm (range, 5–60 mm), and location was as follows: sigmoid colon, 304 (79%); ascending colon, 25 (7%); rectum, 23 (6%); descending colon, 18 (5%); and transverse colon, 14 (3%). Three‐hundred and forty patients (89%) were followed up and available for recurrence rate analysis. Among them, 159 (72%) patients in the head invasion group and 95 (79%) patients in the stalk invasion group were followed up for more than 36 months. In contrast, 21 (6%) patients were followed up for <12 months as shown in Table 1.
Table 1.
Clinical characteristics of 384 patients with pedunculated type early invasive colorectal cancer
| Head invasion | Stalk invasion | Total | |
|---|---|---|---|
| Total number, n (%) | 240 (63) | 144 (37) | 384 (100) |
| Gender (M/F), n (%) | 183 (76)/57 (24) | 103 (72)/41 (28) | 286 (74)/98 (26) |
| Age (years), mean (range) | 62.1 (36–87) | 63.6 (29–89) | 62.7 (29–89) |
| Size (mm), mean ± SD† (range) | 17.5 ± 7.4 (6–60) | 19.4 ± 9.0 (5–57) | 18.2 ± 8.0 (5–60) |
| Location, n (%) | |||
| Rectum | 11 (5) | 12 (8) | 23 (6) |
| Sigmoid colon | 194 (81) | 110 (76) | 304 (79) |
| Descending colon | 13 (5) | 5 (4) | 18 (5) |
| Transverse colon | 10 (4) | 4 (3) | 14 (3) |
| Ascending colon | 12 (5) | 13 (9) | 25 (7) |
| Treatment strategy, n (%) | |||
| Endoscopic resection | 139 (58) | 15 (10) | 154 (40) |
| Endoscopic resection followed by surgical operation | 67 (28) | 89 (62) | 156 (41) |
| Surgical operation | 34 (14) | 40 (28) | 74 (19) |
| Follow‐up period, median (months) | 43 | 47 | 44 |
| <12 months, n (%) | 17 (8) | 4 (3) | 21 (6) |
| 12–36 months | 43 (20) | 22 (18) | 65 (19) |
| >36 months | 159 (72) | 95 (79) | 254 (75) |
†Standard deviation.
Histopathological characteristics. Among 384 pedunculated type early invasive colorectal cancers, 240 (63%) lesions were diagnosed as head invasion, and 144 (37%) were classified as stalk invasion. There were 54 (14%), 53 (14%), and 52 (14%) positive cases of lymphatic invasion, venous invasion, and poorly differentiated component, respectively (Table 2).
Table 2.
Histopathological characteristics of 384 cases of pedunculated type early invasive colorectal cancer
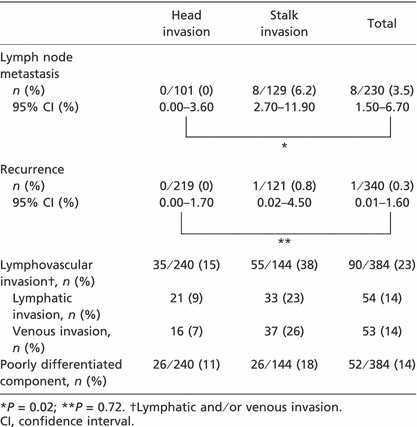
Incidence of lymph node metastasis and recurrence rate. The overall incidence of lymph node metastasis and recurrence rate were 3.5% (8/230; 95% confidence interval CI, 1.5–6.7%) and 0.3% (1/340; 95% CI, 0.01–1.6%), respectively (Table 2). Among lesions diagnosed as head invasion, the incidence of lymph node metastasis and recurrence rate were 0% (0/101; 95% CI, 0.0–3.6%) and 0% (0/219; 95% CI, 0.0–1.7%), as compared with 6.2% (8/129; 95% CI, 2.7–11.9%) and 0.8% (1/121; 95% CI, 0.02–4.50%) in patients with stalk invasion. Head versus stalk invasion: lymph node metastasis, P = 0.02; recurrence, P = 0.72.
Among lesions diagnosed as head invasion, 29 of 101 (29%) were lymphovascular (lymphatic and/or venous) invasion positive, and 72 of 101 (71%) were negative. There were no cases of lymph node metastasis in either group. In contrast, among stalk invasion lesions, 49 of 129 (38%) were lymphovascular invasion positive, whereas 80 of 129 (62%) were negative. There were three of 49 (6.1%) cases of lymph node metastasis in the lymphovascular invasion positive group, and there were five of 80 (6.3%) cases of lymph node metastasis in the lymphovascular invasion negative group, as shown in Table 3. There was no significant difference between lymph node metastasis and lymphovascular invasion.
Table 3.
Lymphovascular invasion among 384 cases of pedunculated type early invasive colorectal cancer with lymph node metastasis
| Head invasion | Stalk invasion | Total | |
|---|---|---|---|
| Lymph node metastasis, n (%) | |||
| ly (+), v (+) | 0/1 (0.0) | 0/14 (0.0) | 0/15 (0.0) |
| ly (+), v (−) | 0/16 (0.0) | 2/17 (11.8) | 2/33 (6.1) |
| ly (−), v (+) | 0/12 (0.0) | 1/18 (5.6) | 1/30 (3.3) |
| ly (−), v (−) | 0/72 (0.0) | 5/80 (6.3) | 5/152 (3.3) |
ly, lymphatic invasion; v, venous invasion.
Risk factors of lymph node metastasis. Clinicopathological factors were compared between lymph node metastasis positive and negative groups. Regarding the depth of invasion, eight stalk invasion cases were identified in the lymph node metastasis positive group, representing a significant difference compared with the negative group (P = 0.02). No significant differences in any other factors were noted between lymph node metastasis positive and negative groups (Table 4).
Table 4.
Comparison of clinicopathological factors between lymph node metastasis positive (+) and negative (−) groups among 384 cases of pedunculated type early invasive colorectal cancer
| Variables | Lymph node metastasis | P‐value |
|---|---|---|
| Depth of invasion | (+) 8/0 | 0.02 |
| (stalk vs head) | (−) 121/101 | |
| Lymphovascular | (+) 3/5 | >0.99 |
| invasion | (−) 75/147 | |
| (ly and/or v [+] vs [−]) | ||
| Poorly differentiated | (+) 1/7 | >0.99 |
| component | (−) 38/184 | |
| Tumor size† | (+) 5/3 | 0.67 |
| (≥20 mm vs <20 mm) | (−) 101/108 |
†Unknown, 13 cases. ly, lymphatic invasion; v, venous invasion.
Discussion
Advances in endoscopic instruments and techniques have allowed increased detection of early stage colorectal cancer, and endoscopic resection is a safe and effective curative treatment for such lesions when there is no risk of lymph node metastasis.
Kudo( 11 ) was the first to classify submucosal invasion of early invasive colorectal cancer as SM1 (upper third of submucosa), SM2 (middle third of submucosa), and SM3 (lower third of submucosa). Since then, Kikuchi et al.( 12 ) have reported lymph node metastasis in 0%, 10%, and 25% of 182 patients with SM1, SM2, and SM3 early invasive colorectal carcinomas, respectively. More recently, Nascimbeni et al. ( 13 ) showed that SM3 invasion had a significantly higher risk of lymph node metastasis compared to SM1–2 by multivariate analysis (SM1, 3%; SM2, 8%; SM3, 23%). The overall risk of lymph node metastasis in early invasive colorectal cancer is approximately 10%, suggesting that endoscopic removal of the vast majority of lesions without surgical intervention could ultimately be curative. In contrast, the rate of lymph node metastasis in patients who underwent additional surgical excision of the colorectum following endoscopic treatment has been reported to be 2.1–25.0%.( 3 , 14 , 15 , 16 , 17 ) This suggests that a significant percentage of patients may undergo unnecessary additional surgery following endoscopic treatment, and more stringent criteria are required to prevent this. Protruding colorectal neoplasms and, more specifically, pedunculated lesions may be easier than non‐pedunculated lesions to detect and remove endoscopically. However, the risk of lymph node metastasis and the prognostic significance of this specific subtype of early invasive colorectal cancer have not been sufficiently examined. This is the first large‐scale multicenter study in Japan to assess the incidence of lymph node metastasis and recurrence of pedunculated type early invasive colorectal cancer.
Conventional measurement of submucosal invasion using SM1–SM3 was originally devised for examination of surgical specimens where the full thickness of the colonic wall was available to the pathologist. Haggitt’s level 2 was used as the baseline to differentiate between head and stalk invasion by Kitajima et al. ( 18 ) and submucosal invasion depth was measured as the vertical distance from this baseline (Haggitt’s line) to the deepest point of invasion. This method of invasion measurement is more appropriate to endoscopically resected specimens where the muscularis propria is not included. According to the data from the Japanese Society for Cancer of the Colon and Rectum, the “so‐called 1000 μm rule of submucosal invasion” is applied to not only non‐pedunculated type but also pedunculated type early invasive colorectal cancers. In our current study, among lesions diagnosed as “stalk invasion”, the incidence of the “<1000 μm group” was under 10%, similar to Kitajima’s data.( 18 ) Moreover, all lymph node metastasis positive cases (eight cases) were classified into the “more than 1000 μm group”. In this study, however, the number of lymph node metastasis positive cases was limited. Therefore, we concluded that more cases with stalk invasion and more cases with lymph node metastasis are necessary to investigate the feasibility of the present 1000 μm rule.
We devised a straightforward description of cancer invasion to either head (above Haggitt’s line) or stalk (below this line) and estimated the risk of lymph node metastasis and recurrence rate for pedunculated type early invasive colorectal cancer according to these groups. In our retrospective study there was no risk of lymph node metastasis in patients with head invasion (0%, 0/101) compared to 6.2% (8/129) of patients with stalk invasion. Furthermore, the recurrence rate during the follow‐up period (mean ± SD, 40.7 ± 24.1 months) in patients with head invasion treated by endoscopic resection was also 0% (0/139; 95% CI, 0.0–2.6%).
In the past 20 years investigators have proposed that the presence of submucosal invasion more than 1000 μm, lymphatic invasion, and/or poor differentiation required additional surgery following endoscopic mucosal resection of early invasive colorectal cancer. Conversely, depth of invasion (stalk invasion) was the only predictive factor for lymph node metastasis in our study. Although our results showed that none of the patients in the head invasion group showed lymph node metastasis, lymphovascular invasion was present in 29 cases in this group and these patients underwent additional surgery. Our results are promising and indicate that the risk of lymph node metastasis in these 29 patients is low, however, prospective studies confirming these findings are required before a change in surgical management is implemented.
It is widely recognized that depressed type (0–IIc) lesions invading into the submucosa display a significantly higher rate of lymph node metastasis in comparison to protruded type (0–Ip and 0–Is), superficial elevated (0–IIa), and flat (0–IIb) lesions.( 6 , 18 , 19 ) Pan et al. ( 20 ) also reported that early invasive colorectal cancers at the fold‐top or with a long distance from the muscularis mucosae to the muscularis propria have a lower tendency to metastasize to lymph nodes. These studies indicate that the lower rate of lymph node metastasis in pedunculated type early invasive colorectal cancers could be elucidated by the presence of a greater muscularis mucosae to muscularis propria distance. Our study also showed a low rate of lymph node metastasis in pedunculated type lesions, although this data was only available for patients who underwent surgical resection (n = 230).
Some controversies with regard to pedunculated type lesions exist. Haggitt et al. (9) stipulated that the presence or absence of a stalk is largely irrelevant histopathologically. Moreover, they commented that the surgeon and pathologist may disagree on stalk length or even existence. Certain factors such as traction force used during removal, retraction of the pedicle following division and shrinkage after fixation could explain this. To avoid contention we imposed strict inclusion criteria in our study allowing only endoscopically diagnosed pedunculated type lesions with an obvious stalk to be eligible.
There are some limitations to our study. First, we retrospectively analyzed the clinical records of all patients who underwent endoscopic resection or surgical resection for pedunculated type colorectal cancers at seven institutes in Japan. The number of examined cases was large compared to previous studies, however, we did not re‐evaluate lymphovascular invasion using immunohistochemical staining for all cases. Routine use of immunohistochemistry should be considered in future retrospective studies. Second, several authors have indicated that early invasive colorectal cancers in the rectum have a higher incidence of lymph node metastasis and local recurrence.( 9 , 12 , 21 ) We were unable to assess this risk in our patients as 79% (304/384) of the pedunculated type lesions were located in the sigmoid colon. Finally, tumor budding, which has also been referred to as sprouting or dedifferentiation( 22 , 23 ) was not evaluated in this study. We evaluated the presence or absence of any poorly differentiated adenocarcinoma component, including that found at the most invasive submucosal margin. This is similar to the focal dedifferentiation reported by Tominaga et al.,( 24 ) however, Sohn et al. ( 25 ) argued that tumor budding should be categorized separately.
In conclusion, all cases with lymph node metastasis or recurrence were categorized into the stalk invasion group in this retrospective multicenter study. Our data suggest that pedunculated type early invasive colorectal cancer diagnosed as head invasion could be managed by endoscopic treatment alone.
Disclosure Statement
None of the authors had any financial relationships relevant to this publication.
Supporting information
Fig. S1. Neoplastic lesions with “superficial” morphology in pedunculated type early invasive colorectal cancer.
Fig. S2. Haggitt’s classification of pedunculated type early invasive colorectal cancer.
Supporting info item
Supporting info item
Acknowledgments
We would like to express our appreciation to Drs Abby Conlin (Department of Gastroenterology, Manchester Royal Infirmary, Manchester, UK) and Tonya Kaltenbach (Division of Gastroenterology, Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA) for their assistance in editing this manuscript.
References
- 1. Morson BC, Whiteway JE, Jones EA et al. Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut 1984; 25: 437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujimori T, Kawamata H, Kashida H. Precancerous lesion of the colorectum. J Gastroenterol 2001; 36: 587–94. [DOI] [PubMed] [Google Scholar]
- 3. Cooper HS. Surgical pathology of endoscopically removed malignant polyps of the colon and rectum. Am J Surg Pathol 1983; 7: 613–23. [DOI] [PubMed] [Google Scholar]
- 4. Kyzer S, Bégin LR, Gordon PH et al. The care of patients with colorectal polyps that contain invasive adenocarcinoma. Cancer 1992; 70: 2044–50. [DOI] [PubMed] [Google Scholar]
- 5. Minamoto T, Mai M, Ogino T et al. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am J Gastroenterol 1993; 88: 1035–9. [PubMed] [Google Scholar]
- 6. Tanaka S, Haruma K, Teixeira CR et al. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol 1995; 30: 710–17. [DOI] [PubMed] [Google Scholar]
- 7. Nusko G, Mansmann U, Partzsch U et al. Invasive carcinoma in colorectal adenomas: multivariate analysis of patient and adenoma characteristics. Endoscopy 1997; 29: 626–31. [DOI] [PubMed] [Google Scholar]
- 8. Participants in the Paris Workshop . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58: S3–43. [DOI] [PubMed] [Google Scholar]
- 9. Haggitt RC, Glotzbach RE, Soffer EE et al. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985; 89: 328–36. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton SR, Aaltonen LA, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press, 2000; 104–19. [Google Scholar]
- 11. Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993; 25: 455–61. [DOI] [PubMed] [Google Scholar]
- 12. Kikuchi R, Takano M, Takagi K et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995; 38: 1286–95. [DOI] [PubMed] [Google Scholar]
- 13. Nascimbeni R, Burgart LJ, Nivatvongs S et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002; 45: 200–6. [DOI] [PubMed] [Google Scholar]
- 14. Coverlizza S, Risio M, Ferrari A et al. Colorectal adenomas containing invasive carcinoma: pathologic assessment of lymph node metastatic potential. Cancer 1989; 64: 1937–47. [DOI] [PubMed] [Google Scholar]
- 15. Colacchio TA, Forde KA, Scantlebury VP. Endoscopic polypec‐tomy: inadequate treatment for invasive colorectal carcinoma. Ann Surg 1981; 194: 704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolff WI, Shinya H. De nitive treatment of “malignant” polypsof the colon. Ann Surg 1975; 182: 516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nivatvongs S, Rojanasakul A, Reiman HM et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum 1991; 34: 323–8. [DOI] [PubMed] [Google Scholar]
- 18. Kitajima K, Fujimori T, Fujii S et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004; 39: 534–43. [DOI] [PubMed] [Google Scholar]
- 19. Kudo S, Kashida H, Tamura T et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg 2000; 24: 1081–90. [DOI] [PubMed] [Google Scholar]
- 20. Pan W, Terai T, Abe S et al. Location of early colorectal cancers at fold‐top may reduce the risk of lymph node metastasis. Dis Colon Rectum 2006; 49: 579–87. [DOI] [PubMed] [Google Scholar]
- 21. Sticca RP, Rodriguez‐Bigas M, Penetrante RB et al. Curative resection for stage I rectal cancer: natural history, prognostic factors, and recurrence patterns. Cancer Invest 1996; 14: 491–7. [DOI] [PubMed] [Google Scholar]
- 22. Shimomura T, Ishiguro S, Konishi H et al. New indication for endoscopic treatment of colorectal carcinoma with submucosal invasion. J Gastroenterol Hepatol 2004; 19: 48–55. [DOI] [PubMed] [Google Scholar]
- 23. Fujimori T, Fujii S, Saito N et al. Pathological diagnosis of early colorectal carcinoma and its clinical implications. Digestion 2009; 79: 40–51. [DOI] [PubMed] [Google Scholar]
- 24. Tominaga K, Nakanishi Y, Nimura S et al. Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum 2005; 48: 92–100. [DOI] [PubMed] [Google Scholar]
- 25. Sohn DK, Chang HJ, Park JW et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol 2007; 60: 912–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Neoplastic lesions with “superficial” morphology in pedunculated type early invasive colorectal cancer.
Fig. S2. Haggitt’s classification of pedunculated type early invasive colorectal cancer.
Supporting info item
Supporting info item


