Abstract
Overexpression of the enhancer of zeste homolog 2 (EZH2) protein, a known repressor of gene transcription, has been reported to be associated with biological malignancy of prostate cancer and several other cancers. The purpose of this study was to examine the expression of EZH2 and analyze its relationship with the clinicopathological features of human gastric cancers. Expression levels of EZH2 mRNA and protein were examined in 13 gastric cancer cell lines and in 83 surgically removed human gastric cancer tissues. Immunohistochemical analysis of the 83 tissue samples and corresponding non‐cancerous gastric mucosa showed that EZH2 was more highly expressed in the cancerous than in the non‐cancerous tissues, and the expression levels of EZH2 were highly correlated with tumor size, depth of invasion, vessel invasion, lymph node metastasis and clinical stages. Univariate analysis of survival rate calculated by the Kaplan‐Meier method revealed that gastric cancer patients with high‐level EZH2 expression had poorer prognosis than those expressing no or low levels of EZH2 (P = 0.0271). These findings suggest that overexpression of EZH2 may contribute to the progression and oncogenesis of human gastric cancers, and thus immunohistochemical study of EZH2 expression may serve as a new biomarker for predicting the prognosis of gastric cancers. (Cancer Sci 2006; 97: 484 – 491)
Although gastric cancer has gradually decreased in prevalence, it still accounts for a large portion of cancer‐related deaths in Japan. The most informative prognostic factor is the tumor stage, which involves both the depth of invasion and the extent of metastasis. The size and histological type of the tumor may also be useful factors. Despite the complexity of stomach carcinogenesis, a number of molecular studies have been performed in a search for additional prognostic factors. As a result, several proteins, such as transforming growth factor alpha (TGFα), epidermal growth factor receptor (EGFR), c‐met, c‐erbB2, cyclin E, p27Kip1 and CDC25B, have been identified as markers of the malignancy of gastric cancer.( 1 , 2 , 3 ) The search for molecular factors that are highly correlated with prognosis may lead to the discovery of factors that can help to predict not only patient survival, but also the tumor response to specific anticancer drugs. One new marker that has potential for cancer screening and can be a predictor of patient survival is enhancer of zeste homolog 2 (EZH2).
EZH2, also called histone lysine methyltransferase (HKMT), was cloned as one of the polycomb group genes.( 4 ) The function of EZH2 is to catalyze the subunit of the polycomb repressor complex by methylating lysine 9 and 27 of histone H3.( 5 , 6 , 7 , 8 ) Although EZH2, which by itself lacks enzyme activity, it is assumed to associate with specific polypeptides present in the polycomb repressive complexes 2 and 3 (PRC2/3), constructing an EZH2 complex to work as a repressor gene in various organs.( 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 )
Recently, close correlation between overexpression of EZH2 and progression of prostate cancer was reported by Varambally et al. in a study using cDNA microarray analysis.( 13 ) An association of EZH2 overexpression with the biological malignancy of tumors has also been reported for several other cancers, including breast cancer, hepatocellular carcinoma, bladder carcinoma and lymphomas.( 10 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 )
In the present study, we examined the mRNA and protein expression of EZH2 in human gastric cancer cell lines. We then conducted EZH2 immunohistochemical analysis of human gastric cancer tissues and analyzed the relationship between EZH2 expression and clinicopathological factors. Furthermore, the association between EZH2 expression and the prognosis of gastric cancer patients was investigated.
Materials and Methods
Cell cultures
Thirteen cell lines derived from human gastric carcinoma were used. Eight gastric cancer cell lines of the HSC series (HSC‐57, tubular adenocarcinoma; HSC‐42, HSC‐58 and HSC‐59, poorly differentiated adenocarcinoma; HSC‐40, HSC‐44, HSC‐45 and HSC‐60, signet ring cell carcinoma) and SH101‐P4 (tubular adenocarcinoma) were established by one of the authors (K.Y).( 23 , 24 ) Three cell lines of the MKN series (MKN‐1, adenosquamous cell carcinoma; MKN‐7 and MKN‐74, tubular adenocarcinoma) were provided by Dr T. Suzuki (Fukushima Medical University, Fukushima).( 25 , 26 ) The TMK‐1 cell line (poorly differentiated adenocarcinoma) was a gift from Dr W. Yasui (Hiroshima University, Hiroshima).( 27 ) The cells were maintained in RPMI1640 (Life Technologies, Grand Island, NY, USA) supplemented with 1 mM L‐glutamine, 10% fetal bovine serum (FBS; Life Technologies) and 12.5 µg/mL gentamicin (Sigma, St. Louis, MO, USA) under humidified 5% CO2 in air at 37°C.
Tissue samples
A total of 83 gastric cancer tissue samples and adjacent non‐cancerous gastric mucosa specimens, surgically removed at Kobe University Hospital, were used in the immunohistochemical analysis. Informed consent was obtained from all patients before surgery. All resected specimens were fixed in 10% formalin and embedded in paraffin. Clinicopathological information was obtained from medical charts and histopathological examination was performed according to the Japanese Classification of Gastric Carcinoma.( 28 ) Tumor size was divided into two groups according to the mean size (40 mm). Fresh non‐cancerous gastric mucosa specimens were obtained at autopsy and, after written informed consent was obtained, were frozen immediately.
RNA extraction and quantitative reverse transcription‐polymerase chain reaction (RT‐PCR) analyses
Total RNAs from gastric cancer cell lines (1 × 106) were isolated using an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Tissue samples were homogenized and total RNA was prepared using a guanidine thiocyanate/cesium method. Quantitative RT‐PCR was performed with a SYBR Green real‐time Quantitative RT‐PCR assay kit (Qiagen) on RNA extracts obtained from gastric cancer cell lines and non‐cancerous gastric mucosa using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primer set used for RT‐PCR amplification of the EZH2 was as follows: forward, 5′‐GCG CGG GAC GAA GAA TAA TCA T‐3′; reverse, 5′‐TAC ACG CTT CCG CCA ACA AAC T‐3′. As an internal control, the levels of β‐actin expression were also analyzed (forward, 5′‐CCA CGA AAC TAC CTT CAA CTC C‐3′; reverse, 5′‐TCA TAC TCC TGC TGC TTG CTG ATC C‐3′). A master mix (50 µL) of the following reaction components was prepared to the indicated end concentration: 25 µL of 2 × QuantiTect SYBR Green RT‐PCR Master Mix, 10 ng of total RNA, 1 mM of the primer pair, reverse transcriptase, and 0.5 µL of QuantiTect RT Mix. They were mixed and amplified for 30 cycles with the following regimen: reverse transcription at 50°C for 30 min; denaturation at 94°C for 30 s; annealing at 60°C for 30 s; extension at 72°C for 1 min.
Western blot analysis
Cells or tissues were lyzed in buffer containing 50 mM Tris‐HCl (pH 7.4), 125 mM NaCl, 0.1% Triton‐X (Wako Pure Chemical Industries, Osaka, Japan) and 5 mM EDTA containing both 1% (v/v) protease inhibitor and 1% (v/v) phosphatase inhibitor cocktail II (Sigma). Forty micrograms of each extracted protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE), followed by electrotransfer onto a PVDF membrane (Millipore, Bedford, MA, USA). Rabbit polyclonal antibody to EZH2 (Upstate, Charlottesville, VA, USA) was used for the primary antibody. As a control, an antibody to β‐actin (DAKO, Glostrup, Denmark) was also used. Horseradish peroxidase‐conjugated sheep antirabbit IgG antibody (Amersham Biosciences, Piscataway, NJ, USA) was used as a secondary antibody for enhanced chemiluminescence (ImmunoStar Reagents, Wako Pure Chemical Industries, Chuo‐ku, Osaka, Japan).
Immunohistochemical analysis
Immunohistochemical analyses were performed with rabbit polyclonal antibody to EZH2 (Upstate). Tissue sections of 4 µm in thickness were cut from each paraffin block. Deparaffinized tissue sections were immersed in 10 mM citrate buffer (pH 6.0) and autoclaved for 15 min at 121°C for antigen retrieval. Endogenous peroxidase activity was blocked by 0.03% hydrogen peroxide. Following incubation with 0.01 M phosphate‐buffered saline (PBS) (pH 7.2) containing 5% bovine serum albumin (BSA) blocking buffer, the sections were incubated with primary antibody overnight at 4°C. After rinsing with 0.05 M Tris‐HCl (pH 7.6), the sections were sequentially incubated with biotinylated secondary antibody (Histofine kit; Nichirei, Tokyo, Japan) for 30 min, streptavidin peroxidase for 15 min and 3,3‐diaminobenzidine for 15 min with an LSAB2 Kit (DAKO). The sections were then counterstained with Mayer's hematoxylin.
The degree of immunoreactivity of EZH2 was categorized as follows: high reactivity, more than 50% of cells showing intense immunoreactivity in their nuclei; low reactivity, 50% of fewer cells showing intense immunoreactivity in their nuclei. The mean percentage of positive tumor cells was determined in at least five areas at high power field.
Statistical analyses
Statistical significance was evaluated with the χ2 and Mann–Whitney U‐tests. Survival rate curves were drawn according to the Kaplan‐Meier method, and differences between the curves were analyzed by applying the log‐rank test. The date of resection was considered day zero. The terminal event for cancer‐related survival was death attributable only to cancer, and the significance level was set at 5% for each analysis.
Results
Expression of EZH2 in human gastric cancer cell lines
We first examined the expression of EZH2 in 13 gastric cancer cell lines and non‐cancerous gastric mucosa at the mRNA and protein levels using quantitative RT‐PCR and western blot analyses, respectively. All of the 13 gastric cancer cell lines expressed some level of EZH2 mRNA (Fig. 1A). In eight of the cell lines‐∖HSC‐60, HSC‐44PE, HSC‐45, HSC‐59, HSC‐60, MKN‐74, TMK‐1 and SH101‐P4‐∖, the expression levels of EZH2 mRNA were more than 20‐fold higher than those in the non‐cancerous gastric mucosa. Among the 13 cell lines, HSC‐60 cells demonstrated the highest level of EZH2 mRNA expression, while MKN‐1 cells showed the lowest.
Figure 1.
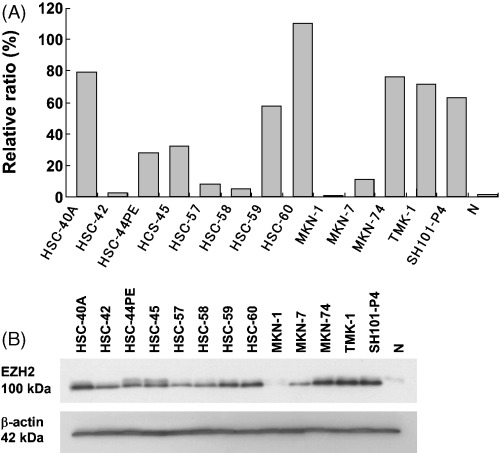
Expression of enhancer of zeste homolog 2 (EZH2) in 13 human gastric cancer cell lines. (A) Expression levels of EZH2 mRNA were quantitatively verified by real‐time reverse transcription‐polymerase chain reaction (RT‐PCR). The correction values of EZH2 expression were calculated by dividing the EZH2 amounts by the amount of internal control (β‐actin) concurrently examined on the same samples. (B) Expression levels of EZH2 protein by western blot analysis. Single 100 kDa band was detected in all cell lines. β‐actin was used as internal control for equal loading. N, non‐cancerous gastric mucosa.
EZH2 protein expressions in these 13 cell lines and non‐cancerous gastric mucosa were determined by western blot analysis using the anti‐EZH2 antibody to confirm the specificity of the antibody (Fig. 1B). As expected, each cell line showed a band with 100 kDa molecular weight whose intensity corresponded to its expression level of EZH2 mRNA.
Immunohistochemical analysis
Next, we examined EZH2 expression in gastric cancer tissues and their corresponding non‐cancerous gastric mucosa by immunohistochemistry with the same anti‐EZH2 antibody used in the western blot analysis. Consistent with the results of the western blot analyses, the non‐cancerous gastric mucosa showed faint EZH2 immunoreactivity restricted to the nuclei of glandular epithelial cells. Weak membranous staining was the characteristic feature for goblet cells of intestinal metaplasia. Also, some of the intestinalized cells showed intense nuclear staining, but none of the cases satisfied the criteria of grading of EZH2 staining as ‘high’. The percentage of the intense nuclear immunoreactivity was used for the evaluation of EZH2 expression in gastric cancer cells. A representative result for each group is shown in Fig. 2A–C (negative, low and high expression groups, respectively). In most of the gastric cancer tissues examined, EZH2‐specific signals were mainly located in the nuclei. However, some gastric cancers showed EZH2 immunoreactivity not only in the nuclei but also in the cytoplasm. Immunoreactivity of EZH2 in cytoplasm was graded as high if the more then 50% of the cells showed intense immunoreactivity in their cytoplasm. Twenty‐seven percent of the gastric cancer tissues (22 out of 83 cases) showed the cytoplasmic staining of EZH2, and 18% (15 out of 84 cases) of the cases showed the intense staining in both the cytoplasm and nuclei. There was no correlation between the nuclear and cytoplasmic expression of EZH2 (P = 0.2021) by χ2‐test. Interestingly, several tissues showed very strong EZH2 immunoreactivities both in the nucleus and cytoplasm of tumor cells forming intravascular emboli (Fig. 2n,o).
Figure 2.
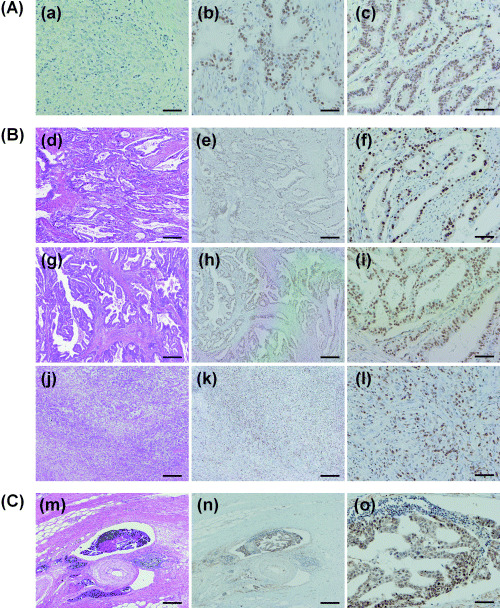
Immunohistochemical analysis of enhancer of zeste homolog 2 (EZH2) protein expression in gastric cancer tissues. (A) Representative results of immunostaining used for standard. (a) Negative staining of EZH2 in cancer cells. (b) Low level of EZH2 expression in nuclei of cancer cells. (c) High level of EZH2 expression in nuclei of cancer cells. (B) Representative results of EZH2 staining in different histological types of the tissues. (d) (e) (f): tubular adenocarcinoma. (g) (h) (i): papillary adenocarcinoma. (j) (k) (l): poorly differentiated adenocarcinoma. (C) An invasive gastric cancer cells forming intravascular emboli. EZH2 immunoreactivity was dominantly positive in the nuclei of the gastric cancer cells (f, i, l). Nuclear and cytoplasmic EZH2 immunoreactivity was detected in cancer cells forming intravascular emboli (n, o). Bar 500 µm (a, b, d, e, g, h, j, k, m, n). Bar 100 µm (c, f, i, l, o). a, b, c, e, f, g, h, i, k, l, n, o, immunoperoxidase stain. d, g, j, m, hematoxylin and eosin stain.
Correlation of EZH2 expression levels with clinicopathological parameters
Immunohistochemical expression levels and their associations with clinicopathological features in the 83 gastric cancers tissues samples are summarized in Table 1. More than half of the cases (47 cases, 56.6%) belonged to the high expression group. Conversely, none of the corresponding normal mucosa expressed high levels of EZH2 immunoreactivity. High levels of EZH2 expression in gastric cancer tissue were correlated with more malignant phenotypes including tumor size (= 40 mm versus = 39 mm; P = 0.0006), depth of invasion (pT1+pT2 versus pT3+pT4; P = 0.0096), lymphatic invasion (P = 0.0013), venous invasion (P = 0.0022), lymph node metastasis (P = 0.0023), and clinical stages (P = 0.0012). There was no significant correlation between cytoplasmic EZH2 immunoreactivity and clinicopathological factors in the gastric carcinoma tissue samples examined (data not shown).
Table 1.
Expression of enhancer of zeste homolog 2 (EZH2) in gastric cancer tissues and its correlation with clinicopathological parameters
| Number of cases | EZH2 expression † | P value* | ||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| n | (%) | n | (%) | |||
| Normal mucosa | 41 | 41 | (100) | 0 | (0) | <0.0001 |
| Gastric cancer | 83 | 36 | (43.4) | 47 | (56.6) | |
| Clinicopathological characteristics | ||||||
| Sex | ||||||
| Male | 59 | 23 | (39.0) | 36 | (61.0) | 0.2057 |
| Female | 24 | 13 | (54.2) | 11 | (45.8) | |
| Age (years) ‡ | ||||||
| ≥68 | 43 | 16 | (37.2) | 27 | (62.8) | 0.2400 |
| ≤67 | 40 | 20 | (50.0) | 20 | (50.0) | |
| Tumor size ‡ | ||||||
| ≥40 mm | 32 | 8 | (25.0) | 24 | (75.0) | 0.0006 |
| ≤39 mm | 51 | 28 | (54.9) | 23 | (45.1) | |
| Histological type § | ||||||
| Pap | 8 | 3 | (37.5) | 5 | (62.5) | 0.0023 |
| Tub | 37 | 13 | (35.1) | 24 | (64.9) | |
| Por | 27 | 9 | (33.3) | 18 | (66.7) | |
| Sig | 10 | 10 | (100) | 0 | (0) | |
| Muc | 1 | 1 | (100) | 0 | (0) | |
| Depth of invasion § | ||||||
| pT1+pT2 | 49 | 27 | (55.1) | 22 | (29.6) | 0.0096 |
| pT3+pT4 | 34 | 9 | (26.5) | 25 | (73.5) | |
| Lymphatic invasion § | ||||||
| (–) | 26 | 18 | (69.2) | 8 | (30.8) | 0.0013 |
| (+) | 57 | 18 | (31.6) | 39 | (68.4) | |
| Venous invasion § | ||||||
| (–) | 35 | 22 | (62.9) | 13 | (37.1) | 0.0022 |
| (+) | 48 | 14 | (29.2) | 34 | (70.8) | |
| Extent of lymph node metastasis § | ||||||
| N0 | 33 | 22 | (66.7) | 11 | (33.3) | 0.0023 |
| N1 | 24 | 4 | (16.7) | 20 | (83.3) | |
| N2 | 21 | 8 | (38.1) | 13 | (61.9) | |
| N3 | 5 | 2 | (40.0) | 3 | (60.0) | |
| Stage § | ||||||
| I | 30 | 21 | (70.0) | 9 | (30.0) | 0.0012 |
| II | 9 | 1 | (11.1) | 8 | (88.9) | |
| III | 23 | 9 | (39.1) | 14 | (60.9) | |
| IV | 21 | 5 | (23.8) | 16 | (76.2) | |
Data were analyzed by the χ2‐test and P < 0.05 was considered to be significant.
Degree of immunoreactivity of EZH2 was evaluated as follows: low, negative or <50% of tumor cells showing intense nuclear immunoreactivity; high, >50% of cells showing intense immunoreactivity in their nuclei.
‡ Tumor size and age were divided into two groups according to the median value.
§ Histological type, depth of invasion, stage, lymphatic invasion, venous invasion and extent of lymph node metastasis were determined according to the Japanese Classification of Gastric Cancer. Muc, mucinous adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; sig, signet ring cell carcinoma; tub, tubular adenocarcinoma.
Correlation of EZH2 expression levels with patient survival after surgery
Finally, we performed a prognostic study in 64 patients who received curative surgery from 1999 to 2002 and were followed up for 1–52 months. Seventeen of these patients died from gastric cancer at between 2 and 30 months (mean 11.4 months). The mean follow‐up time of the remaining 47 cases was 20 months. The results of the univariate analysis of survival rate calculated by the Kaplan‐Meier method are shown in Table 2 and Fig. 3. The calculation showed that the size of the tumor, depth of invasion, lymph node metastasis, distant metastasis, stage, and level of EZH2 expression were associated with an increased risk of death (Table 2). As shown in Fig. 3A, cases with gastric cancer expressing high levels of EZH2 had a worse prognosis than those expressing no or low levels of EZH2 (P = 0.0271). To compare the effect of EZH2 expression and the histological type of gastric cancer on the prognosis, we divided the cases into two groups: cohesive type (papillary adenocarcinoma, tubular adenocarcinoma, solid‐type poorly differentiated adenocarcinoma and mucinous adenocarcinoma) and non‐cohesive type (non‐solid type poorly differentiated adenocarcinoma, signet‐ring cell carcinoma). The survival rate was calculated for each group by the Kaplan‐Meier method and the results are shown in Fig. 3B. No statistically significant differences were observed between the two types (P = 0.7975). In addition, although cases of gastric cancer exhibiting high levels of EZH2 expression tend to have poorer prognosis than those with low levels of EZH2 expression when the T1 cases were excluded from Fig. 3A, a significant statistical difference was not observed between them (Fig. 3C, P = 0.6220).
Table 2.
Relationship between clinicopathological features of gastric cancer and survival
| Deaths/total | P‐value* | ||
|---|---|---|---|
| Sex | Male | 13/46 | 0.8311 |
| Female | 4/18 | ||
| Age (years) † | = 68 | 8/31 | 0.6178 |
| = 67 | 9/33 | ||
| Tumor size † | = 40 mm | 13/22 | <0.0001 |
| = 39 mm | 4/42 | ||
| Differentiation ‡ | Well | 8/34 | 0.7109 |
| Moderate/poor | 9/30 | ||
| Type § | Cohesive | 13/48 | 0.7975 |
| Non‐cohesive | 4/16 | ||
| Depth of invasion ¶ | pT1+pT2 | 3/39 | <0.0001 |
| pT3+pT4 | 14/25 | ||
| Lymph node metastasis ¶ | N0 | 3/27 | 0.0038 |
| N1+N2+N3 | 14/37 | ||
| Distant metastasis ¶ | M0 | 13/57 | 0.0003 |
| M1 | 4/7 | ||
| Stage ¶ | I+II+III | 7/47 | <0.0001 |
| IV | 10/17 | ||
| EZH2 expression | Low | 3/24 | 0.0271 |
| High | 14/40 | ||
Data were analyzed using the Kaplan‐Meier method and the log‐rank test was used to analyze differences in outcome. P < 0.05 was considered to be significant.
† Tumor size and age were divided into two groups according to the median value.
‡ Tumor was graded as well, moderately, or poorly differentiated and typed according to the World Health Organization classification system.
§ Cohesive type: papillary adenocarcinoma, tubular adenocarcinoma, solid type poorly differentiated adenocarcinoma and mucinous adenocarcinoma. Non‐cohesive type: non‐solid type poorly differentiated adenocarcinoma, signet‐ring cell carcinoma.
¶ Histological type, depth of invasion, extent of lymph node metastasis, distant metastasis and stage were determined according to the Japanese Classification of Gastric Cancer. EZH2, enhancer of zeste homolog 2.
Figure 3.
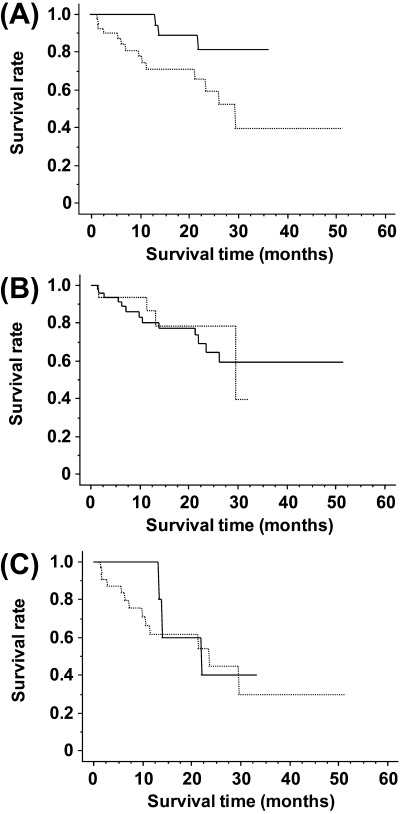
Kaplan‐Meier analysis of gastric cancers. (A) Cancer‐related survival rates showed significant difference between (…) high expression (n = 40) versus (‐) low and negative expression (n = 24) of enhancer of zeste homolog 2 (EZH2) in gastric cancer. P = 0.0271 log–rank (Mantel‐Cox). (B) Cancer‐related survival rates of patients whose histological type showed (‐) cohesive type (n = 48) or (…) non‐cohesive type (n = 16). P = 0.7975 log–rank (Mantel‐Cox). In 64 cases that had undergone curative surgery from 1999 to 2002 and followed up in our hospital, the mean follow‐up time of the 54 surviving cases was 20 months (range 1–52 months). Remaining 18 cases died between 2 and 30 months (mean 11.4 months). (C) Cancer‐related survival rates showed significant difference between (…) high expression (n = 32) versus (‐) low and negative expression (n = 8) of EZH2 in gastric cancer without the T1 cases. Statistical correlation was P = 0.6220 log–rank (Mantel‐Cox). In 40 cases that had undergone curative surgery from 1999 to 2002 and followed up in our hospital, the mean follow‐up time of the 24 surviving cases was 20 months (range 1–52 months). Remaining 16 cases died between 2 and 30 months (mean 11.4 months).
Discussion
The overexpression of EZH2 in advanced prostate cancer in comparison with benign prostate tissues and organ‐confined tumors was reported using cDNA microarray analysis.( 13 ) Because high EZH2 protein levels have been strongly associated with the aggressiveness and patient outcome of prostate cancer, it was proposed that the deregulation of EZH2 expression might promote the malignant transformation of normal cells involving DNA transcription.( 10 , 11 , 13 , 14 , 29 , 30 ) An association between EZH2 overexpression and the biological malignancy of the tumor has also been reported for breast cancer, lymphoma, hepatocellular carcinoma and bladder carcinoma.( 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 )
In the present study, the expression levels of EZH2 protein determined by western blot analyses were in good agreement with those of EZH2 mRNA determined by quantitative real‐time RT‐PCR in 13 gastric cancer cell lines. These results suggested that expression of the EZH2 protein was regulated primarily in the transcriptional process, and we decided to use this anti‐EZH2 antibody in the following immunohistochemical study. Immunohistochemical analysis was carried out to investigate if overexpression of EZH2 was actually reflected in the gastric cancer specimens taken surgically. We found that 56.6% (47 out of 83 cases) of gastric cancer tissues showed high expression of EZH2, which agreed with the previously reported frequency (44%, 8 out of 18 cases) by tissue microarray analysis.( 31 ) In the tumor cells, EZH2 immunoreactivity was mainly located in the nucleus, while some of the sections showed cytoplasmic staining with nuclear dominant patterns, which is also in good agreement with the previously reported results on breast cancer and prostate cancer.( 13 , 14 , 30 )
To examine the clinical use of EZH2 protein as a marker of gastric cancer progression, the associations between EZH2 and clinicopathological factors were evaluated. High levels of EZH2 expression in gastric cancer tissues were significantly associated with several clinicopathological factors including. tumor size, depth of invasion, vessel invasion, lymph node metastasis and clinical stage. These results strongly suggest that the association of EZH2 protein expression to tumor growth and cell invasion in gastric cancer is as reported for prostate cancer, breast cancer, bladder carcinoma and lymphomas. The implications of EZH2 in tumor growth and cell invasion may be explained by the existence of several target genes of EZH2. It is known that EZH2 works to suppress several genes, and a number of the target genes of EZH2 have been revealed by DNA microarrays.( 13 , 32 ) These include not only cell proliferation genes, but also metastasis‐suppressing genes such as Rho GTPase‐activating protein 1.( 13 )
Most of the preceding studies described the role of nuclear EZH2 in tumor progression. In addition to nuclear EZH2, we noted the expression of cytosolic EZH2, because cytosolic EZH2 has been recently reported to play a role in actin polymerization.( 33 ) Interestingly, we found that gastric cancer cells forming intravascular tumor emboli showed very strong cytosolic EZH2 immunoreactivity. This phenomenon may support the idea that the cells expressing cytoplasmic EZH2 have higher motility and therefore a greater ability to invade via the actin polymerization pathway.
In consequence, the results in this study showed there were no correlation between distant metastasis and cytosolic EZH2 expression. We speculated that the mechanism by which EZH2 increases the ability to move and invade is due to its property of polymerizing actins, but this property is not sufficient for establishment of distant metastasis because of improper changes in actin polymerizing balance. To establish distant metastasis, actin needs to be not only polymerized but also severed.( 34 ) This notion is reminiscent of gelsolin, an actin‐binding protein that regulates dynamic changes in the actin cytoskeleton by severing actin filaments into smaller pieces. Gelsolin by itself does not associated with lymph node metastasis, but becomes a prognostic factor when it coexists with erbB2/EGFR, receptors promoting actin polymerization.( 35 ) The factors that cooperate with EZH2 in actin dynamics are not understood yet. Evaluation of such factors and EZH2 together would be useful for predicting distant metastasis.
Although there was no relation between distant metastasis and cytoplasmic EZH2 expression in the present study (data not shown), the survival rate calculated by Kaplan‐Meier analysis revealed a significant correlation between nuclear EZH2 expression and prognosis. Several studies have been reported showing that the histological type of gastric cancers appeared to influence their prognosis.( 36 , 37 , 38 , 39 ) Because cancer cells having a lesser ability to aggregate (non‐cohesive type) are generally regarded as a type of gastric cancer with highly malignant potential in comparison with those having greater ability to aggregate (cohesive type), we wondered if high levels of EZH2 expression in different histological types of cancer consist with the correlation between high EZH2 expression and clinically malignant phenotypes of gastric cancers. Our analysis using the Kaplan‐Meier method demonstrated a significant correlation between EZH2 expression and prognosis, but did not demonstrate a correlation between histological type and prognosis. Additionally, because T1‐early gastric cancer cases may enhance patients’ outcome, we calculated the prognosis without the T1 cases. However, the number of the cases was not enough to give confidence in the obtained results; there was a slight tendency for poorer prognosis in the group with high levels of EZH2 expression.
Pathological staging is known as useful determinant of patient prognosis after curative surgery for gastric cancer and is a key factor affecting the choice of postoperative strategy.( 40 , 41 , 42 , 43 ) The present results support the notion that EZH2 expression could be used to screen a patient with aggressive gastric cancer and poor prognosis. Therefore, clinical therapy can be planned along with EZH2 expression level even though there was no metastasis detected at the time of surgery or primary diagnosis.
In summary, the present study showed for the first time the high correlation of EZH2 expression with poorer prognosis, and the applicability of screening EZH2 expression as a prognostic determinant. Furthermore, EZH2 might be a molecular target in the clinical treatment of gastric cancer; accordingly, further studies will be required to confirm these findings.
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (14–8), by a Grant‐in‐Aid for Scientific Research (B) 14370070 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Terry Fox Run Foundation for Cancer Research. The authors are grateful to Ms A. Obata for her technical assistance.
References
- 1. Yasui W, Yokozaki H, Shimamoto F, Tahara H, Tahara E. Molecular‐pathological diagnosis of gastrointestinal tissues and its contribution to cancer histopathology. Pathol Int 1999; 49: 763–74. [DOI] [PubMed] [Google Scholar]
- 2. Yasui W, Oue N, Kuniyasu H, Ito R, Tahara E, Yokozaki H. Molecular diagnosis of gastric cancer: present and future. Gastric Cancer 2001; 4: 113–21. [DOI] [PubMed] [Google Scholar]
- 3. Yokozaki H, Yasui W, Tahara E. Genetic and epigenetic changes in stomach cancer. Int Rev Cytol 2001; 204: 49–95. [DOI] [PubMed] [Google Scholar]
- 4. Chen H, Rossier C, Antonarakis SE. Cloning of a human homolog of the Drosophila enhancer of zeste gene (EZH2) that maps to chromosome 21q22.2. Genomics 1996; 38: 30–7. [DOI] [PubMed] [Google Scholar]
- 5. Cao R, Wang L, Wang H et al. Role of histone H3 lysine 27 methylation in Polycomb‐group silencing. Science 2002; 298: 1039–43. [DOI] [PubMed] [Google Scholar]
- 6. Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 2002; 111: 185–96. [DOI] [PubMed] [Google Scholar]
- 7. Kuzmichev A, Nishioka K, Erdjument‐Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of Zeste protein. Genes Dev 2002; 16: 2893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muller J, Hart CM, Francis NJ et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 2002; 111: 197–208. [DOI] [PubMed] [Google Scholar]
- 9. Kuzmichev A, Jenuwein T, Tempst P, Reinberg D. Different EZH2‐containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol Cell 2004; 14: 183–93. [DOI] [PubMed] [Google Scholar]
- 10. Tonini T, Bagella L, D’Andrilli G, Claudio PP, Giordano A. Ezh2 reduces the ability of HDAC1‐dependent pRb2/p130 transcriptional repression of cyclin A. Oncogene 2004; 23: 4930–7. [DOI] [PubMed] [Google Scholar]
- 11. Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex biomarker approach for determining risk of prostate‐specific antigen‐defined recurrence of prostate cancer. J Natl Cancer Inst 2003; 95: 661–8. [DOI] [PubMed] [Google Scholar]
- 12. Cao R, Zhang Y. The functions of E (Z)/EZH2‐mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 2004; 14: 155–64. [DOI] [PubMed] [Google Scholar]
- 13. Varambally S, Dhanasekaran SM, Zhou M et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419: 624–9. [DOI] [PubMed] [Google Scholar]
- 14. Kleer CG, Cao Q, Varambally S et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 2003; 100: 11606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raaphorst FM, Meijer CJ, Fieret E et al. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia 2003; 5: 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudo T, Utsunomiya T, Mimori K et al. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer 2005; 92: 1754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weikert S, Christoph F, Kollermann J et al. Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med 2005; 16: 349–53. [PubMed] [Google Scholar]
- 18. Arisan S, Buyuktuncer ED, Palavan‐Unsal N, Caskurlu T, Cakir OO, Ergenekon E. Increased expression of EZH2, a polycomb group protein, in bladder carcinoma. Urol Int 2005; 75: 252–7. [DOI] [PubMed] [Google Scholar]
- 19. Raaphorst FM, Van Kemenade FJ, Blokzijl T et al. Coexpression of BMI‐1 and EZH2 polycomb group genes in Reed‐Sternberg cells of Hodgkin's disease. Am J Pathol 2000; 157: 709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visser HP, Gunster MJ, Kluin‐Nelemans HC et al. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol 2001; 112: 950–8. [DOI] [PubMed] [Google Scholar]
- 21. Van Kemenade FJ, Raaphorst FM, Blokzijl T et al. Coexpression of BMI‐1 and EZH2 polycomb‐group proteins is associated with cycling cells and degree of malignancy in B‐cell non‐Hodgkin lymphoma. Blood 2001; 97: 3896–901. [DOI] [PubMed] [Google Scholar]
- 22. Dukers DF, Van Galen JC, Giroth C et al. Unique polycomb gene expression pattern in Hodgkin's lymphoma and Hodgkin's lymphoma‐derived cell lines. Am J Pathol 2004; 164: 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yanagihara K, Seyama T, Tsumuraya M, Kamada N, Yokoro K. Establishment and characterization of human signet ring cell gastric carcinoma cell lines with amplification of the c‐myc oncogene. Cancer Res 1991; 51: 381–6. [PubMed] [Google Scholar]
- 24. Yanagihara K, Ito A, Toge T, Numoto M. Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res 1993; 53: 5815–21. [PubMed] [Google Scholar]
- 25. Hojo H. Establishment of cultured cell lines of human stomach cancer – origin and their morphological characteristics. Niigata Igakukai Zasshi 1977; 91: 737–52 (in Japanese). [Google Scholar]
- 26. Motoyama T, Hojo H, Watanabe H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn 1986; 36: 65–83. [DOI] [PubMed] [Google Scholar]
- 27. Ochiai A, Yasui W, Tahara E. Growth‐promoting effect of gastrin on human gastric carcinoma cell line TMK‐1. Jpn J Cancer Res 1985; 76: 1064–71. [PubMed] [Google Scholar]
- 28. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma‐ 2nd English Edition. Gastric Cancer 1998; 1: 10–24. [DOI] [PubMed] [Google Scholar]
- 29. Sellers WR, Loda M. The EZH2 polycomb transcriptional repressor – a marker or mover of metastatic prostate cancer? Cancer Cell 2002; 2: 349–50. [DOI] [PubMed] [Google Scholar]
- 30. Foster CS, Falconer A, Dodson AR et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene 2004; 23: 5871–9. [DOI] [PubMed] [Google Scholar]
- 31. Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB‐E2F pathway, essential for proliferation and amplified in cancer. Embo J 2003; 22: 5323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen H, Tu SW, Hsieh JT. Down‐regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J Biol Chem 2005; 280: 22437–44. [DOI] [PubMed] [Google Scholar]
- 33. Su IH, Dobenecker MW, Dickinson E et al. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell 2005; 121: 425–36. [DOI] [PubMed] [Google Scholar]
- 34. Lambrechts A, Van Troys M, Ampe C. The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol 2004; 36: 1890–909. [DOI] [PubMed] [Google Scholar]
- 35. Thor AD, Edgerton SM, Liu S, Moore DH 2nd, Kwiatkowski DJ. Gelsolin as a negative prognostic factor and effector of motility in erbB‐2‐positive epidermal growth factor receptor‐positive breast cancers. Clin Cancer Res 2001; 7: 2415–24. [PubMed] [Google Scholar]
- 36. Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer 2000; 89: 1418–24. [PubMed] [Google Scholar]
- 37. Hyung WJ, Noh SH, Lee JH et al. Early gastric carcinoma with signet ring cell histology. Cancer 2002; 94: 78–83. [DOI] [PubMed] [Google Scholar]
- 38. Arai T, Esaki Y, Inoshita N et al. Pathologic characteristics of gastric cancer in the elderly. a retrospective study of 994 surgical patients. Gastric Cancer 2004; 7: 154–9. [DOI] [PubMed] [Google Scholar]
- 39. Nakamura K, Ogoshi K, Makuuchi H. Focus on the histologic diversity in primary and lymph node lesions and the outcome of gastric cancer. J Exp Clin Cancer Res 2004; 23: 15–23. [PubMed] [Google Scholar]
- 40. Kim JP, Kim YW, Yang HK, Noh DY. Significant prognostic factors by multivariate analysis of 3926 gastric cancer patients. World J Surg 1994; 18: 872–8. [DOI] [PubMed] [Google Scholar]
- 41. Soreide JA, Van Heerden JA, Burgart LJ, Donohue JH, Sarr MG, Ilstrup DM. Surgical aspects of patients with adenocarcinoma of the stomach operated on for cure. Arch Surg 1996; 131: 481–8. [DOI] [PubMed] [Google Scholar]
- 42. Kajiyama Y, Tsurumaru M, Udagawa H et al. Prognostic factors in adenocarcinoma of the gastric cardia: pathologic stage analysis and multivariate regression analysis. J Clin Oncol 1997; 15: 2015–21. [DOI] [PubMed] [Google Scholar]
- 43. Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer 1999; 85: 2119–23. [DOI] [PubMed] [Google Scholar]


