Abstract
A randomized controlled trial was conducted to evaluate the efficacy of high‐dose chemotherapy (HDC) as consolidation of the treatment of high‐risk postoperative breast cancer. Patients under 56 years of age with stage I to IIIB breast cancer involving 10 or more axillary lymph nodes were eligible. The primary endpoint was relapse‐free survival (RFS). Between May 1993 and March 1999, 97 patients were enrolled, and two patients became ineligible. The median age of the 97 patients was 46 years (range 27–55 years), and 72 (74%) were premenopausal. The median number of involved axillary nodes was 16 (range 10–49). All patients had undergone a radical mastectomy. Major characteristics were well balanced between the treatment arms. Forty‐eight patients in the standard‐dose (STD) arm received six courses of cyclophosphamide, doxorubicin, and 5‐fluorouracil followed by tamoxifen. Forty‐nine patients were assigned to undergo HDC with cyclophosphamide and thiotepa after six courses of cyclophosphamide, doxorubicin, and 5‐fluorouracil followed by tamoxifen; however, 15 of these patients (31%) did not undergo HDC. HDC was well tolerated without any treatment‐related mortality. At a median follow‐up of 63 months, the 5‐year RFS of 47 eligible patients in the STD arm and 48 eligible patients in the HDC arm was 37% and 52% on an intent‐to‐treat basis, respectively (P = 0.17). Five‐year overall survival of all randomized patients was 62% for the STD arm and 63% for the HDC arm (P = 0.78). Although the prespecified values of the two arms were not so accurate as to allow detection of the observed difference, no advantage of HDC was observed in terms of RFS or overall survival. (Cancer Sci 2008; 99: 145–151)
Preclinical studies have suggested that doses of cytotoxic chemotherapy correlate with the cure of cancer patients.( 1 ) Among several kinds of dose‐intensification strategies, high‐dose chemotherapy (HDC) with autologous hematopoietic stem cell support has been extensively investigated in clinical oncology. In addition, HDC was shown to produce survival advantages in certain types of malignant neoplasms, including relapsed aggressive non‐Hodgkin's lymphoma responding to salvage chemotherapy.( 2 ) and untreated multiple myeloma( 3 , 4 ) in randomized controlled studies.
Adjuvant chemotherapy has been shown to improve relapse‐free survival (RFS) and overall survival (OS) in patients with primary breast cancer( 5 ) and dose‐intensification was found to be associated with superior outcomes in some populations.( 6 ) However, the prognosis of patients with extensive axillary lymph node involvement is still poor despite conventional‐dose adjuvant chemotherapy. Thus, such patients have been considered to be appropriate candidates for clinical trials of HDC.
Several uncontrolled studies have suggested a survival advantage for HDC in the adjuvant treatment of high‐risk primary breast cancer with extensive axillary lymph node involvement.( 7 , 8 , 9 , 10 , 11 ) At the time of writing, 12 adequately conducted randomized controlled trials comparing HDC with standard‐dose (STD) or conventional‐dose chemotherapy in high‐risk postoperative breast cancer patients have been reported.( 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ) In 10 of them, the advantage of HDC was not shown. However, two of them have shown improved RFS from HDC( 18 , 22 ) and one study has shown an OS benefit.( 22 ) Thus, its role in the treatment of high‐risk primary breast cancer is still inconclusive and deserves further attention.
Based on the promising results of uncontrolled phase II trials of HDC for high‐risk primary breast cancer, especially those of the Duke series including patients enrolled into the Cancer and Leukemia Group B (CALGB) study 8782, reported by Peters et al.( 8 ) phase I/II studies of cyclophosphamide and thiotepa with autologous bone marrow reinfusion( 24 , 25 ) and our own earlier feasibility study of HDC of cyclophosphamide and thiotepa with autologous stem cell reinfusion against metastatic breast cancer( 26 ) the Japan Clinical Oncology Group (JCOG)( 27 ) conducted a randomized controlled study to evaluate the efficacy of HDC of cyclophosphamide and thiotepa as consolidation of the treatment for high‐risk postoperative breast cancer.
Patients and Methods
Patients. The study was designed for women between 15 and 55 years of age with breast cancer, stage I to IIIB, involving 10 or more axillary nodes, histologically confirmed by level II or further dissection. Eligible patients had to have a performance status rating of 0 or 1 according to the Eastern Cooperative Oncology Group (ECOG) criteria.( 28 ) Exclusion criteria were prior chemotherapy, radiotherapy, and endocrine therapy. Patients were required to have adequate bone marrow, hepatic, renal, cardiac, and respiratory functions (leukocyte count ≥3.5 × 109/L; hemoglobin ≥10 g/dL; platelet count ≥100 × 109/L; aspartate aminotransferase and alanine aminotransferase ≤4 times the upper normal limit; total bilirubin ≤1.5 times the upper normal limit; blood urea nitrogen and serum creatinine within normal limits; creatinine clearance ≥60 mL/min; no severe cardiac disorder on electrocardiogram; ejection fraction ≥50%; and PaO2 ≥70 mmHg). Physical examination, chest X‐ray, abdominal ultrasound examination, brain computed tomography and a radionuclide bone scan had to be negative for distant metastases. Negative result for bone marrow aspiration or biopsy from the posterior iliac bone was also required.
Patients meeting any one of the following criteria were excluded from the trial: contralateral breast cancer; active concurrent cancer; active peptic ulcer; seropositive for hepatitis B virus surface antigen, hepatitis C virus antibody, or HIV antibody; liver cirrhosis; pulmonary fibrosis or chronic obstructive lung disease; severe psychiatric disorder; diabetes mellitus requiring insulin treatment; uncontrollable hypertension (diastolic pressure ≥110 mmHg); hypercalcemia (serum Ca ≥11 mg/dL); pregnancy or lactation; history of cardiac failure or renal failure; or evidence of concurrent bacterial and fungal infection.
This clinical trial was planned to be conducted at 11 centers belonging to the Autologous Bone Marrow Transplantation Study Group and the Breast Cancer Study Group of JCOG. The JCOG 9208 study protocol and the informed consent document complying with JCOG guidelines and policies were approved by the Clinical Trial Review Committee of JCOG and by the institutional review committee of each participating institution before the start of the study.( 27 ) All patients provided their written or oral consent before the start of the study. Registration involved a telephone call or facsimile from the participating physicians to the JCOG Statistical/Data Center, National Cancer Center, Tokyo, Japan (1991–1997, Statistical Center; 1998–, Data Center).( 27 ) The attending physicians were responsible for submitting periodic data reports on toxicity, relapse, and survival.
Treatment. As shown in Fig. 1, eligible patients were randomly assigned to the STD or HDC arm at the time of enrolment by minimization method to balance the numbers of positive axillary nodes (10–19 or 20–), menopausal status (pre or post) and institution between the arms.
Figure 1.

Trial design of Japan Clinical Oncology Group study, JCOG 9208. ABMT, autologous bone marrow transplantation; CAF, cyclophosphamide, doxorubicin, 5‐fluorouracil; G‐CSF, granulocyte colony‐stimulating factor; HDC, high‐dose chemotherapy; PBSC, peripheral blood stem cell; STD, standard‐dose; TAM, tamoxifen.
Patients assigned to the STD arm were planned to receive six courses of cyclophosphamide, doxorubicin and 5‐fluorouracil (CAF) at 21‐day intervals. Each course consisted of intravenous injection with cyclophosphamide 500 mg/m2, doxorubicin 40 mg/m2, and 5‐fluorouracil 500 mg/m2. The first course of CAF chemotherapy had to be initiated within 10 weeks after primary surgery.
Patients assigned to the HDC arm underwent bone marrow procurement under general anesthesia before CAF chemotherapy within 9 weeks after primary surgery. Typically, 1 week after primary surgery, they received the first course of CAF together with lenograstim (granulocyte colony‐stimulating factor) to collect peripheral blood stem cells (PBSC) as previously described.( 29 ) Lenograstim was given subcutaneously daily from day 8 after CAF chemotherapy until the day of the last leukapheresis. Leukapheresis was carried out once or twice when the leukocyte count increased to greater than 10 × 109/L as described previously.( 26 ) At least 3 weeks after the sixth course of CAF chemotherapy, the patients underwent HDC consisting of cyclophosphamide 2000 mg/m2/day and thiotepa 200 mg/m2/day for three consecutive days (days –5 to –3). The doses of cyclophosphamide and thiotepa were determined based on the results of combination phase I/II studies( 24 , 25 ) and our own feasibility study.( 26 ) Autologous bone marrow and PBSC were thawed and infused on day 0 and 1, respectively. All patients received oral antibiotics, antifungal agents, sulfamethoxazole/trimethoprim and oral acyclovir (200 mg × 5, daily) prophylactically. Irradiated platelet transfusions were given to maintain the platelet count above 20 × 109/L, and irradiated red blood cells were given if necessary. Then 5 µg/kg lenograstim was started on day 2.
Following the above‐described therapy, all patients received tamoxifen 20 mg/day for at least 2 years, irrespective of receptor status. Radiation therapy was not planned. All toxicities were graded according to the toxicity grading criteria of JCOG,( 30 ) a modified and expanded version of the National Cancer Institute – Common Toxicity Criteria version 1.0.
Baseline evaluation included staging examination (mammography, bone scintigram, brain computed tomography, chest X‐ray, abdominal ultrasonography, and bone marrow aspiration/biopsy), complete medical history, physical examination, complete blood cell count, serum chemistry, urinary analysis, tumor marker, and estrogen receptor/progesterone receptor. Restaging evaluation, including chest X‐ray, bone scintigram, abdominal ultrasonography, and tumor marker, was conducted every 3–4 months for the first 3 years, and every 6 months for the subsequent 2 years. Central monitoring was carried out every 6 months throughout the study.
Study design and statistical analysis. The primary endpoint was RFS and secondary endpoints were OS and toxicity. RFS was defined as the time from randomization to the first observation of relapse or death due to any cause. OS was defined as the time from randomization to the time of death due to any cause. Survival curves were estimated by the Kaplan–Meier method and compared using the log–rank test.
All eligible patients were analyzed as a data set. To detect a 40% increase in RFS at 5 years of the HDC arm compared with 30% of the STD arm at a significance level of 5% by two‐sided log–rank test and a power of 80%, 25 patients are required in each arm. Three years of accrual time and 4 years of follow‐up time from the last patient enrolment were assumed initially. As up to 25% of patients in the HDC arm might fail to receive HDC, we estimated a requirement of 100 patients in total (50 patients in each arm) in order to have sufficient statistical power at the beginning of the study.
Patient enrolment into this trial was closed in March 1999, and the actual accrual period was 5.8 years. The follow‐up time from the last patient enrolment was amended to 2 years, as approved by the JCOG Data and Safety Monitoring Committee in September 2000.( 27 ) Statistical re‐calculation revealed 90% power to detect a 30% increase in RFS at 5 years or 60% power to detect a 20% increase with a significance level of 5% by one‐sided log–rank test. No interim analysis was carried out.
Results
Patients. Between May 1993 and March 1999, a total of 97 patients were enrolled from eight institutions. Two patients were ruled ineligible, as one had stage IV disease and the other was enrolled after the start of chemotherapy. Median age was 46 years (range 27–55 years), and 72 patients (74%) were premenopausal. The median number of involved axillary nodes was 16 (range 10–49), and 41 patients (42%) had 20 or more positive axillary nodes. All patients had undergone a radical mastectomy. Forty‐eight patients were assigned to receive six courses of CAF (STD arm), and 49 patients were assigned to receive additional HDC with autologous stem cell support (HDC arm). The treatment groups were well balanced in terms of characteristics such as age, menopausal status, performance status, number of positive axillary nodes, stage, and hormone receptor status (Table 1).
Table 1.
Characteristics of all randomized patients in the Japan Clinical Oncology Group study, JCOG 9208
| Treatment arm | Standard‐dose | High‐dose | |
|---|---|---|---|
| No. of enrolled patients | 48 | 49 | |
| Median age in years (range) | 47 (27–55) | 46 (29–55) | |
| Menopause | Pre/post | 34/14 | 38/11 |
| PS | 0/1 | 41/7 | 46/3 |
| No. of positive axillary nodes | Median (range) | 18 (10–46) | 16 (10–49) |
| 10–19 | 28 | 28 | |
| 20– | 20 | 21 | |
| Stage | I | 2 | 2 |
| IIA | 8 | 12 | |
| IIB | 18 | 16 | |
| IIIA | 10 | 9 | |
| IIIB | 10 | 9 | |
| IV | 0 | 1 † | |
| ER | +/–/unknown | 29/19/0 | 25/22/2 |
| PgR | +/–/unknown | 25/22/1 | 22/24/3 |
Ineligible. ER, estrogen receptor; No., number; PgR, progesterone receptor; PS, performance status (0 or 1 according to the Eastern Cooperative Oncology Group criteria( 28 )).
Fifteen patients (31%) in the HDC arm did not receive HDC, including seven recurrences during or immediately after CAF therapy, seven refusals and one ineligible patient (Fig. 2). One patient in the HDC arm did not receive high‐dose cyclophosphamide on day –3 due to the development of grade 4 arrhythmia (complete atrioventricular block). In addition to the one ineligible, five patients in the STD arm did not complete the planned six courses of CAF therapy, consisting of three recurrences and two refusals. Therefore, of the 97 patients enrolled, 76 (80%) of 95 eligible patients completed the planned treatments.
Figure 2.
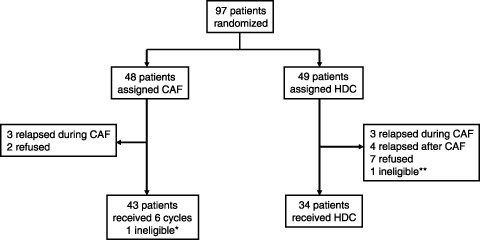
Trial profile of Japan Clinical Oncology Group study, JCOG 9208. *Registered after the start of cyclophosphamide, doxorubicin, 5‐fluorouracil (CAF; violation). **Bone marrow involvement was revealed before the start of CAF.
Major deviations from the protocol were: CAF chemotherapy given despite the presence of grade 2 leukopenia (four patients in the STD arm and nine in the HDC arm); CAF given despite hepatic transaminase elevation >4 times the upper normal limit (one patient in each arm); interval shortening and/or prolongation between the cycles of CAF (four patients in the STD arm and three in the HDC arm); initiation of CAF more than 10 weeks after primary surgery (one HDC patient); and a larger dose (140% of the planned doses) of cyclophosphamide and 5‐fluorouracil in the first cycle of CAF (one HDC patient).
RFS and OS. Seven years after patient recruitment was completed, 52 (54%) of the 97 enrolled patients were alive. Sixty‐one (64%) of the 95 eligible patients relapsed or died, 33 (70%) of 47 patients in the STD arm and 28 (58%) of 48 in the HDC arm. Primary analysis was carried out for all 95 eligible patients. At 5 years, RFS of 47 eligible patients in the STD arm and 48 eligible patients in the HDC arm was 37% (95% confidence interval [CI], 23–51%) and 52% (95% CI, 37–66%), respectively (two‐sided log–rank, P = 0.17) (Fig. 3). Estimated median RFS time was 32 months (95% CI, 23–79 months) for the STD arm and 70 months (95% CI, 36 months–) for the HDC arm. Five‐year survival of all randomized patients was 62% (95% CI, 48–76%) for the STD arm and 63% (95% CI, 50–77%) for the HDC arm (P = 0.78) (Fig. 4). Estimated median survival time was 87 months for the STD arm (95% CI, 55 months–) and was 110 months for the HDC arm (95% CI, 57 months–).
Figure 3.
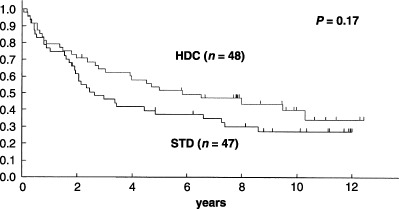
Relapse‐free survival (RFS) of all eligible patients in the Japan Clinical Oncology Group study, JCOG 9208. At 5 years, the intent‐to‐treat RFS of 47 eligible patients in the standard‐dose (STD) arm and 48 eligible patients in the high‐dose chemotherapy (HDC) arm was 37% and 52%, respectively (one‐sided log–rank, P = 0.17). Estimated median RFS time was 36 months for the STD arm and 60 months for the HDC arm.
Figure 4.

Overall survival (OS) of all randomized patients in the Japan Clinical Oncology Group study, JCOG 9208. Five‐year OS of all randomized patients was 62% for the standard‐dose arm and 63% for the high‐dose chemotherapy arm (one‐sided log–rank, P = 0.78).
Toxicity. The HDC treatment was well tolerated, without any treatment‐related mortality. All 34 patients receiving HDC actually developed grade 4 leukopenia and grade 4 neutropenia; 27 (79%) developed grade 4 and the other seven grade 3 thrombocytopenia. Hematological status was restored in all patients. Non‐hematological toxicities of HDC in 34 patients are shown in Table 2. Three patients developed grade 4 non‐hematological toxicities. One developed grade 4 diarrhea on day 4 (9 days after the start of HDC) and recovered 2 days later. Another showed transient grade 4 elevation of hepatic transaminase on day 13 (18 days after the start of HDC). The third patient developed grade 4 arrhythmia (complete atrioventricular block) on day –3 (the third day of HDC), and completely recovered by day 11 (14 days later).
Table 2.
Non‐hematological toxicities of high‐dose chemotherapy in 34 patients in the Japan Clinical Oncology Group study, JCOG 9208
| Toxicity | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) | Any grade (%) |
|---|---|---|---|---|---|
| Nausea/vomiting | 3 (9) | 9 (26) | 22 (65) | 0 | 34 (100) |
| Diarrhea | 10 (29) | 11 (32) | 9 (26) | 1 (3) | 31 (91) |
| Mucositis | 16 (47) | 3 (9) | 5 (15) | 0 (0) | 24 (71) |
| Arrhythmia | 3 (9) | 1 (3) | 1 (3) | 0 (0) | 6 (18) |
| Infection | 9 (26) | 9 (26) | 2 (6) | 1 (3) | 20 (59) |
| Bilirubin | 0 | 4 (12) | 1 (3) | 0 (0) | 5 (15) |
| AST | 15 (44) | 12 (35) | 5 (15) | 0 (0) | 32 (94) |
| ALT | 10 (29) | 13 (38) | 7 (21) | 1 (3) | 31 (91) |
No therapy‐related death was observed during high‐dose chemotherapy. All toxicities were graded according to the toxicity grading criteria of JCOG,( 30 ) a modified and expanded version of the National Cancer Institute – Common Toxicity Criteria version 1.0. AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Of 93 patients who actually underwent CAF therapy, seven patients (8%) developed grade 4 neutropenia, but none developed grade 4 non‐hematological toxicities. All the toxicities of CAF therapy were transient.
Discussion
In the present phase III study, we evaluated the efficacy of HDC in high‐risk postoperative patients involving 10 or more axillary nodes, using a common CAF regimen as an induction therapy, and HDC as a consolidation after CAF therapy. So far, 13 randomized controlled studies to evaluate the use of HDC in high‐risk primary breast cancer have been reported( 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ) including the first report of our study.( 31 ) In the present report, we have updated the analysis of the study, now with a median follow‐up of 63 months. However, our study was unable to show any advantage of HDC in terms of RFS or OS.
In our first report, the 4‐year RFS of the STD arm was 43% and that of the HDC arm was 61%, showing a trend favoring the latter, although there was no statistical significance between the two arms (P = 0.12).( 31 ) In this analysis, the 5‐year RFS of the STD and HDC arms was 37% and 52%, respectively, again without statistical significance. When we designed this randomized study in 1992, we anticipated a 5‐year RFS of 30% for the STD arm, based on the results of consecutive clinical trials conducted by CALGB (CALGB 7581 and CALGB 8082)( 8 , 32 , 33 ) and the historical series in the National Cancer Center Hospital in Japan; the 5‐year RFS and 10‐year RFS of patients involving 10 or more axillary nodes were 30% and 19%, respectively (unpublished data). However, we expected a 5‐year RFS of 70% for the HDC arm, based on the results of the phase II study by the Duke group.( 8 ) When we took a closer look at these results, and in particular the selection biases in phase II studies, it seemed likely that the expected difference in RFS between the two arms was too large. As the present study was small and did not have sufficient statistical power to detect small differences (90% power to detect 30% increase in RFS at 5 years or 60% power to detect 20% increase with a significance level of 5% by one‐sided test), there remains a possibility that a smaller advantage for the HDC was missed. However, the absence of a trend favoring the HDC arm in OS (P = 0.75) suggests that the survival advantage for the HDC would be minimal even if it exists.
The 4‐year RFS of 61% for the HDC arm was similar to that of the collected data of the Autologous Blood and Marrow Transplant Registry (ABMTR).( 34 ) However, the RFS data for the HDC arm in the present study was inferior to that of the Duke series (63% of 5‐year RFS in the present study versus 71% in 5‐year event‐free survival [EFS] in the Duke series).( 16 ) In the Duke series, only 10% of patients had 20 or more axillary node metastases (median, 14), whereas it was 41% of patients in the present study (median, 16). The higher RFS in the Duke series could be explained partly because they contained more patients with lower risk than the present study. Another possible explanation is that cyclophosphamide and thiotepa of the HDC regimen used in the present study might be less active than cyclophosphamide, carmustine, and cisplatin used in the Duke series.( 7 , 8 ) The cyclophosphamide and thiotepa regimen was most common in HDC for stage II or III or inflammatory breast cancer, followed by the cyclophosphamide, thiotepa, and carboplatin regimen, according to analysis of ABMTR.( 34 ) Although these two regimens have never been directly compared in a randomized fashion, the analysis of 3451 metastatic breast cancer patients in ABMTR suggested that the HDC regimen did not affect prognosis.( 34 ) Furthermore, two other studies recruiting patients with 10 or more positive axillary nodes( 19 , 20 ) showed a 6‐year RFS of 48% and a 4‐year RFS of 52% for the HDC arms, respectively, similar to our results.
In contrast to the RFS results in the HDC arm, the 5‐year RFS of 37% for the STD arm was higher than initially anticipated. According to the abstract for the annual meeting of ASCO in 1992 by Peters et al.( 8 ) 3‐year EFS of the historical control series from CALGB using adjustment for duration of follow‐up and selected for age less than 56 years, involvement of 10 or more axillary nodes, and freedom from failure of at least 5 months was 30% in CALGB 8082 and 38% in CALGB 7581.( 8 , 32 , 33 ) In an intergroup phase III study, 6‐year RFS of 257 patients with 10 or more positive nodes in the conventional‐dose arm was 46%( 19 ) and in a German study, 4‐year RFS in the conventional‐dose arm was 42%.( 20 ) Thus, it is unlikely that RFS in the conventional‐dose arm was too high in the present study. As Peto commented on the trend towards a sizeable reduction in breast cancer mortality during the last decade, small improvements might add up to a large beneficial effect( 35 ) in addition to patient selection( 36 , 37 ) and stage migration.( 38 )
In the present study, all patients received tamoxifen 20 mg/day for at least 2 years, irrespective of receptor status. In the German study( 20 ) tamoxifen was not planned in the initial protocol, although it was amended.to prescribe tamoxifen for patients with positive hormone‐receptor status simultaneously in the HDC and STD arms. According to the Dutch study protocol( 15 ) all patients originally received tamoxifen (40 mg/day) for 2 years. Because of the increasing evidence for treatment with tamoxifen in hormone receptor‐positive patients, the protocol was amended and only patients with hormone receptor‐positive cancer continued to receive tamoxifen for an additional 3 years. On the contrary, in the ECOG study( 19 ) tamoxifen (20 mg/day) was to be given for 5 years to hormone receptor‐positive patients in line with current recommendations. Furthermore, in the present study, adjuvant radiotherapy was originally prohibited, as regional radiotherapy had not been established when the protocol was designed. In the German study( 20 ) as well as the Dutch study( 15 ) it was not initially specified. In contrast, 50 Gy of regional radiotherapy was to be given in the ECOG study.( 19 ) Thus, even in terms of tamoxifen treatment and regional radiotherapy after chemotherapy, protocols in the trials were varied. The results from the single trials and the meta‐analysis were inconclusive. HDC should be further investigated in the context of contemporary therapies such as taxanes, dose‐dense therapy, hormonal therapy, and radiotherapy.
Of 49 patients assigned to the HDC arm, 15 patients (31%) did not undergo the HDC, which was more than expected (up to 25%). Seven had relapsed before HDC, and seven refused it. When we compared the 69% (34/49) of patients in the HDC arm actually receiving HDC with the results of large studies (96%[264/274] in a Scandinavian study,( 14 ) 90%[397/442] in a Dutch national phase III study,( 15 ) 84%[214/254] in the ECOG study,( 19 ) and 82%[123/150] in the German study( 20 )), fewer patients could complete HDC in the present study. In the US intergroup trial,( 16 ) randomization was carried out after completion of the induction chemotherapy. This might have been a better option for the present trial.
In the present study, the effectiveness of HDC as consolidation was not confirmed in patients with high‐risk postoperative breast cancer involving 10 or more axillary nodes. In the PEGASE 01 trial (n = 314) enrolling patients with eight or more positive axillary nodes, 3‐year RFS was 71% and 55% (P = 0.002) for the HDC and STD arms, respectively.( 18 ) Recently, Nitz et al. published the most successful results of HDC in the West German Study Group study.( 22 ) In that study, tandem HDC was compared with dose‐dense chemotherapy in 403 patients with at least nine positive nodes (mean, 17.6). Patients in the HDC arm received two cycles of standard‐dose EC (epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2) at 2‐week intervals followed by two cycles of HDC with epirubicin 90 mg/m2, cyclophosphamide 3000 mg/m2, and thiotepa 400 mg/m2 every 21 days with autologous hematopoietic stem cell support. Patients in the control arm received dose‐dense chemotherapy with four cycles of standard‐dose EC followed by three cycles of cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, and 5‐fluorouracil 600 mg/m2 at 2‐week intervals. With a median follow‐up time of 48.6 months, 4‐year EFS was 60% in the HDC arm and 44% in the control arm (P = 0.00069). The 4‐year OS rates were 75% and 70% (P = 0.02), respectively. Although an early and rapidly cycled tandem HDC might be a promising approach to be prospectively examined, the efficacy of HDC in the treatment of high‐risk primary breast cancer nonetheless remains inconclusive.
Retrospective subgroup analyses to find subsets with more benefit from HDC have been reported, but because of the limited sample size this could not be carried out in the present study. In the Dutch study, patients with HER2‐negative disease benefited from HDC with a hazard ratio (HR) of 0.68 for RFS (P = 0.002) and 0.72 for OS (P = 0.02).( 39 ) In the West German Study Group trial, retrospective subgroup analyses for triple negative patients showed that tandem HDC did significantly better than the control arm in terms of RFS (HR = 0.31) and OS (HR = 0.35, P = 0.011).( 40 )
In the present study, no treatment‐related death occurred in either treatment arm. The ABMTR database reported that 3% of patients treated with HDC died within 100 days after transplantation in stage II or III or inflammatory breast cancer.( 34 ) Peters et al. reported a treatment‐related mortality of 12% in the Duke series( 7 ) and 7% in the HDC arm in the US intergroup trial.( 16 ) Although the present trial was the first multi‐institutional study using HDC for primary breast cancer patients in Japan, HDC could be safely used by the JCOG members.
List of Participants
K. Tobinai (study chairman), M. Narabayashi, K. Takeyama, T. Yokozawa, M. Shimoyama, M. Ando, N. Katsumata, T. Watanabe, I. Adachi, R. Tanosaki, T. Fukutomi, S. Akashi, T. Nanasawa (National Cancer Center Hospital, Tokyo); T. Tajima, Y. Tokuda, A. Okumura (Tokai University School of Medicine, Isehara); M. Sano, T. Chou, H. Makino (Niigata Cancer Center Hospital, Niigata); Y. Sasaki, T. Igarashi, T. Ohtsu, K. Itoh, H. Fujii, H. Minami, S. Imoto (National Cancer Center Hospital East, Kashiwa); Y. Morishima, M. Ogura, Y. Kagami, H. Taji, S. Miura, H. Murai (Aichi Cancer Center, Nagoya); S. Okamoto, A. Ishida, Y. Ikeda, T. Ikeda, K. Enomoto (Keio University School of Medicine, Tokyo); M. Ogita (Sapporo National Hospital, Sapporo); M. Kasai, Y. Kiyama, N. Kobayashi (Sapporo Hokuyu Hospital, Sapporo); T. Kobayashi (Jikei University of School of Medicine, Tokyo); S. Takashima (chairman of the Breast Cancer Study Group of JCOG) (National Shikoku Cancer Center, Matsuyama); M. Niimi, N. Ishizuka, H. Fukuda (JCOG Data Center, National Cancer Center Research Institute, Tokyo).
Acknowledgments
We are grateful to Ms. K. Tajima (JCOG Statistical Center, National Cancer Center) and Mr N. Ishizuka (JCOG Data Center, National Cancer Center) for data management, and to Dr Y. Ohashi (University of Tokyo) for valuable advice concerning the study design and statistical considerations. We are greatly appreciative of all of the members of the Autologous Bone Marrow Transplantation Study Group and the Breast Cancer Study Group of JCOG and the JCOG Statistical/Data Center participating in this long‐term study. This study was supported by Grants‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (2S‐1, 5S‐1, 8S‐1, 11S‐1, 14S‐1, 14S‐5, 17S‐1, 17S‐5) (1990–present), for the Second‐Term Comprehensive Ten‐Year Strategy for Cancer Control (H10‐Gan‐027, H12‐Gan‐012) from the Ministry of Health, Labor and Welfare (1994–present), and for Basic Research from the Science and Technology Agency (1991–1993). Chugai Pharmaceutical Co. is acknowledged for supplying lenograstim for the accompanying lenograstim study for PBSC mobilization.
References
- 1. Skipper HE. Dose intensity versus total dose chemotherapy: an experimental basis. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia, PA: Lippincott, 1990: 43–64. [PubMed] [Google Scholar]
- 2. Philip T, Guglielmi C, Hagenbeek A et al . Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–5. [DOI] [PubMed] [Google Scholar]
- 3. Attal M, Harousseau J‐L, Stoppa A‐M, Sotto J‐J et al ., for The Intergroupe Français du Myélome . A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med 1996; 335: 91–9. [DOI] [PubMed] [Google Scholar]
- 4. Child JA, Morgan GJ, Davies FE et al ., for the Medical Research Council Adult Leukaemia Working Party . High‐dose chemotherapy with hematopoietic stem‐cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–83. [DOI] [PubMed] [Google Scholar]
- 5. Clarke M, Collins R, Darby S, Davies C et al ., Early Breast Cancer Trialists’ Collaborative Group (EBCTCG ). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–717. [DOI] [PubMed] [Google Scholar]
- 6. Wood WC, Budman DR, Korzun AH et al . Dose and dose intensity of adjuvant chemotherapy for stage II, node‐positive breast carcinoma. N Engl J Med 1994; 330: 1253–9. [DOI] [PubMed] [Google Scholar]
- 7. Peters WP, Ross M, Vredenburgh JJ et al . High‐dose chemotherapy and autologous bone marrow support as consolidation after standard‐dose adjuvant therapy for high‐risk primary breast cancer. J Clin Oncol 1993; 11: 1132–43. [DOI] [PubMed] [Google Scholar]
- 8. Peters WP, Ross M, Vredenburgh J et al . High‐dose alkylating agents and autologous bone marrow support (ABMS) for stage II/III breast cancer involving 10 or more axillary lymph nodes (Duke and CALGB 8782). Proc Am Soc Clin Oncol 1992; 11: 58a (Abstract). [Google Scholar]
- 9. Peters W, Berry D, Vredenburgh JJ et al . Five‐year follow‐up results of high‐dose combination alkylating agents with ABMT as consolidation after standard‐dose CAF for primary breast cancer involving 10 axillary lymph nodes. Proc Am Soc Clin Oncol 1995; 14: 317 (Abstract). [Google Scholar]
- 10. Gianni A, Siena S, Bregni M et al . Five‐year results of high‐dose sequential adjuvant chemotherapy in breast cancer with 10 positive nodes. Proc Am Soc Clin Oncol 1995; 14: 90 (Abstract). [DOI] [PubMed] [Google Scholar]
- 11. Bearman SI, Overmoyer BA, Bolwell BJ et al . High‐dose chemotherapy with autologous peripheral blood progenitor cell support for primary breast cancer in patients with 4–9 involved axillary lymph nodes. Bone Marrow Transplant 1997; 20: 931–7. [DOI] [PubMed] [Google Scholar]
- 12. Schrama JG, Faneyte IF, Schornagel JH et al . Randomized trial of high‐dose chemotherapy and hematopoietic progenitor‐cell support in operable breast cancer with extensive lymph node involvement: final analysis with 7 years of follow‐up. Ann Oncol 2002; 13: 689–98. [DOI] [PubMed] [Google Scholar]
- 13. Hanrahan EO, Broglio K, Frye D et al . Randomized trial of high‐dose chemotherapy and autologous hematopoietic stem cell support for high‐risk primary breast carcinoma. Follow‐up at 12 years. Cancer 2006; 106: 2327–36. [DOI] [PubMed] [Google Scholar]
- 14. Bergh J, Wiklund T, Erikstein B et al. , for the Scandinavian Breast Group 9401 study . Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow‐supported high‐dose chemotherapy as adjuvant treatment for high‐risk breast cancer: a randomised trial. Lancet 2000; 356: 1384–91. [DOI] [PubMed] [Google Scholar]
- 15. Rodenhuis S, Bontenbal M, Beex LVAM et al ., for the Netherlands Working Party on Autologous Transplantation in Solid Tumors . High‐dose chemotherapy with hematopoietic stem‐cell rescue for high‐risk breast cancer. New Engl J Med 2003; 349: 7–16. [DOI] [PubMed] [Google Scholar]
- 16. Peters WP, Rosner GL, Vredenburgh JJ et al . Prospective, randomized comparison of high‐dose chemotherapy with stem‐cell support versus intermediate‐dose chemotherapy after surgery and adjuvant chemotherapy in women with high‐risk primary breast cancer: a report of CALGB 9082, SWOG 9114, and NCIC MA‐13. J Clin Oncol 2005; 23: 2191–200. [DOI] [PubMed] [Google Scholar]
- 17. Gianni A, Bonadonna G. Five‐year results of the randomized clinical trial comparing standard versus high‐dose myeloablative chemotherapy in the adjuvant treatment of breast cancer with >3 positive nodes (LN+). Proc Am Soc Clin Oncol 2001; 20: 21a (Abstract). [Google Scholar]
- 18. Roche H, Viens P, Biron P, Lotz J‐P, Asselain B. High‐dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control 2003; 10: 42–7. [DOI] [PubMed] [Google Scholar]
- 19. Tallman MS, Gray R, Robert NJ et al . Conventional adjuvant chemotherapy with or without high‐dose chemotherapy and autologous stem‐cell transplantation in high‐risk breast cancer. N Engl J Med 2003; 349: 17–26. [DOI] [PubMed] [Google Scholar]
- 20. Zander AR, Kröger N, Schmoor C et al . High‐dose chemotherapy with autologous hematopoietic stem‐cell support compared with standard‐dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: first results of a randomized trial. J Clin Oncol 2004; 22: 2273–83. [DOI] [PubMed] [Google Scholar]
- 21. Leonard RCF, Lind M, Twelves C et al . Conventional adjuvant chemotherapy versus single‐cycle, autograft‐supported, high‐dose, late‐intensification chemotherapy in high‐risk breast cancer. J Natl Cancer Inst 2004; 96: 1076–83. [DOI] [PubMed] [Google Scholar]
- 22. Nitz UA, Mohrmann S, Fischer J et al ., for the West German Study Group . Comparison of rapidly cycled tandem high‐dose chemotherapy plus peripheral‐blood stem‐cell support versus dose‐dense conventional chemotherapy for adjuvant treatment of high‐risk breast cancer: results of a multicentre phase III trial. Lancet 2005; 366: 1935–44. [DOI] [PubMed] [Google Scholar]
- 23. Basser RL, O’Neill A, Martinelli G et al .: International Breast Cancer Study Group . Multicycle dose‐intensive chemotherapy for women with high‐risk primary breast cancer: results of International Breast Cancer Study Group Trial 15‐95. J Clin Oncol 2006; 24: 370–8. [DOI] [PubMed] [Google Scholar]
- 24. Williams SF, Bitran JD, Kaminer L et al . A phase I–II study of bialkylator chemotherapy, high‐dose thiotepa, and cyclophosphamide with autologous bone marrow reinfusion in patients with advanced cancer. J Clin Oncol 1987; 5: 260–5. [DOI] [PubMed] [Google Scholar]
- 25. Eder JP, Antman K, Elias A et al . Cyclophosphamide and thiotepa with autologous bone marrow transplantation in patients with solid tumors. J Natl Cancer Inst 1988; 80: 1221–6. [DOI] [PubMed] [Google Scholar]
- 26. Kohno A, Takeyama K, Narabayashi M et al . Low‐dose granulocyte colony‐stimulating factor enables the efficient collection of peripheral blood stem cells after disease‐oriented, conventional‐dose chemotherapy for breast cancer, malignant lymphoma and germ cell tumor. Bone Marrow Transplant 1995; 15: 49–54. [PubMed] [Google Scholar]
- 27. Shimoyama M, Fukuda H, Saijo N, Yamaguchi N. Japan Clinical Oncology Group (JCOG). Jpn J Clin Oncol 1998; 28: 158–62. [DOI] [PubMed] [Google Scholar]
- 28. Oken MM, Creech RH, Tormey DC et al . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55. [PubMed] [Google Scholar]
- 29. Narabayashi M, Takeyama K, Fukutomi T et al . A dose‐finding study of lenograstim (glycosylated rHuG‐CSF) for peripheral blood stem cell mobilization during postoperative adjuvant chemotherapy in patients with breast cancer. Jpn J Clin Oncol 1999; 29: 285–90. [DOI] [PubMed] [Google Scholar]
- 30. Tobinai K, Kohno A, Shimada Y et al . Toxicity grading criteria of the Japan Clinical Oncology Group. Jpn J Clin Oncol 1993; 23: 250–7. [PubMed] [Google Scholar]
- 31. Tokuda Y, Tajima T, Narabayashi M et al . Randomized phase III study of high‐dose chemotherapy with autologous stem cell support as consolidation in high‐risk postoperative breast cancer. Japan Clinical Oncology Group (JCOG9208). Proc Am Soc Clin Oncol 2001; 20: 38a (Abstract). [Google Scholar]
- 32. Tormey DC, Weinberg VE, Holland JF et al . A randomized trial of five and three drug chemotherapy and chemoimmunotherapy in women with operable node positive breast cancer. J Clin Oncol 1983; 1: 138–45. [DOI] [PubMed] [Google Scholar]
- 33. Lichtman SM, Budman D, Bosworth J et al . Adjuvant therapy of stage II breast cancer treated with CMFVP, radiation therapy and VATH following lumpectomy. Am J Clin Oncol 1991; 14: 317–21. [DOI] [PubMed] [Google Scholar]
- 34. Antman KH, Rowlings PA, Vaughan WP et al . High‐dose chemotherapy with autologous hematopoietic stem‐cell support for breast cancer in North America. J Clin Oncol 1997; 15: 1870–9. [DOI] [PubMed] [Google Scholar]
- 35. Peto R. Update of worldwide evidence on the adjuvant treatment of breast cancer. Eur J Cancer 2002; 38 (Suppl 3): S22. 12409061 [Google Scholar]
- 36. Rahman ZU, Frye DK, Buzdar AU et al . Impact of selection process on response rate and long‐term survival of potential high‐dose chemotherapy candidates treated with standard‐dose doxorubicin‐containing chemotherapy in patients with metastatic breast cancer. J Clin Oncol 1997; 15: 3171–7. [DOI] [PubMed] [Google Scholar]
- 37. Garcia‐Carbonero R, Hidalgo M, Paz‐Ares L et al . Patient selection in high‐dose chemotherapy trials: relevance in high‐risk breast cancer. J Clin Oncol 1997; 15: 3178–84. [DOI] [PubMed] [Google Scholar]
- 38. Crump M, Goss PE, Prince M, Girouard C. Outcome of extensive evaluation before adjuvant therapy in women with breast cancer and 10 or more positive axillary lymph nodes. J Clin Oncol 1996; 14: 66–9. [DOI] [PubMed] [Google Scholar]
- 39. Rodenhuis S, Bontenbal M, Van Hoesel QG et al . Efficacy of high‐dose alkylating chemotherapy in HER2/neu‐negative breast cancer. Ann Oncol 2006; 17: 588–96. [DOI] [PubMed] [Google Scholar]
- 40. Nitz UA, Gluz O, Herr A et al . Retrospective analysis of WSG AM01 tandem high dose chemotherapy trial in high risk primary breast cancer: a hypothesis generating study. Proc Am Soc Clin Oncol 2006; 24: 44s (Abstract). [Google Scholar]


