Abstract
Tumor hypoxia has a pronounced effect on malignant progression and metastatic spread of human tumors. As carbonic anhydrases (CA) 9 and 12 are induced by the low‐oxygen environment within tumors, we investigated the relationship between the expression of these two CA and the presence of metastatic lymph nodes (LN) in uterine cervical cancer. CA9/CA12 expression was evaluated histochemically in primary cervical cancer tissues of 73 patients who underwent laparoscopic LN staging and two patients with clinical staging before definitive radiotherapy at the National Cancer Center, Korea. We also evaluated CA9 expression in 33 patients with pathologically confirmed metastatic LN. CA9 expression in the primary tumors was significantly associated with LN metastasis (P = 0.03) and poorer disease‐free survival (relative risk, 6.1; 95% confidence interval, 1.3–28.3, P = 0.02, multivariate analysis), whereas CA12 expression did not show such a relationship. In addition, 21 of 24 metastatic LN revealed similar CA9 expression (P = 0.001), suggesting that CA9‐expressing tumor cells had a higher metastatic potential. CA9 was expressed in 45 of 75 (60%) primary tumors, with positive tumor cells observed predominantly in the area away from the blood vessels. In contrast, CA12 expression was observed in only 29 of 74 primary tumors (39%), without a specific pattern. These findings indicate that expression of CA9, but not CA12, in tumors is associated with the presence of LN metastases and poorer prognosis. Selective application of new treatment modalities based on CA9 expression to prevent LN metastases may improve overall treatment outcome in patients with uterine cervical cancer. (Cancer Sci 2007; 98: 329–333)
Hypoxia is known to cause treatment resistance and promote the selective survival of metastatic phenotype.( 1 ) Carbonic anhydrase (CA) 9 is the most specific and strongly overexpressed gene in response to hypoxia in human cancer cells, suggesting it may be a surrogate marker of hypoxia in various human cancers.( 1 , 2 , 3 , 4 , 5 ) IGFBP‐5, another hypoxia‐overexpressed gene, has been found to show a four‐fold increase under 1.5% hypoxia, and is related to axillary lymph node (LN) metastases in patients with breast cancer.( 6 ) We previously reported that expression of CA9 mRNA in uterine cervical cancer (UCC) is a strong predictor for poorer metastasis‐free survival.( 7 ) To further examine whether expression of CA9 and CA12 is associated with LN metastases in UCC, we assayed their expression patterns in tumor tissues by immunohistochemical staining. Because we found that CA9 expression was associated with LN metastases, we assayed CA9 expression in primary cervical cancer and matching metastatic LN specimens from the same individuals. To understand the different patterns of expression of these two isoenzymes, we assayed expression in a series of archived paraffin‐embedded cervical tissues comprising various stages of preinvasive epithelial lesions.
Materials and methods
Patients and tissue samples. Seventy‐five patients diagnosed with UCC between September 2001 and June 2005 were included in this study. Laparoscopic LN staging involved a full lymphadenectomy, including a complete dissection of external and internal iliac LN and obturator group, common iliac LN (CILN), and para‐aortic LN (PAN). This surgical procedure has been implemented under our institutional clinical trial protocol since 2001.( 8 , 9 ) The present study was carried out with the approval of the Institutional Review Board of the National Cancer Center (NCC) of Korea, and written informed consent was obtained from each individual patient. The patients were grouped according to their LN status either as ‘positive pelvic LN with or without PAN and/or CILN’ or ‘distant LN (PAN or CILN involvement)’. For 33 of 38 patients who were surgically confirmed to have positive LN, serial microscopic examinations were carried out on individual LN samples cut at 2‐mm intervals. Slides for LN specimens were not available for the other five patients, due to the loss of paraffin blocks (n = 3) or the presence of micrometastases only (n = 2). Another 75 samples, including 12 of normal cervical mucosa, 25 of cervical intraepithelial neoplasia (CIN) I, 15 of CIN II, and 23 of CIN III, were randomly selected from the archived tissue blocks of the Department of Pathology, NCC.
CA9 and CA12 immunohistochemical staining. Tissue samples were composed of four or more pieces obtained by multiple punch biopsies, measuring approximately 3 × 3 mm each. All pieces from each tumor were formalin‐fixed and paraffin‐embedded into a single block, and 4‐µm sections were prepared from the tumors and LN. Immunostaining was carried out using the avidin–biotin peroxidase complex method. After dewaxing, the samples were incubated with a 1:50 dilution of M75 for CA9 (a kind gift from Dr S. Pastorekova, Institute of Virology, Slovak Academy of Sciences, Slovak Republic) for 30 min at room temperature. For antigen retrieval of CA12, the slides were boiled in retrieval solution (DAKO target retrieval solution; DAKO, Carpinteria, CA, USA) at 98°C for 15 min. Polyclonal antibody against human recombinant CA12 (a gift from Dr W. Sly, Department of Biochemistry, St Louis University, St Louis, MO, USA) was used at a 1:1600 dilution. Cytoplasmic or membranous staining with moderate or strong intensity was regarded as expression of CA9 or CA12. CA9 immunostaining was scored as grade 0 for ≤5%, grade 1 for 6–10%, grade 2 for 11–30%, and grade 3 for >30%, taking into account the percentage area of tumor cells in all pieces of specimen from each patient mounted in one slide. CA12 expression was scored as positive (>5% of tumor areas) or negative (≤5%).
Statistical analysis. All statistical analyses were carried out using SPSS software, version 12.0 (SPSS, Chicago, IL, USA). Disease‐free survival (DFS) was compared with expression of CA9 or CA12 and other clinicopathological factors using the Kaplan–Meier method and the Cox regression model. The Cox multiple regression model was used for multivariate analyses. The statistical significance of associations between CA9 and CA12 staining and the clinicopathological characteristics of the patients was assessed by χ2‐tests. Statistical significance was defined as P < 0.05.
Results
CA9 and CA12 expression in relation to clinicopathological parameters. CA9 expression was associated with the presence of tumor‐positive LN, classified either as ‘positive pelvic LN with or without PAN and/or CILN’ or ‘distant LN (PAN or CILN involvement)’ (both P = 0.03; Table 1). Younger patients (≤45 years old) had a significantly higher frequency of CA9 expression than older patients (P = 0.01). CA12 (P = 0.02), but not CA9, expression was associated with more differentiated histology. Follow‐up periods of the patients ranged from 12 to 58 months (average 30.56 ± 11.2 months). CA9 expression was significantly associated with poor DFS (P = 0.03; Fig. 1a) and the grade of CA9 expression also showed the same tendency (P = 0.05; Fig. 1b). In contrast, CA12 expression was not associated with patient survival (P = 0.84; Fig. 1c). In addition, the presence of positive pelvic LN with or without PAN and/or CILN (relative risk [RR] = 4.50, 95% confidence interval [CI], 1.25–16.19, P = 0.02) or the presence of PAN and/or CILN involvement (RR = 5.38, 95% CI, 1.95–14.84, P = 0.001) and advanced stage (RR = 7.56, 95% CI, 2.80–20.43, P < 0.001) were unfavorable prognostic factors (Table 2). Multivariate analysis demonstrated that CA9 expression and patient age were significantly associated with poorer DFS (RR = 6.12, 95% CI, 1.33–28.26, P = 0.02, and RR = 5.43, 95% CI, 1.20–24.62, P = 0.03, respectively).
Table 1.
Expression of carbonic anhydrase (CA) 9 and CA12 in cervical cancer: correlation with clinicopathological characteristics
| Characteristic | Total no. | CA9 expression | P‐value† | CA12 expression | P‐value† | ||
|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | ||||
| Age (years) | |||||||
| ≤45 | 30 | 7 | 23 | 0.01 | 15 | 14 | 0.22 |
| >45 | 45 | 23 | 22 | 30 | 15 | ||
| Histology | |||||||
| SCC | 65 | 28 | 37 | 0.29 | 39 | 25 | 1.0 |
| AC or ASC | 10 | 2 | 8 | 6 | 4 | ||
| Differentiation | |||||||
| WD | 19 | 7 | 12 | 0.51 | 9 | 10 | 0.02 |
| MD | 42 | 19 | 23 | 23 | 18 | ||
| PD | 14 | 4 | 10 | 13 | 1 | ||
| Size of tumor (cm) | |||||||
| ≤4 | 26 | 12 | 14 | 0.46 | 18 | 8 | 0.32 |
| >4 | 49 | 18 | 31 | 27 | 21 | ||
| FigO stage | |||||||
| I | 10 | 6 | 4 | 0.10 | 5 | 5 | 0.75 |
| II | 53 | 22 | 31 | 33 | 20 | ||
| III–IV | 12 | 2 | 10 | 7 | 4 | ||
| Positive pelvic LN with or without PAN and/or CILN | |||||||
| No | 34 | 19 | 15 | 0.03 | 20 | 14 | 0.51 |
| Yes | 38 | 10 | 28 | 23 | 25 | ||
| Distant LN only (PAN or CILN involvement) | |||||||
| No | 54 | 26 | 28 | 0.03 | 31 | 23 | 0.42 |
| Yes | 21 | 4 | 17 | 14 | 6 | ||
| Distant metastasis | |||||||
| No | 64 | 29 | 35 | 0.04 | 39 | 25 | 1.0 |
| Yes | 11 | 1 | 10 | 6 | 4 | ||
| Local recurrence | |||||||
| No | 69 | 28 | 41 | 1.0 | 41 | 27 | 1.0 |
| Yes | 6 | 2 | 4 | 4 | 2 | ||
By Fisher's exact test. AC, adenocarcinoma; ASC, adenosquamous cell carcinoma; CILN, common iliac lymph node; LN, lymph node; MD, moderately differentiated; PAN, para‐aortic lymph node; PD, poorly differentiated; SCC, squamous cell carcinoma; WD, well differentiated.
Figure 1.
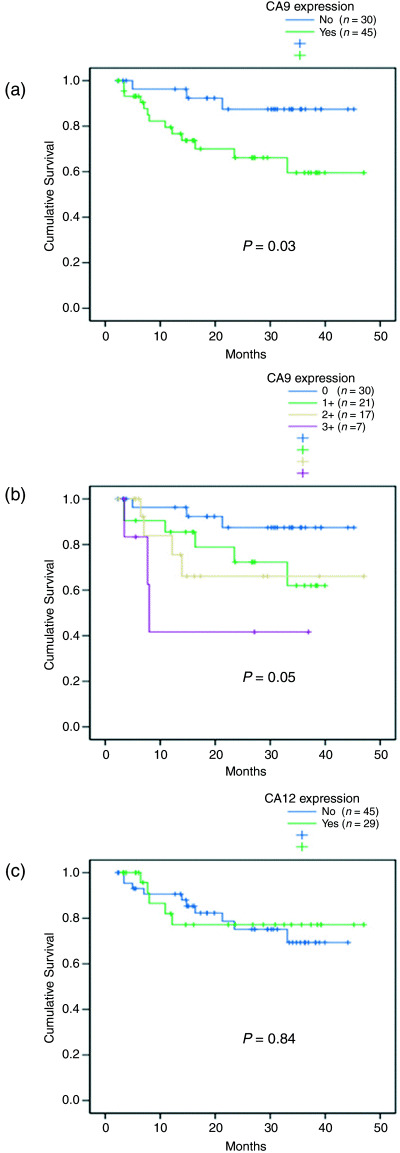
(a) Disease‐free survival (DFS) relative to positive or negative carbonic anhydrase (CA) 9 expression. (b) Patient stratification according to grade of CA9 expression. (c) DFS relative to CA12 expression. P‐values are for log rank tests.
Table 2.
Univariate analysis and final Cox multiple regression model of clinicopathological prognostic factors in 75 patients with cervical cancer following radiotherapy
| Clinicopathological factors | Disease‐free survival | |
|---|---|---|
| Relative risk (95% confidence interval) | P‐value† | |
| Univariate analysis | ||
| CA9 (CA9 expression vs no expression) | 3.707 (1.053–13.048) | 0.041 |
| CA12 (CA9 expression vs no expression) | 0.897 (0.306–2.629) | 0.843 |
| FigO stage (stages I, II vs stages III, IV) | 7.562 (2.80–20.437) | <0.001 |
| Age (≤45 years vs ≥45 years) | 2.831 (0.806–9.945) | 0.105 |
| Histological type (squamous cell carcinoma vs other type) | 0.331 (0.044–2.515) | 0.285 |
| Differentiation (well or moderate vs poor) | 0.856 (0.244–3.006) | 0.808 |
| Tumor size (≤4 cm vs >4 cm) | 1.565 (0.504–4.858) | 0.438 |
| LN metastasis (metastasis vs no metastasis) | 4.505 (1.254–16.191) | 0.021 |
| Multivariate analysis | ||
| CA9 expression | 6.124 (1.327–28.264) | 0.020 |
| Age | 5.432 (1.199–24.623) | 0.028 |
Univariate analysis by Cox proportional hazards model and final Cox multiple regression model after backward stepwise elimination with variables eliminated at P < 0.1.
CA9 and CA12 expression in the primary cervical cancer and in LN. CA9 and CA12 expression was detected in 60% (45/75) and 39% (29/74) of primary tumors, respectively. Grade 1 CA9 expression was detected in 21 patients (21/45, 47%), grade 2 in 17 (17/45, 38%) and grade 3 in seven (7/45, 15%). Only 20% (15/75) of the patients expressed both CA9 and CA12 in their tumors, and the two proteins were not colocalized within each tumor. CA9 was predominantly expressed in areas distant from blood vessels or perinecrotic areas, whereas CA12 did not show any consistent pattern. Typical examples of CA9 and CA12 expression are shown in Fig. 2. CA9 expression in primary cervical cancer tissues was compared to those of tumor‐positive resected LN in 33 patients with LN metastases. In general, LN harboring metastatic tumor cells showed a similar grade of CA9 expression as their matched primary cervical tumors (Table 3). The sensitivity, specificity, positive predictive value and negative predictive value of CA9 expression for LN metastasis were 65.1, 65.5, 73.6 and 55.8%, respectively. Twenty‐three of 33 patients (67.7%) with metastatic LN had CA9‐positive LN, which translates into 88% (21/24) of patients with CA9‐positive primary tumors. In contrast, two of the nine CA9‐negative tumors (22%) with positive LN metastases showed grade 1 CA9 expression in one of four and five resected LN, respectively (P = 0.001; Tables 3,4, [link]).
Figure 2.
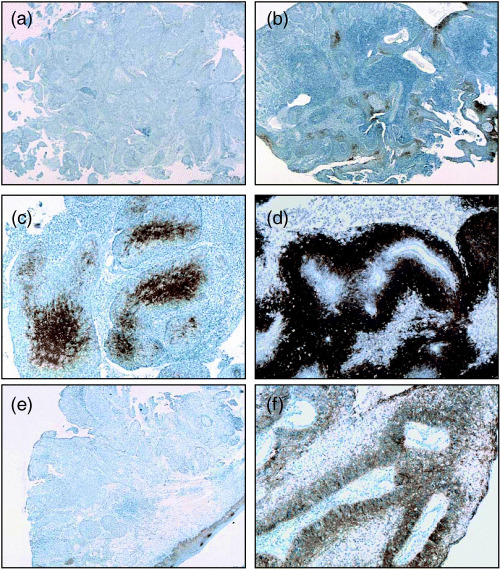
Expression of (a–c) carbonic anhydrase (CA) 9 and (e,f) CA12 in uterine cervical cancer. (a) Negative for CA9, (b) grade 1, (c) grade 2, (d) grade 3, (e) negative for CA12, and (f) positive for CA12. (b,c) CA9 was expressed in the cytoplasm of tumor cells. CA9 expression was concentrated in the central area of tumor cell nests. (f) CA12 was expressed at the cytoplasmic membrane. Original magnification: (a,b,e) ×100, (c,d,f) ×200.
Figure 3.
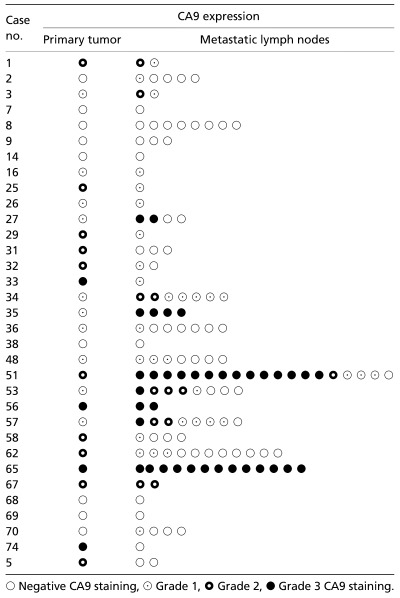
Carbonic anhydrase (CA) 9 expression in primary cervical cancer and matched lymph nodes
Table 4.
Carbonic anhydrase (CA) 9 expression in primary cervical cancer and their matched metastatic lymph nodes (n = 33)
| CA9 expression in LNs | CA9 expression in cervical cancer | Total no. | |
|---|---|---|---|
| Yes† | No | ||
| Yes | 21 | 2 | 23 |
| No | 3 | 7 | 10 |
| Total | 24 | 9 | 33 |
If CA9 was expressed in at least one among multiple tumor‐positive lymph nodes, it was regarded as expression positive.
CA9 and CA12 expression in cervical intraepithelial neoplasia. CA9 was expressed with increasing frequency as the grade of CIN increased (3/25 [12%] for CIN I, 4/15 [27%] for CIN II, 12/23 [52%] for CIN III, P = 0.002 for trend), whereas normal cervix did not show any CA9 expression (Fig. 3). Weak expression of CA9 was also observed in reserve cell hyperplasia or immature squamous metaplasia in the area combined with chronic cervicitis. In contrast, CA12 was expressed frequently in normal cervix (10/12, 83%), as well as in all low‐grade CIN lesions examined (25/25 for CIN I and 15/15 for CIN II), and in 70% (16/23) of CIN III lesions. Interestingly, CA12 expression decreased as CIN grade increased (P = 0.004 for trend; Fig. 3) and was further decreased in invasive cancer (40%).
Figure 3.
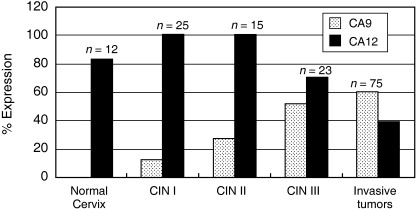
Carbonic anhydrase (CA) 9/CA12 expression in cervical intraepithelial neoplasia (CIN) and normal cervix tissues in comparison with invasive cervical tumors. CA9 expression increased as CIN grade increased, with no expression in normal cervical mucosa and highest expression in invasive cancer. In contrast, CA12 expression decreased in higher‐grade CIN, but was detected in 80% of normal cervical mucosae.
Discussion
We have investigated the expression of CA9 and CA12 in cervical malignancies and the value of CA9 and CA12 expression as a predictive marker for LN metastases. Identifying genes associated with the development of LN metastases provides a new prospect in the treatment of UCC as LN metastasis is the strongest clinical parameter predicting poorer 5‐year survival in this disease.( 10 ) We found that CA9 expression was associated with a higher incidence of metastatic LN. We then explored whether CA9‐expressing tumor cells constituted the subpopulation of cells with higher metastatic potential when compared with CA9 non‐expressing tumor cells. By examining each metastatic LN for CA9 expression, we found that metastatic LN from patients with CA9‐expressing primary tumors mostly had a similar extent of CA9 expression, which supports our hypothesis. However, there were varied degrees of expression within the LN group from the same patients, suggesting that CA9 expression may be influenced by individual tumor microenvironment within the LN.
There have been several other studies comparing CA9 expression and prognosis in human solid tumors, with one study showing a close correlation between high CA9 expression and poorer metastasis‐free survival (MFS),( 3 ) whereas another failed to show such an association.( 11 ) Our findings support the results of the former study, in that we found that CA9 was predictive of MFS and the extent of expression was linearly related to poorer DFS. These two studies also showed a discrepancy in the relationship between CA9 expression and intratumoral O2 tension. In the study failing to show an association between CA9 expression and poorer clinical outcomes,( 11 ) however, single tumor biopsy samples were used for most patients, which may not have represented the characteristics of the entire tumors.
CA9 has a role in anchorage‐independent tumor cell growth, facilitating invasion of cancer cells into the extracellular matrix by modulating the functions of E‐cadherin.( 12 , 13 , 14 ) Strong correlations have been demonstrated between CA9 mRNA expression and the metastasis of UCC( 7 ) and vulvar cancer.( 15 ) Although CA9 mRNA expression cannot show the level of tissue hypoxia, it is indicative of CA9 enzyme activity in individual tumors, which is important in the degradation of extracellular matrix and tumor cell invasion in the tumor‐specific microenvironment.
Our finding of an association between CA12 expression and more differentiated histology and less invasive cancer suggests that CA12 expression may be driven predominantly by factors related to tissue differentiation, rather than by hypoxia. This finding is in agreement with results showing that CA12 was more highly expressed in less aggressive, more differentiated types of breast cancer cells and tissues.( 16 ) CA12 has also been reported to be upregulated by treatment with 1,25‐dihydroxyvitamin D3, which has prodifferentiation and antiproliferative effects on cell cultures.( 17 ) Further studies are needed to unravel the role of CA12 in various cancer tissues.
In conclusion, we found that CA9 expression was associated with a higher incidence of LN metastases in UCC. Selective application of new treatment modalities based on tumor CA9 expression levels to prevent LN metastases may improve the overall treatment outcome of patients with UCC.
Acknowledgment
This work was implemented with the support of National Cancer Center Grant 0510572.
Dr S Lee is presently working at the Department of Pathology, Kyong Hee University, Korea, Dr I. O. Han is currently working at the College of Medicine, Inha University, Incheon, Korea and Dr J. W. Roh is at the Department of Obstetrics and Gynecology, Dongguk University International Hospital, Gyeonggi, Korea.
References
- 1. Kizaka‐Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci 2003; 94: 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olive PL, Aquino‐Parsons C, MacPhail SH et al. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res 2001; 61: 8924–9. [PubMed] [Google Scholar]
- 3. Loncaster JA, Harris AL, Davidson SE et al. Carbonic anhydrase (CAIX) expression, a potential new intrinsic marker of hypoxia: correlates with tumor oxygen measurement and prognosis in locally advanced carcinoma of the cervix. Cancer Res 2001; 61: 6394–9. [PubMed] [Google Scholar]
- 4. Lal A, Peters H, St Croix B et al. Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst 2001; 93: 1337–43. [DOI] [PubMed] [Google Scholar]
- 5. Vordermark D, Brown JM. Endogenous markers of tumor hypoxia: predictors of clinical radiation resistance? Strahlenther Onkol 2003; 179: 801–11. [DOI] [PubMed] [Google Scholar]
- 6. Hao X, Sun B, Hu L et al. Differential gene and protein expression in primary breast tissue microarray analysis. Cancer 2004; 100: 1110–22. [DOI] [PubMed] [Google Scholar]
- 7. Kim JY, Shin HJ, Kim TH et al. Tumor‐associated carbonic anhydrases are linked to metastases in primary cervical cancer. J Cancer Res Clin Oncol 2006; 132: 302–8. [DOI] [PubMed] [Google Scholar]
- 8. Roh JW, Seo SS, Lee S et al. Role of positron emission tomography in pretreatment lymph node staging of UCC: a prospective surgicopathologic correlation study. Eur J Cancer 2005; 41: 2086–92. [DOI] [PubMed] [Google Scholar]
- 9. Chung HH, Lee S, Sim JS et al. Pretreatment laparoscopic surgical staging in locally advanced cervical cancer: preliminary results in Korea. Gynecol Oncol 2005; 97: 468–75. [DOI] [PubMed] [Google Scholar]
- 10. Stehman FB, Bundy BN, DiSaia PJ, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with radiation therapy I. Cancer 1991; 67: 2776–85. [DOI] [PubMed] [Google Scholar]
- 11. Hedley D, Pintilie M, Woo J et al. Carbonic anhydrase IX expression, hypoxia, and prognosis in patients with uterine cervical carcinomas. Clin Cancer Res 2003; 9: 5666–74. [PubMed] [Google Scholar]
- 12. Ivanov S, Liao SY, Ivanova A et al. Expression of hypoxia‐inducible cell‐surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 2001; 158: 905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wykoff CC, Beasley NJ, Watson PH et al. Hypoxia‐inducible expression of tumor‐associated carbonic anhydrases. Cancer Res 2000; 60: 7075–83. [PubMed] [Google Scholar]
- 14. Svastova E, Zilka N, Zat’ovicova M et al. Carbonic anhydrase IX reduces E‐cadherin‐mediated adhesion of MDCK cells via interaction with β‐catenin. Exp Cell Res 2003; 290: 332–45. [DOI] [PubMed] [Google Scholar]
- 15. Kowalewska M, Radziszewski J, Kulik J et al. Detection of carbonic anhydrase 9‐expressing tumor cells in the lymph nodes of vulvar carcinoma patients by RT‐PCR. Int J Cancer 2005; 116: 957–62. [DOI] [PubMed] [Google Scholar]
- 16. Wykoff CC, Beasley N, Watson PH et al. Expression of the hypoxia‐inducible and tumor‐associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol 2001; 158: 1011–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wood RJ, Tchack L, Angelo G, Pratt RE, Sonna LA. DNA microarray analysis of vitamin D‐induced gene expression in a human colon carcinoma cell line. Physiol Genomics 2004; 17: 122–9. [DOI] [PubMed] [Google Scholar]


