Abstract
ZSTK474 is a novel orally applicable phosphoinositide 3‐kinase‐specific inhibitor that strongly inhibits cancer cell proliferation. To further explore the antitumor effect of ZSTK474 for future clinical usage, we studied its combined effects with radiation. The proliferation of HeLa cells was inhibited by treatment with X‐rays alone or ZSTK474 alone. Combination treatment using X‐rays then ZSTK474 given orally for 8 days, starting 24 h post‐irradiation, significantly enhanced cell growth inhibition. The combined effect was also observed for clonogenic survival with continuous ZSTK474 treatment. Western blot analysis showed enhanced phosphorylation of Akt and GSK‐3β by X‐irradiation, whereas phosphorylation was inhibited by ZSTK474 treatment alone. Treatment with ZSTK474 after X‐irradiation also inhibited phosphorylation, and remarkably inhibited xenograft tumor growth. Combined treatment with X‐rays and ZSTK474 has greater therapeutic potential than radiation or drug therapy alone, both in vitro and in vivo. (Cancer Sci 2011; 102: 1176–1180)
Phosphoinositide 3‐kinases (PI3Ks) are a family of lipid kinase that phosphorylates the 3‐hydroxyl group of the inositol ring of phosphoinositides.( 1 ) As PI3Ks have been shown to be important targets in cancer therapy, development of PI3K inhibitors has attracted attention from both academic and industrial researchers. Recently, several novel PI3K inhibitors have been developed and some of them are now undergoing clinical trial.( 2 )
Among the novel PI3K inhibitors, ZSTK474 has unique properties.( 1 , 3 , 4 , 5 , 6 ) ZSTK474 is a pan‐PI3K inhibitor: it inhibits all four PI3K isoforms in an ATP‐competitive manner. Among all of the PI3K isoforms, PI3Kδ was inhibited most potently by ZSTK474. PI3K‐related kinases are a group of protein kinases with a catalytic core structure similar to PI3K, but they lack the lipid kinase activity. This group includes mTOR, DNA‐PK, ATM, and ATR proteins, of which the latter three are known to be involved in DNA damage‐related responses. The inhibition activity of ZSTK474 against DNA‐PK and mTOR was determined, and was shown to be far weaker compared with that observed against PI3K. These results indicate that ZSTK474 was the most specific agent among these PI3K inhibitors.( 1 , 4 , 6 ) The inhibition selectivity of ZSTK474 for PI3K over DNA‐PK was significantly higher than other PI3K inhibitors such as NVP‐BEZ235, PI‐103, and LY294002.( 6 ) In vitro, ZSTK474 induced marked G0/G1 arrest in various human cancer cells without any obvious apoptosis.( 3 , 5 ) This pan‐PI3K inhibitor, given orally, showed potent in vivo antitumor efficacy on cancer xenografts at both early and advanced stages, without obvious toxicity being observed.( 3 )
ZSTK474 strongly inhibited tumor growth. Thus, to consider the clinical usage of ZSTK474, its combination with another therapy is a practical choice. In single drug therapy, the use of multiple agents, such as chemotherapeutic drugs and radiation, increases effectiveness and potency in clinical cancer therapy. Radiation can be used as an agent to increase the anticancer effect of ZSTK474, as ZSTK474 effectively inhibits the downstream of the PI3K pathway.
In general, the combination of chemotherapy and radiotherapy can be classified into three categories based on the time sequence of the treatments: neoadjuvant (chemotherapy before radiotherapy); concurrent; and adjuvant (chemotherapy after radiotherapy) chemo‐radiotherapy. The time sequence of the treatments usually affects the results. It is known that the sensitivity of cells to radiation depends on the cell cycle phases.( 7 ) The cells are most sensitive to low linear energy transfer (LET) ionizing radiation when irradiated at G2/M phase and are most resistant when irradiated at late S phase. Other phases, such as G1 and early S, show intermediate sensitivity. As ZSTK474 induces arrest in the G0/G1 phase of the cell cycle, where radiation sensitivity is not the highest, ZSTK474 was not used in this study in the typical manner of radiation sensitizers. In addition, ZSTK474 is a specific PI3K inhibitor, and it showed weaker inhibitory activity against DNA‐PKcs, which is thought to be essential for the double strand break (DSB) repair process after radiation exposure.
We then examined the time sequence where ZSTK474 was applied not immediately, but much later after irradiation. In this study, significant combination effects of X‐rays and ZSTK474 were observed both in vitro and in vivo.
Materials and Methods
Chemicals. ZSTK474 was synthesized in the Central Research Laboratory, Zenyaku Kogyo Co. (Tokyo, Japan). For in vitro studies, ZSTK474 was dissolved in DMSO. For animal experiments, ZSTK474 was suspended in 5% hydroxypropyl cellulose in water as a solid dispersion form.( 3 )
Animals. All animal experiments were carried out at the National Institute of Radiological Sciences (NIRS; Chiba, Japan), conformed to institutional guidelines, and were approved by the Institutional Animal Care and Use Committee of NIRS. Male nude mice (BALB/c‐nu/nu) were obtained at 6 weeks of age from CLEA Japan (Tokyo, Japan). The mice were housed in a temperature‐ and humidity‐controlled room at 23 ± 1°C and 55 ± 5%, respectively, and maintained on a 12:12 light:dark cycle. The mice were kept five per cage. They received acidified water and diet (MB‐1; Funabashi Farm, Funabashi, Japan) ad libitum during the experimental period.
Cell cultures. The HeLa cell line (human cervix epithelium carcinoma) was obtained from the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). The cells were grown in DMEM (Sigma‐Aldrich, Tokyo, Japan) supplemented with 10% FBS and antibiotic–antimycotic (Gibco‐Invitrogen, Tokyo, Japan) at 37°C with 5% CO2.
X‐irradiation. HeLa cells in dishes were X‐irradiated with a Shimadzu Pantak HF‐320 at a dose rate of 0.95 Gy/min (200 kVp, 20 mA, 0.5 mm Al + 0.5 mm Cu filter). For the irradiation of mice, nude mice bearing xenograft tumors at the right hind leg were placed on a Lucite plate and the tumors were X‐irradiated at a dose rate of 1.27 Gy/min with the Shimadzu Pantak HF‐320 (200 kVp, 20 mA, 0.5 mm Al + 0.5 mm Cu filter).
Cell viability assay. HeLa cells in 60 mm dishes were prepared at various cell densities 1 day before X‐irradiation. One day later, the cells were X‐irradiated (2 Gy) at room temperature (day 0). At 24 h after X‐irradiation (day 1), the cells were exposed to ZSTK474 (1 μM). When counting cell numbers, the medium in the dish was transferred to 15 mL plastic tubes to count the number of dead cells. The remaining cells attached to the dishes were washed with PBS and the PBS was transferred to the plastic tubes to combine with the medium. The cells on the dishes were trypsinized for 5 min at 37°C and the cell suspensions were combined with the medium in the plastic tubes. The combined suspensions were centrifuged at 110g for 5 min at 4°C with a M160‐IV centrifuge (Sakuma, Tokyo, Japan). The pellet was suspended in 0.5 mL PBS and the number of cells was counted under a microscope (Olympus IX‐70 Fluorescence microscope; Olympus, Tokyo, Japan) on days 0, 1, 2, 3, 4, 5, and 8. The relative growth rates were calculated based on the number of cells counted at the time of X‐irradiation (day 0). Each point on the cell growth curve represents the mean and the standard deviation from four samples.
Clonogenic cell survival assay. The clonogenic cell survival rate was determined by the colony‐forming assay. Immediately after irradiation, cells were washed with PBS, trypsinized, diluted, and seeded in 60‐mm dishes at various cell densities. At 24 h after irradiation, the cells were exposed to ZSTK474 for 24 h, then the medium was changed to ZSTK474‐free medium and incubated at 37°C for 2 weeks (transient exposure). Alternatively, the cells were exposed to ZSTK474 at 24 h after irradiation, then continuously exposed for 2 weeks during the incubation (continuous exposure). After 2 weeks of incubation, colonies were stained with crystal violet dissolved in methanol. Colonies containing more than 50 cells were counted directly by eye or by a scanner. NIH Image software (Image J, http://rsb.info.nih.gov/ij/) was used for the analysis of number and area of colony. The surviving fraction was calculated based on the plating efficiency determined from the control (no X‐irradiation, drug only). Each point on the survival curve represents the mean surviving fraction from three dishes. Clonogenic survival curves are representative of independent triplicate experiments.
Western blot analysis. Preparation of samples for Western blot analysis was carried out according to the following method.( 3 , 8 ) The sample dishes were frozen in liquid nitrogen, and the cells were lysed in a buffer containing 10 mM Tris–HCl at pH 7.4, 50 mM NaCl, 50 mM NaF, 30 mM sodium pyrophosphate, 50 mM sodium orthovanadate, 5 mM EDTA, 1 mM PMSF, 0.5% Nonidet P‐40, 0.1% SDS, and one complete protease inhibitor cocktail tablet (Roche Diagnostics, Tokyo, Japan). Proteins in cell extracts were separated by SDS‐PAGE) at a constant voltage (150 V), then electrotransferred onto nitrocellulose membranes (Invitrogen, Tokyo, Japan) at 30 V. The membranes were incubated with a primary antibody overnight at 4°C, and a secondary antibody for 1 h at 37°C. Finally, the blots were visualized by the enhanced chemiluminescence method (GE Healthcare, Tokyo, Japan) according to the manufacturer’s instructions. Primary antibodies used for immunoblotting were as follows: rabbit polyclonal antibodies for Akt, phosphorylated Akt (phosphorylated residue Ser473), phosphorylated Akt (phosphorylated residue Thr308), phosphorylated glycogen synthase kinase 3β (GSK‐3β, phosphorylated residue Ser9), and β‐actin. Secondary antibodies used were goat polyclonal anti‐rabbit IgG‐HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
In vivo study for tumor growth in xenograft model. Nude mice (BALB/c‐nu/nu, 8‐week‐old) were injected s.c. with 106 HeLa cells in 100 μL PBS. Approximately 2 weeks after inoculation, when the tumors had reached an average volume of 150 mm3, the mice were divided into four groups: group 1, control (n = 6 + 2); group 2, X‐rays (n = 6 + 2); group 3, ZSTK474 (n = 6 + 2); and group 4, X‐rays + ZSTK474 (n = 6 + 2). On day 0, the tumors on the right hind legs of mice were X‐irradiated at 8 Gy (groups 2 and 4). 24 hours after 8 Gy irradiation on the tumor, the mice began an oral inoculation regime of 100 mg/kg ZSTK474, given 11 times in 15 days, every day from day 1 to day 15 except for days 3, 4, 10, and 11. Body weight of the mice was measured before each dose of ZSTK474. ZSTK474 was suspended in 5% hydroxypropyl methylcellulose in water as a solid dispersion form at just before use. The size of palpable tumors in the mice (n = 6 per group) was measured with calipers every 2–4 days. The tumor volume (V) was calculated as V (mm3) = length (mm) × width (mm)2/2. Animals were killed when tumors exceeded 1000 mm3 in size or became severely necrotic.
Immunohistochemical analysis. Two mice from each group on day 2 and two mice from groups 2, 3, and 4 on day 15 were used for immunohistochemical (IHC) analysis of tumors.( 5 ) For these experiments, each group involved an additional two mice (total of eight mice). For IHC analysis, tumor tissues were fixed in 10% neutral formalin and embedded in paraffin. Tissue sections (4 μm) were deparaffinized in xylene and rehydrated through graded ethanol solutions. Antigen retrieval was carried out using 10 mM Tris–EDTA buffer (pH 9.0). Specific antibody against phosphorylated Akt (Ser473) (Cell Signaling Technology, Danvers, MA, USA) were used for hybridization and the bound antibodies were visualized by a Dako EnVision kit (Dako Cytomation, Glostrup, Denmark) containing a secondary HRP‐conjugated anti‐rabbit polymer antibody complex. Sections were counterstained with Mayer’s hematoxylin.
Statistical analysis. The statistical analysis of mean values was carried out using Student’s t‐test. Differences with a P‐value <0.05 were considered statistically significant. Data shown are the mean and SD.
Results
Effect of combined treatment on in vitro HeLa cell proliferation. Effects of treatment with X‐rays alone, ZSTK474 alone, or the combination of both agents on HeLa cell proliferation were examined in vitro. The cells were irradiated with X‐rays at 2 Gy (day 0) and 24 h later (day 1) treated with 1 μM ZSTK474 dissolved in DMSO. The number of cells was counted on days 1, 2, 3, 4, 5, and 8. As shown in Figure 1, the cell proliferation was inhibited by the treatment with X‐rays alone, ZSTK474 alone, and the combination of both agents. The inhibition of cell proliferation by X‐irradiation alone was 52% and 58% on days 4 and 8, respectively. The inhibition by ZSTK474 alone was 55% and 69% on days 4 and 8, respectively. Interestingly, a dramatic inhibitory effect was observed on day 8 of the combination treatment. The inhibition by treatment using X‐rays and ZSTK474 was 76% and 91% on days 4 and 8, respectively.
Figure 1.
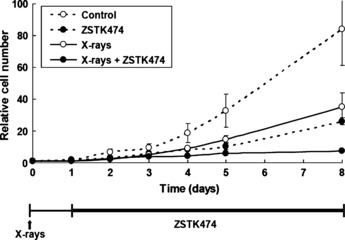
Effect of treatment with X‐rays alone, ZSTK474 alone, or a combination of both agents on the proliferation of HeLa cells. The cells were prepared in 60‐mm dishes at various cell densities 1 day before X‐irradiation. For treatment with X‐rays alone, the cells were X‐irradiated (2 Gy) 1 day later (day 0). For treatment with ZSTK474 alone, 2 days later (day 1), the cells were exposed to 1 μM ZSTK474. For X‐rays + ZSTK474, the cells were treated with 1 μM ZSTK474 24‐h after 2 Gy X‐irradiation. The number of cells was counted on days 0, 1, 2, 3, 4, 5, and 8 under a microscope. The relative values of the number of the cells based on the number on the day of X‐irradiation (day 0) are shown.
Effect of combined treatment of X‐rays and ZSTK474 on clonogenic cell survival. Clonogenic cell survival was examined for HeLa cells by colony‐forming assay. Figure 2(A) shows a representative result of clonogenic cell survival in three independent experiments for transient (24 h) ZSTK474 treatment. No significant difference was observed by the transient treatment with ZSTK474. In contrast, when cells were treated with ZSTK474 continuously (continuous treatment), the surviving fraction was lower compared to samples without the drug (Fig. 2B). The continuous treatment of cells with ZSTK474 decreased the size of colonies (Fig. 2C). The size was even smaller for colonies formed after the combined treatment using X‐rays and ZSTK474 (Fig. 2C).
Figure 2.
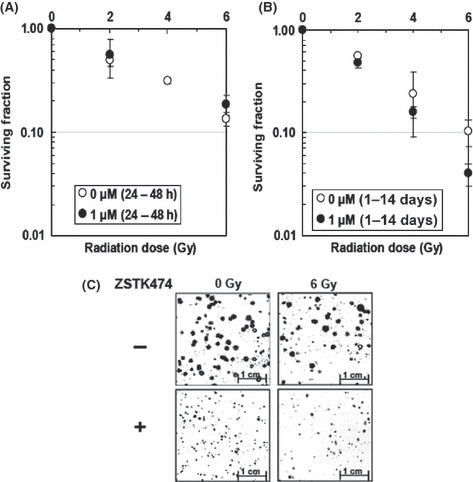
(A) Clonogenic survival rate of HeLa cells treated with ZSTK474 (transient treatment; 24 h) after X‐irradiation. The cells were irradiated at the indicated doses of X‐ray, then 1 day later they were exposed to 1 μM ZSTK474 for 24 h. After changing the medium to ZSTK474‐free medium, the cells were incubated at 37°C for 2 weeks for analysis of their colony‐forming abilities. Open circles, control; closed circles, ZSTK474 treatment. Each point represents the mean survival fraction from three dishes. The graph is representative of three independent experiments. (B) Clonogenic survival rate of cells treated with ZSTK474 (continuous treatment; 14 days) after X‐irradiation. The cells were irradiated at the indicated doses of X‐ray, then 1 day later exposed to 1 μM ZSTK474 continuously. The cells were incubated at 37°C for 2 weeks for analysis of their colony‐forming abilities. Open circles, control; closed circles, ZSTK474 treatment. Each point represents the mean survival fraction from three dishes. The graph is representative of three independent experiments. (C) Microphotographs of colonies taken 2 weeks after treatment. Upper left, control; upper right, 6 Gy X‐rays only; lower left, 1 μM ZSTK474 only; lower right, combination of X‐rays and ZSTK474.
Effect of treatment with X‐rays and/or ZSTK474 on Akt phosphorylation. Figure 3 shows in vitro effects of X‐irradiation alone, ZSTK474 alone, or in combination on phosphorylation of Akt protein in HeLa cells. The Akt phosphorylation was almost completely inhibited by the treatment using 1 μM ZSTK474. Irradiation with 6 Gy X‐rays enhanced the phosphorylation at Thr308 of Akt, although phosphorylation at Ser473 did not increase significantly. The phosphorylation of GSK‐3β, a downstream molecule of Akt, was also inhibited significantly by ZSTK474 treatment. X‐irradiation slightly increased the phosphorylation of GSK‐3β. Treatment with ZSTK474 1 day after X‐irradiation greatly decreased the phosphorylation of Akt and GSK‐3β.
Figure 3.
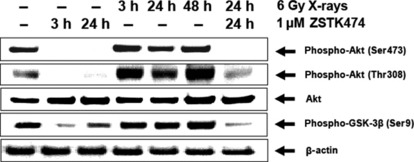
Phosphorylation of Akt and GSK‐3β proteins in HeLa cells treated with X‐rays alone, ZSTK474 alone, or a combination of both agents. The cells were treated as indicated and analyzed by Western blotting. For the conditions without ZSTK474, DMSO was added. Lane 1 is a control (without X‐irradiation or ZSTK474 treatment). The cells were harvested at 3 h or 24 h after drug exposure (lanes 2 and 3). The cells were harvested at 3, 24, or 48 h after 6 Gy X‐irradiation (lanes 4, 5, and 6). The cells were irradiated with 6 Gy X‐rays followed by treatment with 1 μM ZSTK474 for 24 h beginning 1 day after irradiation (lane 7).
Effect of combined treatment against in vivo tumor growth in HeLa xenografts. The combined effect of X‐rays and ZSTK474 against tumor growth of a HeLa xenograft model was examined. HeLa cells were inoculated on a hind leg of each mouse. When the tumor size reached approximately 150 mm3, the tumor region was irradiated once with X‐rays. One day later, treatment with ZSTK474 began, given orally. As shown in Figure 4(A), single local X‐irradiation at 8 Gy alone or continuous oral administration of 100 mg/kg ZSTK474 alone inhibited the growth of s.c. implanted human HeLa tumors. X‐irradiation alone or ZSTK474 alone induced only partial inhibition of tumor growth. However, the combined treatment resulted in significant total tumor growth inhibition (Fig. 4B). There were no enhancements of toxicity judged by loss of body weight in treated mice during the combined therapy (Fig. 4C). To assess toxicity, Yaguchi et al. ( 3 ) evaluated toxic effects for normal organs by measuring the body weight of mice and the H&E staining of femur bone marrow sections. Furthermore, chronic oral inoculation of ZSTK474 at a higher dose (800 mg/kg) over 4 weeks again reduced body weight within a tolerable range, without toxic effects to critical organs. The researchers concluded that ZSTK474 given orally to mice had strong antitumor activity against human cancer xenografts without toxic effects to critical organs.( 3 )
Figure 4.
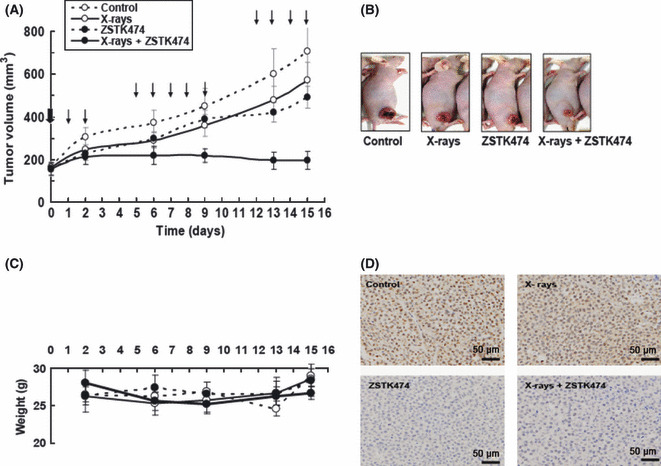
(A) Xenograft growth of HeLa cells in nude mice treated as indicated. At the bold arrow (day 0), the tumors were irradiated with 8 Gy X‐rays (groups 2 and 4). Beginning 1 day after X‐irradiation (day 1), ZSTK474 (100 mg/kg) was given orally every day (groups 3 and 4) until day 15 except for days 3, 4, 10, and 11. We irradiated the tumor on day 0, then started the first drug dosage on days 1 and 2. The following week, we gave the agent everyday from day 5 to day 9. In the third week, we gave the agent every day from day 12 to day 15. Mice were killed on day 15. Subsequently, those tumor tissues were extracted for immunohistochemical analysis. Points are mean of tumor volume (mm3) of each treatment (n = 6). Bars show SD. (B) Weight change of the mice during the time course shown. (C) Photographs of mice on day 15, showing those with tumor size close to the average for each group. The mice were anesthetized. (D) Effect of treatment with X‐rays alone, ZSTK474 alone, or in combination on phosphorylation of Akt measured by immunohistochemical analysis of HeLa xenografts on day 2. Upper left, control; upper right, 8 Gy X‐rays; lower left, ZSTK474 100 mg/kg; lower right, X‐rays + ZSTK474.
In vivo effects of X‐rays and ZSTK474 on Akt signaling in tumors were examined using the IHC method. As shown in Figure 4D, ZSTK474 clearly inhibited the phosphorylation of Akt in mouse tumor on day 2. Continuous treatment with ZSTK474 after X‐irradiation also inhibited the phosphorylation of Akt. Similar results were also obtained on day 15 (data not shown).
Discussion
Ionizing radiation activates the PI3K/Akt pathway,( 9 , 10 ) so the PI3K survival pathway can be a major target for combined treatments. Many reports have shown the radiosensitizing effect of low molecule PI3K inhibitors in vitro.( 11 , 12 ) However, the inhibitors extensively studied so far, such as wortmannin and LY294002, lack specificity and showed unacceptable toxicity in animal studies.( 13 ) Recently, a new generation of PI3K inhibitors, including ZSTK474, has been developed and their in vivo anticancer effect has been closely examined.( 3 , 14 , 15 , 16 , 17 , 18 ) Furthermore, their combination with other agents might be desirable for more adequate usage of the drugs in future clinical therapy. In the present study, we have clearly showed that the combination of X‐rays and ZSTK474 (treated 24 h after X‐rays) remarkably inhibited the proliferation of human cancer cells (HeLa) and the growth of the xenograft tumor. Only 100 mg/kg ZSTK474 was required to almost completely inhibit tumor growth in vivo if the tumor was X‐irradiated once before treatment. A higher concentration (400 mg/kg) was required for a similar effect with ZSTK474 alone.( 3 )
Treatment of non‐small‐cell lung cancer with a combination of PI3K inhibitor and γ‐irradiation in vivo was reported recently.( 19 ) In the report, dual PI3K/mTOR blockade by BEZ235, a novel, orally applicable pan‐PI3K inhibitor, was shown to effectively sensitize non‐small‐cell lung cancer to the pro‐apoptotic effects of ionizing radiation both in vivo and in vitro, where BEZ235 was treated before irradiation to enhance the radiation effect. As the specificity of BEZ235 to PI3K is not high compared to ZSTK474 and considerable inhibition of DNA‐PK is expected,( 6 ) the radiosensitizing effect of BEZ235 might be partly explained by the inhibition of DNA‐PK, by which the DNA repair process should be disturbed.( 20 ) In the present study, however, we used ZSTK474, a novel, and orally applicable PI3K‐specific inhibitor after X‐ray treatment. The timing of ZSTK474 application was 24 h after irradiation, so the DNA repair process related to DNA‐PK should be completed.( 20 ) Therefore, inhibition of DNA‐PK by ZSTK474, even if such activity is present, is not likely to be the major mechanism for the significant combination effect observed in the present study.
A strong combination effect was observed for in vitro cell proliferation assay when the cells were treated for a longer time with ZSTK474. The colony‐forming assay also showed that continuous treatment, rather than transient treatment (24 h), significantly reduced the surviving fraction after X‐irradiation. This reduction in the surviving fraction might be due to the strong inhibition of cell proliferation by ZSTK474, especially after treatment with X‐rays, rather than the induction of actual cell death. This interpretation is supported by colony sizes, where ZSTK474 treatment alone remarkably reduced the colony sizes, but the effect was stronger for cells irradiated first then treated with the drug. ZSTK474 alone does not induce apoptosis.( 3 , 5 ) A significant difference in the number of apoptotic cells was not observed in the tumor between the samples treated with X‐rays alone and with the combination of X‐rays and ZSTK474 (data not shown).
ZSTK474 alone significantly inhibited phosphorylation of Akt and its downstream molecule GSK‐3β. In contrast, X‐irradiation enhanced the phosphorylation of Akt and GSK‐3β. The activation of PI3K/Akt signaling is associated with the radio‐resistance of many cancer cells.( 21 , 22 , 23 ) When ZSTK474 was applied after X‐irradiation, the enhancement of the activation of Akt and GSK‐3β was reversed and the reduced phosphorylation of these proteins was observed. It is postulated that the activation of the PI3K pathway in irradiated tumors is an important survival strategy for these cells. However, to understand the molecular mechanism(s) of the therapeutic efficacy of this combination, its effects on other survival or death factors should be examined. Therefore, treatment with ZSTK474 after X‐irradiation leading to the inhibition of PI3K pathway, which the irradiated cells try to activate, might be related to effective tumor growth inhibition, observed by the combination treatment in this study.
In this study, we clearly showed that the combination of ZSTK474 with X‐rays (continuous application of ZSTK474 after X‐irradiation) results in dramatic tumor growth inhibition in vitro and in vivo. Although the precise molecular mechanism to explain these results remains to be elucidated, this phenomenon might be worth exploring for clinical application. An alternative sequence, X‐irradiation after ZSTK474, should be examined next. Furthermore, combination with heavy‐ion radiotherapy, an effective cancer therapy modality for certain tumors, is also a fascinating theme.
Disclosure Statement
T. Yamori and R. Okayasu were partly supported by a research grant from Zenyaku Kogyo Co. H. Yoshimi, S. Yaguchi and J. Enami are employee of Zenyaku Kogyo Co.
Acknowledgments
This work was partly supported by: grants from the National Institute of Biomedical Innovation of Japan (5‐13); Grants‐in‐Aid for the Priority Area “Cancer” and “New Research Categories” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20200039, 18015049, 20015048); Grants‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science (17390032 and 20790087); and a grant from the Kobayashi Institute for Innovative Cancer Chemotherapy. We thank Ms. Mitsuko Takusagawa of NIRS for her help with cell culture and clonogenic survival assays.
References
- 1. Kong D, Okamura M, Yoshimi H, Yamori T. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3‐kinase inhibitor. Eur J Cancer 2009; 45: 857–65. [DOI] [PubMed] [Google Scholar]
- 2. Kong D, Yamori T. Advances in development of phosphatidylinositol 3‐kinase inhibitors. Curr Med Chem 2009; 16: 2839–54. [DOI] [PubMed] [Google Scholar]
- 3. Yaguchi S, Fukui Y, Koshimizu I et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3‐kinase inhibitor. J Natl Cancer Inst 2006; 98: 545–56. [DOI] [PubMed] [Google Scholar]
- 4. Kong D, Yamori T. ZSTK474 is an ATP‐competitive inhibitor of class I phosphatidylinositol 3 kinase isoforms. Cancer Sci 2007; 98: 1638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dan S, Yoshimi H, Okamura M, Mukai Y, Yamori T. Inhibition of PI3K by ZSTK474 suppressed tumor growth not via apoptosis but G0/G1 arrest. Biochem Biophys Res Commun 2009; 379: 104–9. [DOI] [PubMed] [Google Scholar]
- 6. Kong D, Yaguchi S, Yamori T. Effect of ZSTK474, a novel phosphatidylinositol 3‐kinase inhibitor, on DNA‐dependent protein kinase. Biol Pharm Bull 2009; 32: 297–300. [DOI] [PubMed] [Google Scholar]
- 7. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 8. Yamori T, Iizuka Y, Takayama Y et al. Insulin‐like growth factor I rapidly induces tyrosine phosphorylation of a Mr 150 000 and a Mr 160 000 protein in highly metastatic mouse colon carcinoma 26 NL‐17 cells. Cancer Res 1991; 51: 5859–65. [PubMed] [Google Scholar]
- 9. Sunavala‐Dossabhoy G, Fowler M, De Benedetti A. Translation of the radioresistance kinase TLK1B is induced by gamma‐irradiation through activation of mTOR and phosphorylation of 4E‐BP1. BMC Mol Biol 2004; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reits EA, Hodge JW, Herberts CA et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubota N, Ozawa F, Okada S, Inada T, Komatsu K, Okayasu R. The phosphatidylinositol 3‐kinase inhibitor wortmannin sensitizes quiescent but not proliferating MG‐63 human osteosarcoma cells to radiation. Cancer Lett 1998; 133: 161–7. [DOI] [PubMed] [Google Scholar]
- 12. Hosoi Y, Miyachi H, Matsumoto Y et al. A phosphatidylinositol 3‐kinase inhibitor wortmannin induces radioresistant DNA synthesis and sensitizes cells to bleomycin and ionizing radiation. Int J Cancer 1998; 78: 642–7. [DOI] [PubMed] [Google Scholar]
- 13. Gupta AK, Cerniglia GJ, Mick R et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys 2003; 56: 846–53. [DOI] [PubMed] [Google Scholar]
- 14. Garlich JR, De P, Dey N et al. A vascular targeted pan phosphoinositide 3‐kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res 2008; 68: 206–15. [DOI] [PubMed] [Google Scholar]
- 15. Maira SM, Stauffer F, Brueggen J et al. Identification and characterization of NVP‐BEZ235, a new orally available dual phosphatidylinositol 3‐kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 2008; 7: 1851–63. [DOI] [PubMed] [Google Scholar]
- 16. Folkes AJ, Ahmadi K, Alderton WK et al. The identification of 2‐(1H‐indazol‐4‐yl)‐6‐(4‐methanesulfonyl‐piperazin‐1‐ylmethyl)‐4‐morpholin ‐4‐yl‐thieno[3,2‐d]pyrimidine (GDC‐0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 2008; 51: 5522–32. [DOI] [PubMed] [Google Scholar]
- 17. Fan QW, Cheng CK, Nicolaides TP et al. A dual phosphoinositide‐3‐kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN‐mutant glioma. Cancer Res 2007; 67: 7960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ihle NT, Williams R, Chow S et al. Molecular pharmacology and antitumor activity of PX‐866, a novel inhibitor of phosphoinositide‐3‐kinase signaling. Mol Cancer Ther 2004; 3: 763–72. [PubMed] [Google Scholar]
- 19. Konstantinidou G, Bey EA, Rabellino A et al. Dual phosphoinositide 3‐kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non‐small cell lung cancer harboring K‐RAS mutations. Cancer Res 2009; 69: 7644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okayasu R, Suetomi K, Ullrich RL. Wortmannin inhibits repair of DNA double‐strand breaks in irradiated normal human cells. Radiat Res 1998; 149: 440–5. [PubMed] [Google Scholar]
- 21. Tanno S, Yanagawa N, Habiro A et al. Serine/threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Res 2004; 64: 3486–90. [DOI] [PubMed] [Google Scholar]
- 22. Tomioka A, Tanaka M, De Velasco MA et al. Delivery of PTEN via a novel gene microcapsule sensitizes prostate cancer cells to irradiation. Mol Cancer Ther 2008; 7: 1864–70. [DOI] [PubMed] [Google Scholar]
- 23. Li HF, Kim JS, Waldman T. Radiation‐induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol 2009; 4: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]


