Abstract
Glioma is the most frequent and malignant primary human brain tumor with dismal prognosis despite multimodal therapy. Resveratrol and quercetin, two structurally related and naturally occurring polyphenols, are proposed to have anticancer effects. We report here that resveratrol and quercetin decreased the cell number in four glioma cell lines but not in rat astrocytes. Low doses of resveratrol (10 µM) or quercetin (25 µM) separately had no effect on apoptosis induction, but had a strong effect on caspase 3/7 activation when administered together. Western blot analyses showed that resveratrol (10 µM) and quercetin (25 µM) caused a reduction in phosphorylation of Akt, but this reduction was not sufficient by itself to mediate the effects of these polyphenols. Most important, resveratrol and quercetin chronically administered presented a strong synergism in inducing senescence‐like growth arrest. These results suggest that the combination of polyphenols can potentialize their antitumoral activity, thereby reducing the therapeutic concentration needed for glioma treatment. (Cancer Sci 2009; 100: 1655–1662)
Malignant gliomas comprise the most common and devastating types of primary central nervous system (CNS) tumors and have an incidence of 5–8/100 000 population. The median survival of patients with malignant gliomas is only 12 months after diagnosis.( 1 ) Gliomas are characterized by diffuse infiltrations of the surrounding CNS tissue,( 2 ) and this characteristic is one of the main reasons for the lack of successful treatment of this type of cancer.( 3 ) Nevertheless, the therapeutic strategy for gliomas has remained essentially unchanged for decades due to a limited understanding of the biology of the disease. There is an urgent need to develop mechanism‐based approaches for the management of glioma, i.e. to develop strategies which can eliminate only the damaged (pre‐malignant) or malignant cells without harming normal CNS tissue.( 4 )
Resveratrol (Rsv; 3,5,4′‐trihydroxy‐trans‐stilbene) is found in many plant species such as grapes, berries, and peanuts, and exhibits pleiotropic health beneficial effects, including antioxidant, anti‐inflammatory, cardioprotective, neuroprotective, and antitumor activities.( 5 , 6 , 7 ) The cancer chemopreventive properties of Rsv were first appreciated when Jang et al. (1997)( 5 ) demonstrated that Rsv inhibited cellular events associated with tumor initiation, promotion, and progression. It has been shown that Rsv can induce apoptotic cell death in a number of cancer cell lines, including U251 human glioma cells,( 8 ) RT‐2 rat glioma cells,( 9 ) and the C6 rat glioma cell line.( 10 ) It is thought that Rsv affects whole pathways and sets of intracellular events rather than a single enzyme and is, therefore, less prone to induce resistance in cancer cells.( 11 )
Quercetin (Quer; 3,3′,4′,5,7‐pentahydroxyflavone) is a flavonoid ubiquitous in nature that has recently been described as a potential anticancer agent( 12 ) because of its ability to modulate cell proliferation,( 13 ) survival, and differentiation, targeting key molecules responsible for tumor cell growth such as p53, p21, and Ras in several cancer cell types.( 14 , 15 ) Quer induces little or no toxicity when administered orally or intravenously, and preliminary animal and human data indicate that Quer inhibits tumor growth.( 16 ) We have previously demonstrated that Quer induced necrotic and apoptotic cell death in the U138‐MG human glioma cell line,( 17 ) and Kim et al. (2007) demonstrated that Quer treatment resulted in loss of cell viability in the A172 glioma cell line.( 18 )
Fresh grape skin contains about 50–100 µg/g of Rsv and 40 µg/g of Quer.( 19 , 20 ) In red wine, the concentration of Rsv is in the range of 7–13 µM( 21 ) and Quer is in the range of 7.4 µM,( 22 ) while commercial grape juice contains about 20 µM of trans‐resveratrol.( 23 ) In rats, injection of Rsv or Quer lead to levels of these drugs in the low micromolar range in the CNS, suggesting that they could potentially be used as therapeutic agents against gliomas.( 24 , 25 )
The mechanisms of chemopreventive effects of Rsv and Quer are not well understood. Moreover, studies suggest that targeting regulatory molecules of the cell cycle, apoptosis, and senescence in cancer cells can be an effective strategy for the management of cancer( 26 , 27 , 28 ) and, at present, many works have shown that Rsv and Quer can modulate some of these processes.( 17 , 29 , 30 , 31 ) Both Rsv and Quer have been suggested to extend lifespam of model organisms( 32 , 33 , 34 , 35 ) and to reduce cell senescence in normal cells.( 36 , 37 ) There is only one report showing that Rsv can induce senescence in cancer cells( 31 ) and no such report was found for Quer. Besides, no study considering the interactions of these agents on cell senescence was found in the literature. Here we show that Rsv and Quer cooperate in triggering apoptosis, inhibiting cell proliferation and, most strikingly, in inducing senescence‐like growth arrest in gliomas cell lines.
Matherials and Methods
Cell culture. Rat C6 glioma cells, human U87‐MG, and U138‐MG glioma cells were obtained from ATCC (Rockville, MD, USA). Mouse GL261 glioma cell line was gently provided by Dr Ilker Eyüpoglu, University of Zurich. All culture materials were purchased from Gibco Laboratories (Grand Island, NY, USA). Cells were cultured in DMEM low glucose supplemented with 10% FBS (5% for C6 cells), 1% penicillin/streptomycin, and 0.1% anphoterecin B at 37°C/5% CO2 in a humidified incubator.
Cell treatments. Rsv and Quer were purchased from Sigma Chemical (St. Louis, MO, USA) and administered for 24–72 h. Rsv (10 µM) and Quer (25 µM) were also added together and are referred in the text as cotreatment. The PI3K (Phosphoinositide3‐kinase) inhibitor LY294002 (LY; Cell Signaling, Beverly, MA, USA), was administered (10 µM) for 72 h. The vehicle carrier DMSO (Acros Organics, NJ, USA) was present in the control wells at a maximum concentration of 0.5%.
Astrocyte cultures. Primary astrocyte cultures were prepared as previously described.( 38 ) Briefly, hippocampi of new‐born Wistar rats (1–2 days old) were removed and mechanically dissociated by sequential passage through a Pasteur pipette. Cell suspension was centrifuged at 1000 g for 5 min. The supernatant was discarded, and the pellet resuspended in culture medium containing DMEM with 10% FBS and 1 % penicillin/streptomycin and 0.1% anphoterecin B. Cells were plated at a density of 1.5 × 105 cells/cm2 onto 24‐well plates pretreated with poly‐lysine in 5% CO2 at 37°C, and allowed to grow to confluence (14 days). All animal use procedures were approved by the local Animal Care Committee and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Cell number determination and propidium iodide (PI) staining. The number of cells was counted in a hemocytometer and the value of the treated cells was calculated as a percentage of the control. For PI staining, cells were treated with the drugs in the presence of 6 µM PI (Sigma) for 72 h and images were obtained using a Carl Zeiss Fluorescence Inverted Microscope with a rhodamine filter (Carl Zeiss, Jena, Germany). Results are presented as ratio of cells marked with PI to total cells of at least three fields of three experiments analyzed by Image J software (NIH Image, Rockville, MD, USA).
Mitotic index (MI) determination. Cells were fixed with 70% ethanol for 15 min and stained with 6 µM PI. MI was evaluated by counting at least 300 cells per sample using Image J software and the percentage of cells with condensed chromatin was determined.
Long‐term culture of C6 cells in the presence of Rsv and Quer and determination of population doublings. Cells were plated at each passage in 24‐well plates at a density of 50 000 cells/well and exposed to DMSO or different concentrations of Rsv and Quer with renewal every 48 h of medium and treatments. Confluent cells were passaged, and population doublings (PD) were determined according to the formula PD =[log N(t)*logN(to)]/log 2, where N(t) is the number of cells per well at time of passage, and N(to) is the number of cells seeded at the previous passage.( 31 ) The sum of PDs was then plotted against time of culture.
Colony formation assay. After 6 or 12 days of treatments, cells were trypsinized, counted, and plated at concentration of 1000 cells/well into six‐well plates. After 7 days without treatments, senescence induction was analyzed as described below and cells were stained with Giemsa solution to determinate colony number formation.( 39 ) The percentage of colony forming efficiency was calculated in relation to values of untreated control cells.
Senescence‐associated‐β‐galactosidade (SA‐β‐gal) staining. After 12 days in culture, cells were washed in PBS, fixed in 3% formaldehyde for 15 min at room temperature, washed, and incubated with fresh SA‐β‐gal staining solution containing 1 mg/mL X‐gal (Sigma), 40 mM citric acid/sodium phosphate (pH 6.0), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, and 2 mM MgCl for 12–16 h at 37°C.( 40 ) Results are presented as ratio of SA‐β‐gal‐positive cells to total cells of at least three fields of three experiments.
Caspase 3/7 activity. Measurement of caspase 3/7 activity was done as previously described.( 41 ) After treatments, C6 cells were lysed and 40 µg of protein were incubated with a reaction buffer. The caspase 3/7 substrate Ac‐DEVD‐MCA (Peptide Institute, Osaka, Japan) was present at a final concentration of 20 µM. The cleavage of the fluorogenic synthetic substrate was quantified by fluorimetry in a Spectramax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The fluorescence intensity was calibrated with standard concentrations of MCA, and the caspase 3/7 activity was calculated using the slope of the accumulated fluorogenic product and expressed in picomols per min per mg of protein. Cells treated with 50 µM etoposide (Sigma) for 24 h were used as positive control.
Annexin V staining. Phosphatidylserine externalization was determined by annexin fluorescent signal of Annexin‐V‐FLUOS Staining Kit assay (Roche, Germany) according to the manufacturer's protocol. C6 cells were treated and incubated with PI and annexin for 10 min at room temperature and photographed. Necrotic cells were identified by double PI and annexin fluorescent staining, while apoptotic cells were positive only for annexin.
Immunoblotting. Preparation of protein samples and western blot were performed as described previously with minor modifications.( 7 ) Whole‐cell lysate (40 µg) was electrophoresed and electroblotted onto a PVDF membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The membrane was blocked in 5% skimmed milk/TBST for an hour and probed with Ab to phosphatase and tensin homolog (PTEN) (1:2000 dilution; Cell Signaling), phospho‐Akt (pAkt Ser473) (1:1000; Cell Signaling), and Akt (1:1000; Cell Signaling) for 1 h at room temperature. Bound Ab was detected with appropriate HRP secondary Ab (1:2000; Cell Signaling) using ECL and X‐ray films (Kodak X‐Omat, Rochester, NY, USA). The optical density of the bands was obtained in with Bio‐Rad software (Quantity One; Hercules, CA).
Statistical analysis. All data were expressed as mean ± SEM and were analyzed using anova, followed by SNK post‐hoc test for multiple comparisons. Differences with P < 0.05 were considered significant. Analyses of the data were performed using the software GraphPad INSTAT, (GraphPad Software, San Diego, CA, USA).
Results
Rsv and Quer decreased cell numbers. In search of novel strategies to induce cell death, we investigated the antitumor effect of the natural compounds Rsv and Quer on glioma cell lines. As shown in Figure 1, Rsv and Quer decreased the number of glioma cells in a time‐ and dose‐dependent manner. In C6 cells, after 72 h of 10 µM Rsv and 25 µM Quer treatment, the number of cells was reduced 25% and 54%, respectively. When cells were treated with both compounds at the same concentration and exposure time, the number of cells was reduced 80%, indicating an additive effect of the cotreatment. This reduction was similar to the result observed with higher concentrations of Rsv and Quer. These results were confirmed by MTT assay (data not shown). In other glioma cell lines, the additive effect was not observed, with 50 µM Rsv being the dose that induced the biggest cell number reduction after 72 h of treatment. The difference of response could be explained by the genetic characteristics of each cell line, since U87‐MG and U138‐MG are deleted for PTEN, and U138‐MG is mutant for TP53, whereas C6 has both proteins functional.( 42 , 43 )
Figure 1.
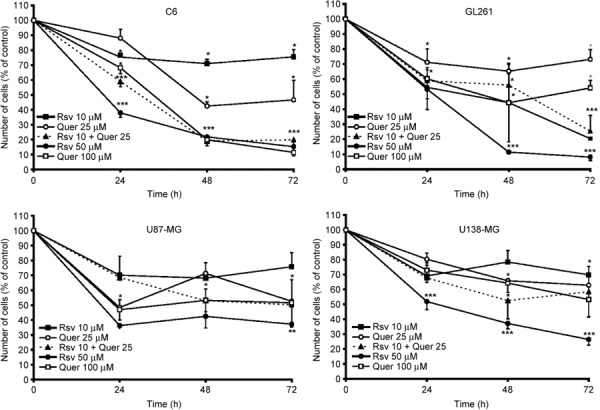
Resveratrol (Rsv) and quercetin (Quer) reduced the number of human, mouse, and rat gliomas. Glioma cell lines from rat (C6), mouse (GL261), and humans (U87‐MG and U138‐MG) were treated with 10 or 50 µM Rsv, 25 or 100 µM Quer, and 10 µM Rsv plus 25 µM Quer for 24, 48, and 72 h; cells were then counted in a hemocytometer. Each time point represents the mean ± SEM of three independent experiments. anova was followed by SNK post test. *P < 0.05, **P < 0.01, ***P < 0.001.
To evaluate whether the cytotoxic effect of Rsv is selective to glioma cell cultures, primary astrocyte cultures and glioma C6 cells were treated for 72 h with Rsv and stained with PI. As shown in Figure 2(a,b), PI incorporation was highest in glioma cells treated with 50 µM Rsv. This effect was not observed in rat astrocyte cultures (Fig. 2c,d). C6 cells treated with 25–100 µM Quer (Fig. 2a,b) presented no significant PI incorporation.
Figure 2.
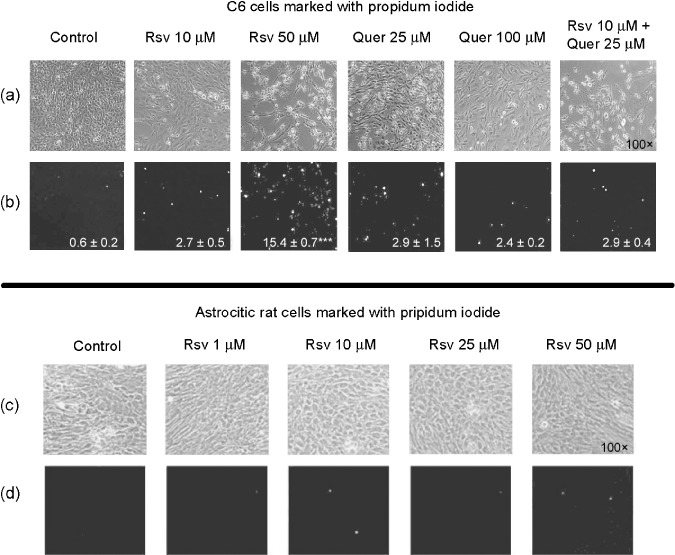
Resveratrol (Rsv) increased propidium iodide (PI) incorporation in C6 cells but not in astrocyte cell cultures. C6 cells were treated with 10 or 50 µM Rsv, 25 or 100 µM quercetin (Quer), and 10 µM Rsv plus 25 µM Quer. Astrocyte cultures were treated with 1, 10, 25, or 50 µM Rsv. At the end of the treatments (72 h), the images were obtained using a Carl Zeiss inverted microscope. Representative light micrographs of C6 (a), astrocyte cell cultures (c), fluorescence micrographs of C6 (b), and astrocyte cell cultures (d) stained with PI (×100) are shown. The numbers below the pictures depict values of PI staining (ratio of PI labeled cells/total number cells, normalized to control) as mean ± SEM of three independent experiments. anova was followed by SNK post test ***P < 0.001.
Rsv activated caspase 3/7. To assess whether cytotoxicity of Rsv and Quer was due to apoptosis induction, we measured the activity of the executioner caspase 3/7( 44 ) after treating C6 cells with these compounds. Rsv (50 µM) increased caspase 3/7 activity after 48 h of treatment at an intensity similar to 50 µM etoposide, used as a positive control (Fig. 3a).( 41 ) This activation was slow, since there was no detectable caspase 3/7 activation before this time (data not shown). Quer (100 µM) was unable to induce caspase 3/7 even at 72 h of treatment. Neither 10 µM Res nor 25 µM Quer alone were able to induce caspase 3/7 activation; however, a strong activation was observed with the cotreatment, suggesting a synergistic effect of these compounds in caspase 3/7 activation (Fig. 3a).
Figure 3.
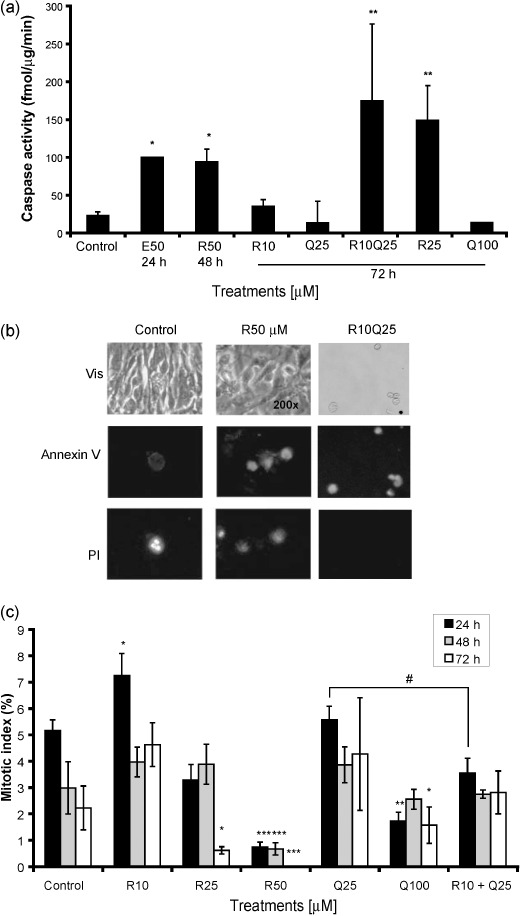
Resveratrol (R) induces caspase 3/7 activation, annexin V staining, and redudes mitotic index. C6 cells were treated with Rsv (10, 25, or 50 µM) and quercetin (Q) (25 or 100 µM) and 10 µM Rsv plus 25 µM Quer for 48 or 72 h. Caspase 3/7 activity was estimated by fluorogenic assay for the effector caspase 3/7 (a). Cells were stained with annexin V‐FLUOS and propidium iodide (PI) and positive cells were observed using an inverted fluorescence microscope (×200). The same fields are shown. Control cells (left panels), 50 µM Rsv (middle panels), and 10 µM Rsv plus 25 µM Quer (right panels) observed under light microscopic image (upper panel), with annexin V staining (middle panel) and PI staining (lower panel) after 72 h of treatments (b). Cells were treated as indicated above for 24, 48, and 72 h and cells in mitosis were visualized by PI staining. The results represent mean ± SEM from three independent experiments. anova was followed by SNK post test. *P < 0.05, **P < 0.01, ***P < 0.001.
To verify if the caspase 3/7 activation was leading to apoptosis, we analyzed annexin V (an apoptosis marker) and PI (necrosis or late apoptosis marker) staining. After 72 h of 50 µM Rsv and cotreatment of Rsv and Quer, there was an increase in annexin V and PI staining, suggesting that the observed caspase 3/7 activation was leading to apoptosis (Fig. 3b).
In order to investigate the effect of Rsv and Quer on cell cycle progression we determined the MI. The MI decreased with 50 µM Rsv and 100 µM Quer treatments but did not decrease with lower doses of Rsv and Quer. Surprisingly, 10 µM Rsv increased the MI in the first 24 h of treatment, but had no effect at later time‐points. Rsv and Quer cotreatment induced a small reduction in MI, which was not observed by these treatments alone (Fig. 3c). Cell cycle distribution indicated that Rsv (50 µM) caused a modest G1 arrest (60 ± 3% compared to 48 ± 5% control cells) while Quer (100 µM) induced an S‐phase accumulation (20 ± 3% compared to 16 ± 2 control cells) at 24 h. These effects were no longer observed at 72 h of treatment (data not shown).
Reduction in pAkt by Rsv and Quer is not essential for their cytostatic effects. Given the evidence that PI3K/Akt is overactivated in a wide range of tumor types and triggers a cascade of responses that include cell growth, proliferation, and survival,( 45 ) we wondered whether this pathway could be involved in Rsv and Quer apoptosis induction and growth arrest. Rsv (10 µM) and Quer (25 µM) reduced the phosphorylation of Akt to a level comparable to the PI3K inhibitor LY without changing the intracellular Akt levels (Fig. 4a). This effect was more intense within 30 min of treatment, but was also observed at 4 h and even at 24 h (Fig. 4a). This reduction in the phosphorylation of Akt was not due to alteration in expression of PTEN, which was not affected by the treatments (Fig. 4b).
Figure 4.
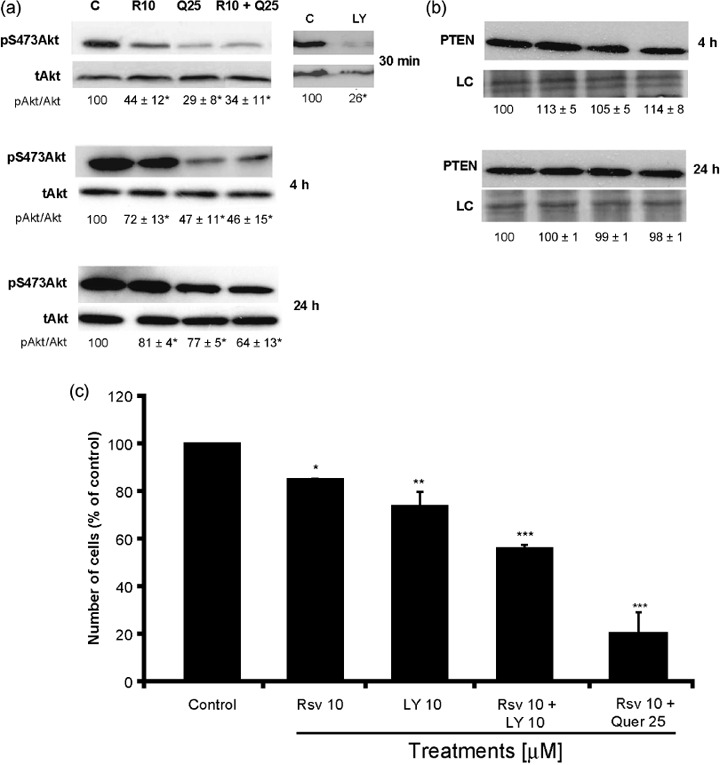
Resveratrol (Rsv) and quercetin (Quer) decreased the phosphorylation of Akt. C6 cells were treated with 10 µM Rsv, 25 µM Quer, or 10 µM Rsv plus 25 µM Quer, for 30 min, 4 h, and 24 h, and with 10 µM LY294002 for 30 min. Lysates were subjected to western blot analysis for pS473Akt and total Akt antibodies (a) and PTEN antibody (b). Representative blots of three independent experiments are shown and the numbers below the blots depict values of densitometric evaluation as mean ± SEM. Number of C6 cells treated as indicated for 72 h (c). The results represent mean ± SEM from three independent experiments. anova was followed by SNK post test. *P < 0.05, **P < 0.01.
To verify if the reduction in the phosphorylation of Akt was responsible for the cell number decrease, we counted cells after LY treatment. While Rsv plus Quer reduced pAkt to 34% of the control and the cell number to 20% of the control, LY reduced the same parameter to 26% and 70% respectively (Fig. 4c). Taken together, these results indicated that there was not a relationship between pAkt reduction and cell number decrease since LY was more potent in reducing pAkt and less effective in decreasing cell number.
Rsv and Quer cooperated in inducing senescence‐like growth arrest in C6 cells. In order to assess a potential pro‐senescence activity of chronically administered Rsv and Quer, we cultivated C6 cells in the presence of Rsv and Quer and determined population doublings during 12 days. Rsv (10 µM) or Quer (25 µM) had only a small effect on the proliferation rate of C6 cells, but Rsv plus Quer lead to a striking reduction in long‐term glioma growth (Fig. 5a). Senescence induction by Rsv plus Quer, measured by the activity of acidic β‐galactosidase, a biochemical marker for lysosome acidification,( 40 ) was cooperative, since each treatment alone produced around 20% of SA‐β‐gal positive cells, whereas combination of Rsv and Quer induced staining in almost all cells (Fig. 5b,c).
Figure 5.
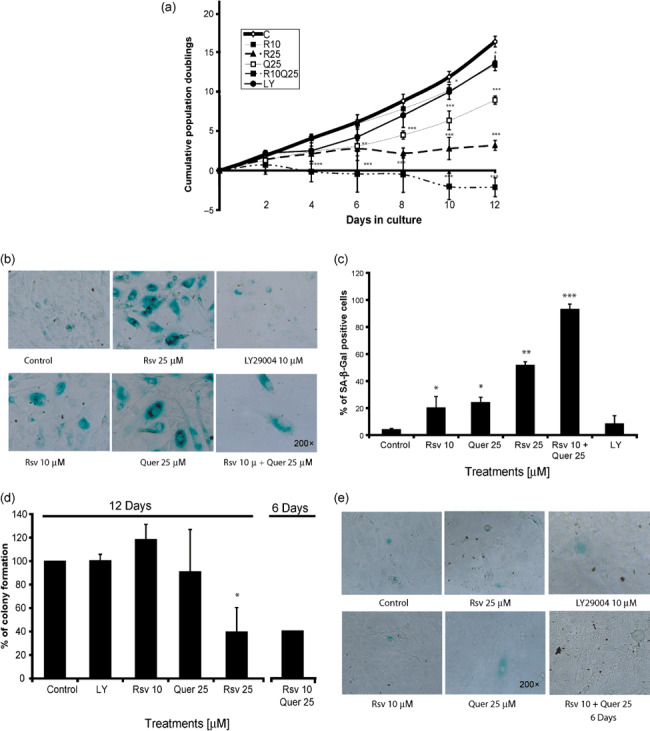
Resveratrol (Rsv) and quercetin (Quer) induce senescence in C6 cells. C6 cells were cultivated in the continuous presence of 10 or 25 µM Rsv, 25 µM Quer, 10 µM Rsv plus 25 µM Quer, or 10 µM LY294002 for 12 days and cumulative population doublings were plotted against time (a). After this time cells were stained for senescence‐associated‐β‐galactosidade (SA‐β‐gal) and photographed (b). The bar graph represents the ratio of SA‐β‐gal‐positive cells to total cells of at least three fields of three independent experiments (c). Colony formation efficiency of C6 cells 7 days after 12 days Rsv or Quer treatment or 6 days of cotreatment (d), and SA‐β‐gal staining at the end of the colony formation assay (e). anova was followed by SNK post test. *P < 0.05, **P < 0.01, ***P < 0.001.
Despite a lack of relationship between pAkt reduction and cell number decrease in the acute treatments, we analyzed whether Akt played a role in chronic treatments. For this reason, we treated cells for 12 days with 10 µM LY and determined population doublings. LY, despite blocking Akt (Fig. 4a), did not affect the proliferation rate of C6 cells and these cells did not present SA‐β‐gal staining (Fig. 5a–c), corroborating the results found by acute treatments.
In order to assess the recovery ability of cells that survived the long‐term Rsv and Quer treatments, we performed colony formation assay. For this, after 12 days of treatments we allowed cells to grow for 7 days without treatment and then we analyzed their ability to form colonies. The colony formation was reduced after treatment with 25 µM Rsv, suggesting that in these cases, a sub‐population of cells that survived the 12‐day treatment were committed to arrest or death, but another sub‐population survived and formed SA‐β‐gal‐negative colonies. (Fig. 5d,e). At the end of 12 days of Rsv and Quer cotreatments, no cells survived to form colonies. Therefore, we performed a test after 6 days of cotreatment followed by 7 days without treatment and observed that the colony formation was reduced by 40%; and as observed with the other treatments, cells that survived were not SA‐β‐gal‐positive (Fig. 5d,e).
Discussion
In the present work we found that Rsv and Quer elicited cytostatic and cytotoxic effects on C6 glioma cell lines by reducing cell growth when administered acutely and chronically. The type of effect elicited was dependent on drug concentration and exposure time. The concentrations at/or above 50 µM Rsv induced necrosis, apoptosis, and cell cycle arrest in early time‐points, whereas lower concentrations of Rsv required the presence of Quer for the induction of long‐term growth arrest and senescence. Others have observed additive effects with acute treatments with Rsv plus Quer in leukemia, breast cancer, and melanomas,( 46 , 47 , 48 ) but these studies did not evaluate long‐term effects of this combination.
Inhibition of apoptosis and cell cycle deregulation are two mechanisms of tumor formation, and many chemopreventive agents act through the induction of apoptosis and cell cycle arrest to block the carcinogenic process.( 49 , 50 , 51 , 52 ) Our data showed that C6 cells treated with 50 µM Rsv for 72 h and Rsv plus Quer induced an increase in caspase 3/7 activity and an augmented annexin V staining. As soon as 24 h after 50 µM Rsv treatment, a strong decrease in MI accompanied by a small reduction in the G1 population was observed. These results support our notion that the cancer preventive effect of Rsv plus Quer may occur through the induction of apoptosis.( 8 , 53 ) Previous works showed that the upregulation of p53‐responsive genes such as p21WAF1/CIP1, p300/CBP, and Apaf1 by Rsv predisposed human prostate cancer (LNCaP) cells to undergo apoptosis.( 54 ) Similarly, Rsv‐ and Quer‐induced apoptosis in HepG2 cells was accompanied by a p53‐dependent increase in Bax and p21.( 55 , 56 )
Cell cycle deregulation and apoptosis are closely related events. The PI3K/Akt pathway has a key role in regulating both processes and, as recently described, is involved in senescence induction.( 57 , 58 ) Besides this, it has been reported that increased PI3K activity due to loss of PTEN, gene amplification, and mutation of catalytic and regulatory subunits of PI3K is common in high grade glioma,( 59 ) and that inhibition of PI3K is cytotoxic in several human glioma cell lines in vitro. ( 60 ) Both Rsv and Quer reduced the phosphorylation of Akt at early time‐points and despite reducing pAkt to a similar level to Rsv or Quer, LY produced only a small reduction in cell numbers with acute and chronic treatments. These results indicate that inhibition of the PI3K/Akt pathway does not play an essential role in Rsv‐ or Quer‐induced cell death. Other studies have found an inhibitory effect of Rsv on the PI3K/Akt pathway, but in this case the effect was mimicked by a PI3K inhibitor.( 61 )
The induction of senescence, irreversible growth arrest, is regarded in cancer cells as a means to halt tumor initiation and progression.( 62 ) Recent studies have revealed some molecular basis for cellular senescence, which may involve the p38 MAPK cascade, and Rb and p53 tumor suppression pathways.( 63 ) Rsv and Quer are known for their antisenescent effects induced by a variety of stresses, with only one report that shows Rsv‐induced senescence in the HCT116 colon cancer cell line( 31 ) and no report of Quer‐induced senescence. The striking synergy observed with low concentrations of Rsv and Quer point to the regulation of converging pathways to the senescence machinery.
Long‐term exposure to low concentrations of Rsv (10–25 µM) or Quer (25 µM) induced senescence‐like growth arrest. More important, the clear additive – even synergistic – effect between Rsv and Quer was observed on cell number decrease, caspase 3/7 activation, long‐term proliferation inhibition, and senescence induction. This indicates that different targets may be involved in the regulation of these features and supports the notion that the combination of Rsv and Quer is an interesting strategy for glioma treatment.
Inhibition of Akt( 57 ) as well as activation of Akt( 58 ) have been linked to increase in senescence. Since we found that Rsv and Quer reduced pAkt, we investigated whether these effects could be involved with long‐term growth arrest and senescence induction. LY, despite dramatically reducing Akt phosphorylation, did not have any effect on cell growth and senescence, indicating that pAkt reduction is not solely responsible for this process.
In this work we observed two types of effects, with high doses of Rsv inducing apoptosis, low doses inducing senescence, and cotreatment with low doses of Rsv and Quer inducing both. It is still not clear what determines if a cell will undergo senescence or apoptosis. Although most cells are capable of both, these processes seem to be mutually exclusive.( 28 ) Cell type, gene expression, and level of tumour suppressor protein p53( 64 , 65 ) appear to be determinant for the cell to undergo apoptosis or senescence. Some studies clearly point to a crosstalk between the processes of apoptosis and cellular senescence;( 66 ) however, the cross‐regulation between these processes is far from understood and further studies are needed to clarify their relationship.
The synergistic induction of senescence and long‐term growth inhibition by Rsv and Quer suggests that the combination of these agents is a good candidate treatment for preclinical studies on glioma tumors. Studies in rodents indicate that both Rsv and Quer cross the intact blood–brain barrier and reach concentrations in the range of 0.1 and 0.3 µmol/kg, respectively.( 24 , 25 ) Glioma growth may break the blood–brain barrier, probably increasing the concentration reachable inside the glioma. It is worthwhile noting that Rsv is not toxic to primary astrocyte cultures and both Rsv and Quer are protective against oxygen and glucose deprivation in hippocampal organotypic cultures,( 7 , 17 ) making these agents ideal for therapeutic intervention in CNS tumors.
Taken together, our studies add a novel facet to the cancer chemopreventive action of Rsv and Quer. Acutely administered high doses of Rsv can induce apotosis and chronically administered Rsv and Quer in subapoptotic doses can induce senescence‐like growth arrest.
Several reports have investigated the effect of individual polyphenols. Because the majority of dietary compounds contain a mixture of polyphenols, it is important to understand their combinatorial effect at dietary concentrations. For a further potential application of Rsv and Quer, isolated or combined, as cancer chemopreventive agents or adjuvants in chemotherapy, more studies focusing on bioavailability, the half‐life of Rsv and Quer, the interaction of both compounds and their metabolites are needed. This is even more necessary when taken into account that senescence induction normally requires the continuous presence of the inducing agents at the tumor site.
Acknowledgments
This work was supported by the Brazilian funding agencies CNPq, FAPERGS, and PROPESQ/UFRGS. We thank Nicolle Barbieri for help with caspase assay and Pítia Ledur for help with preparation of the manuscript.
References
- 1. Avgeropoulos NG, Batchelor TT. New treatment strategies for malignant gliomas. Oncologist 1999; 4: 209–24. [PubMed] [Google Scholar]
- 2. Russell DS, Rubinstein LJ. Pathology of Tumours of the Nervous System. Baltimore: Williams & Wilkins, 1989. [Google Scholar]
- 3. Tonn JC, Goldbrunner R. Mechanisms of glioma cell invasion. Acta Neurochir Suppl 2003; 88: 163–7. [DOI] [PubMed] [Google Scholar]
- 4. Mukhtar H, Ahmad N. Cancer chemoprevention: future holds in multiple agents: contemporary issues in toxicology. Toxicol Appl Pharmacol 1999; 158: 207–10. [DOI] [PubMed] [Google Scholar]
- 5. Jang M, Cai L, Udeani GO et al . Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275: 218–20. [DOI] [PubMed] [Google Scholar]
- 6. Ray PS, Maulik G, Cordis GA, Bertelli AAE, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med 1999; 27: 160–9. [DOI] [PubMed] [Google Scholar]
- 7. Zamin LL, Dillenburg‐Pilla P, Argenta‐Comiran R et al . Protective effect of resveratrol against oxygen‐glucose deprivation in organotypic hippocampal slice cultures: involvement of PI3‐K pathway. Neurobiol Dis 2006; 24: 170–82. [DOI] [PubMed] [Google Scholar]
- 8. Jiang H, Zhang L, Kuo J et al . Resveratrol‐induced apoptotic death in human U251 glioma cells. Mol Cancer The 2005; 4: 554–60. [DOI] [PubMed] [Google Scholar]
- 9. Tseng SH, Lin SM, Chen JC et al . Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin Cancer Res 2004; 10: 2190–202. [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Fei Z, Zhen H, Zhang J, Zhang X. Resveratrol inhibits cell growth and induces apoptosis of rat C6 glioma cells. J Neuro-Oncol 2007; 81: 231–40. [DOI] [PubMed] [Google Scholar]
- 11. Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 2008; 658: 68–94. [DOI] [PubMed] [Google Scholar]
- 12. Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett 2008; 269: 315–25. [DOI] [PubMed] [Google Scholar]
- 13. Hosokawa N, Hosokawa Y, Sakai T et al . Inhibitory effect of quercetin on the synthesis of a possibly cell‐cycle‐related 17‐kDa protein, in human colon cancer cells. Int J Cancer 1990; 45: 1119–24. [DOI] [PubMed] [Google Scholar]
- 14. Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down‐regulation of mutant p53 in the human breast cancer cell line MDA‐MB468. Cancer Res 1994; 54: 2424–8. [PubMed] [Google Scholar]
- 15. Ranelletti FO, Maggiano N, Serra FG et al . Quercetin inhibits p21‐RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int J Cancer 2000; 85: 438–45. [PubMed] [Google Scholar]
- 16. Ferry DR, Smith A, Malkhandi J et al . Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 1996; 2: 659–68. [PubMed] [Google Scholar]
- 17. Braganhol E, Zamin LL, Canedo AD et al . Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs 2006; 17: 663–71. [DOI] [PubMed] [Google Scholar]
- 18. Kim EJ, Choi CH, Park JY, Kang SK, Kim YK. Underlying mechanism of quercetin‐induced cell death in human glioma cells. Neurochem Res 2008; 33: 971–9. [DOI] [PubMed] [Google Scholar]
- 19. Jeandet P, Bessis R, Gautheron B. The production of resveratrol (3,5,4‐trihydroxystilbene) by grape berries in different developmental stages. Am J Enol Viti 1991; 42: 41–6. [Google Scholar]
- 20. Talcott ST, Lee JH. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J Agric Food Chem 2002; 50: 3186–92. [DOI] [PubMed] [Google Scholar]
- 21. Goldberg DM, Yan J, Ng E et al . A global survey of trans‐resveratrol concentrations in commercial wines. Am J Enol Viti 1995; 46: 159–65. [Google Scholar]
- 22. Dragoni S, Gee J, Bennett R, Valoti M, Sgaragli G. Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro . Br J Pharmacol 2006; 147: 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pace‐Asciak CR, Rounova O, Hahn SE, Daimandis EP, Goldberg DM. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta 1996; 246: 163–82. [DOI] [PubMed] [Google Scholar]
- 24. Asensi M, Medina I, Ortega A et al . Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 2002; 33: 387–98. [DOI] [PubMed] [Google Scholar]
- 25. De Boer VCJ, Dihal AA, Van Der Woude H et al . Tissue distribution of quercetin in rats and pigs. J Nutr 2005; 135: 1617–8. [DOI] [PubMed] [Google Scholar]
- 26. Collins K, Jacks T, Pavletich NP. The cell cycle and cancer. Proc Natl Acad Sci USA 1997; 94: 2776–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steinbach JP, Weller M. Apoptosis in gliomas: molecular mechanisms and therapeutic implications. J Neuro-Oncol 2004; 70: 245–54. [DOI] [PubMed] [Google Scholar]
- 28. Campisi J, D’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8: 729–40. [DOI] [PubMed] [Google Scholar]
- 29. Park JW, Choi YJ, Jang MA et al . Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Lett 2001; 163: 43–9. [DOI] [PubMed] [Google Scholar]
- 30. Ahmad N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. Resveratrol causes WAF‐1/p21‐mediated G1‐phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res 2001; 7: 1466–73. [PubMed] [Google Scholar]
- 31. Heiss EH, Schilder YDC, Dirsch VM. Chronic treatment with resveratrol induces redox stress‐ and ataxia telangiectasia‐mutated (ATM)‐dependent senescence in p53‐positive cancer cells. J Biol Chem 2007; 282: 26759–66. [DOI] [PubMed] [Google Scholar]
- 32. Howitz KT, Bitterman KJ, Cohen HY et al . Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–6. [DOI] [PubMed] [Google Scholar]
- 33. Bauer HB, Goulpil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan‐extending interventions in Drosophila melanogaster. Proc Nat Acad Sc USA 2004; 101: 12980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short‐term consumption of a resveratrol‐containing nutraceutical mixture mimics gene expression of long‐term caloric restriction in mouse heart. Exp Gerontol 2008; 43: 859–66. [DOI] [PubMed] [Google Scholar]
- 35. Saul N, Pietsch K, Menzel R, Steinberg CEW. Quercetin‐mediated longevity in Caenorhabditis elegans: Is DAF‐16 involved? Mech Ageing Dev 2008; 129: 611–3. [DOI] [PubMed] [Google Scholar]
- 36. Xia L, Wang XX, Hu XS et al . Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt‐dependent mechanisms. Br J Pharmacol 2008; 15: 5387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin‐1 induces premature cellular senescence in primary cultures of murine fibroblasts stress‐induced premature senescence upregulates the expression of endogenous caveolin‐1. Mol Biol Cell 2002; 13: 2502–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lenz G, Gonçalves D, Luo Z, Avruch J, Rodnight R, Neary J. Extracellular ATP stimulates an inhibitory pathway towards growth factor‐induced cRaf‐1 and MEKK activation in astrocyte cultures. J Neurochem 2001; 77: 1001–9. [DOI] [PubMed] [Google Scholar]
- 39. Hirose Y, Berger MS, Pieper RO. p53 effects both the duration of G2/M arrest and the fate of temozolomide‐treated human glioblastoma cells. Cancer Res 2001; 61: 1957–63. [PubMed] [Google Scholar]
- 40. Dimri GP, Lee X, Basile G et al . A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc Natl Acad Sci USA 1995; 92: 9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horn AP, Gerhardt D, Geyer AB et al . Cellular death in hippocampus in response to PI3K pathway inhibiton and oxygen and glucose deprivation. Neurochem Res 2005; 30: 355–61. [DOI] [PubMed] [Google Scholar]
- 42. Ishii N, Maier D, Merlo A et al . Frequent co‐alterations of TP53, p16/CDKN2A, p14ARF, PTEN Tumor suppressor Genes in Human Glioma Cell lines. Brain Pathol 1999; 9: 469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang L, Wang S, Sung B, Lim G, Mao J. Morphine induces ubiquitin‐proteasome activity and glutamate transporter degradation. J Biol Chem 2008; 283: 21703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vivanco I, Sawyers CL. The phosphatidylinositol 3‐Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501. [DOI] [PubMed] [Google Scholar]
- 46. Ferrer P, Asensi M, Segarra R et al . Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia 2005; 7: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mertens‐Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 2005; 218: 141–51. [DOI] [PubMed] [Google Scholar]
- 48. Schlachterman A, Valle F, Wall KM et al . Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol 2008; 1: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bursch W, Oberhammerand F, Schulte‐Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci 1992; 13: 245–51. [DOI] [PubMed] [Google Scholar]
- 50. Wright SC, Zhong J, Larrick JW. Inhibition of apoptosis as a mechanism of tumor promotion. FASEB J 1994; 8: 654–60. [DOI] [PubMed] [Google Scholar]
- 51. Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell 1994; 78: 539–42. [DOI] [PubMed] [Google Scholar]
- 52. Collins K, Jacks T, Paveltich NP. The cell cycle and cancer. Proc Natl Acad Sci USA 1997; 94: 2776–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nam TW, Yoo CI, Kwon HTKCH, Park JY, Kim YK. The flavonoid quercetin induces apoptosis and inhibits migration through a MAPK‐dependent mechanism in osteoblasts. J Bone Miner Metab 2008; 26: 551–60. [DOI] [PubMed] [Google Scholar]
- 54. Narayanan BA, Narayanan NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: P53‐mediated molecular targets. Int J Cancer 2003; 104: 204–12. [DOI] [PubMed] [Google Scholar]
- 55. Kuo PL, Chiang LC, Lin CC. Resveratrol‐induced apoptosis is mediated by p53‐dependent pathway in Hep G2 cells. Life Sci 2002; 72: 23–34. [DOI] [PubMed] [Google Scholar]
- 56. Tanigawa S, Fuji M, Hou DX. Stabilization of p53 is involved in quercetin‐induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem 2008; 72: 797–804. [DOI] [PubMed] [Google Scholar]
- 57. Thaler S, Hähnel PS, Schad A, Dammann R, Schuler M. RASSF1A mediates p21Cip1/Waf1‐dependent cell cycle arrest and senescence through modulation of the Raf‐MEK‐ERK pathway and inhibition of Akt. Cancer Res 2009; 69: 1748–57. [DOI] [PubMed] [Google Scholar]
- 58. Nogueira V, Park Y, Chen CC et al . Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 2009; 14: 458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mizoguchi M, Nutt CL, Mohapatra G, Louis DN. Genetic alterations of phosphoinositide 3‐kinase subunit genes in human glioblastomas. Brain Pathol 2004; 14: 372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guillard S, Clarke PA, Te Poele R et al . Molecular pharmacology of phosphatidylinositol 3‐kinase inhibition in human glioma. Cell Cycle 2009; 8: 443–53. [DOI] [PubMed] [Google Scholar]
- 61. Poolman TM, Ng LL, Farmer PB, Manson MM. Inhibition of the respiratory burst by resveratrol in human monocytes: correlation with inhibition of PI3K signaling. Free Radic Biol Med 2005; 39: 118–32. [DOI] [PubMed] [Google Scholar]
- 62. Braig M, Schmitt CA. Oncogene‐induced senescence: putting the brakes on tumor development. Cancer Res 2006; 66: 2881–4. [DOI] [PubMed] [Google Scholar]
- 63. Katakura Y. Molecular basis for the cellular senescence program and its application of anticancer therapy. Biosci Biotechnol Biochem 2006; 70: 1076–81. [DOI] [PubMed] [Google Scholar]
- 64. Nelyudova A, Aksenov N, Pospelov V, Pospelova T. By blocking apoptosis, Bcl‐2 in p38‐depen‐dent manner promotes cell cycle arrest and accelerated senescence after DNA damage and serum withdrawal. Cell Cycle 2007; 6: 2171–7. [DOI] [PubMed] [Google Scholar]
- 65. Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin‐induced apoptosis to senescence. Oncogene 2003; 22: 2805–11. [DOI] [PubMed] [Google Scholar]
- 66. Seluanov A, Gorbunova V, Falcovitz A et al . Change of the death pathway in senescent human fibroblasts in response to DNA damage is caused by an inability to stabilize p53. Mol Cell Biol 2001; 21: 1552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


