Abstract
We previously reported that puromycin‐insensitive leucyl‐specific aminopeptidase (PILSAP) is required for vascular endothelial growth factor (VEGF)‐ and basic fibroblast growth factor (bFGF)‐induced angiogenesis and for endothelial differentiation from embryonic stem (ES) cells via the aminopeptidase activity of PILSAP. In this study, we searched for molecules that function during angiogenesis with PILSAP. We performed proteome analysis of nuclear extracts from embryoid bodies (EBs) made from ES cells transfected with mutant PILSAP lacking aminopeptidase activity and mock EBs. We identified pigpen, a 67‐kDa nuclear coiled body component protein. Immunoprecipitation and western blotting demonstrated the binding of PILSAP and pigpen in endothelial cells (ECs), and this interaction was enhanced by VEGF and bFGF. Pigpen was reported to be expressed in actively growing ECs such as those in embryos and tumors. However, whether Pigpen is involved in angiogenesis is not known. Therefore, we examined the effect of pigpen on angiogenesis by silencing pigpen with siRNA (siPigpen). Compared with scrambled RNA (scrPigpen), transfection of siPigpen into mouse ECs inhibited proliferation, migration, and network formation. These results were confirmed with other two siRNAs. Moreover, siPigpen suppressed bFGF‐induced angiogenesis in a Matrigel plug assay, and injection of siPigpen into Lewis lung carcinoma cell tumors implanted subcutaneously into 5‐week‐old C57/BL male mice prevented tumor growth and tumor angiogenesis. These data indicate that Pigpen is involved in angiogenesis and that pigpen may be a target for blocking tumor angiogenesis.
(Cancer Sci 2010; 101: 1170–1176)
New blood vessel formation or angiogenesis is clinically classified into two types, physiological and pathological angiogenesis. Pathological angiogenesis is indispensable for tumor growth and metastasis.( 1 ) To induce angiogenesis, tumors secrete various growth factors and proteolytic enzymes that stimulate endothelial cells (ECs), mobilize endothelial progenitor cells (EPCs) from bone marrow, and degrade the extracellular matrix (ECM) including the basement membrane.( 2 ) Therefore, anti‐angiogenic drugs have been developed to inhibit tumor angiogenesis by targeting ECs. Some angiogenesis inhibitors have been approved for use in progressive or metastatic tumors in combination with chemotherapy. Angiogenesis can be inhibited by blocking angiogenesis factors or their receptors, blocking signaling pathways, or by mimetics or derivatives of endogenous angiogenesis inhibitors. Currently, the most promising target is vascular endothelial growth factor (VEGF). The importance of VEGF has been further demonstrated by the observation that VEGF haploinsufficiency in mice is embryonically lethal due to a defect in blood vessel formation.( 3 , 4 )
Using PCR‐coupled subtractive cDNA cloning, we previously isolated genes that are expressed during in vitro differentiation of mouse embryonic stem (ES) cells to ECs and identified puromycin‐insensitive leucyl‐specific aminopeptidase (PILSAP), whose expression is induced by VEGF.( 5 ) We further demonstrated that PILSAP plays an important role in postnatal angiogenesis.( 5 ) VEGF facilitates PILSAP binding to its substrate, phosphatidylinositol‐dependent kinase 1 (PDK1) via aminopeptidase (AP) activity, allowing complex formation of PILSAP‐PDK1‐S6 kinase (S6K) and resulting in activation of cyclin‐dependent kinase (CDK) 4/6 by phosphorylated S6K. Activated CDK 4/6 then promotes G1–S transition, leading to EC proliferation.( 6 ) PILSAP is also involved in VEGF‐mediated induction of EC migration by activating integrins and focal adhesion kinase( 7 ) as well as increasing adhesion of ECs to the ECM via activation of RhoA.( 8 )
In this study, we searched for molecules that interact with PILSAP and function in angiogenesis, and identified pigpen using proteome analysis of mtPILSAP‐EBs lacking AP activity( 9 ) and mock EBs. Pigpen is a 67‐kDa sepharose‐binding molecule that was previously isolated from ECs( 10 ) and is a nuclear coiled body component protein.( 11 , 12 ) The Cajal bodies, or coiled bodies (CBs) were first discovered as nuclear accessory bodies by Ramóny Cajal and were found to be ubiquitous nuclear inclusions of unknown function.( 12 ) CBs are enriched in rapidly dividing cells with high levels of transcriptional activities.( 13 ) Since the component proteins of CBs such as coilin and the survival of motor neurons (SMN) have been identified, the research into CBs has been greatly advanced and suggests that CBs are involved in coordinating the assembly and maturation of nuclear ribonucleoproteins (RNPs). On the other hand, little is known about the function of another CB component protein, pigpen. However, pigpen is demonstrated to be expressed also in actively growing cells such as ECs in embryos and tumors.( 10 , 14 ) Nuclear injection of anti‐pigpen antibodies inhibits EC division,( 15 ) and therefore pigpen may play a role in angiogenesis. To clarify whether and how pigpen is involved in angiogenesis, we synthesized siRNAs for pigpen. Transfection of the siRNA inhibited angiogenesis in vivo as well as in vitro. Furthermore, siPigpen inhibited tumor angiogenesis and tumor growth in a mouse syngeneic tumor transplant model. These results indicate that pigpen plays a role in angiogenesis via induction of EC proliferation, migration, and network formation, and may be a future target for blocking tumor angiogenesis.
Materials and Methods
Cell culture. Mock and mtPILSAP ES cells were produced and cultured as previously described.( 9 ) The mouse endothelial cell lines MSS31 and MS1 were cultured in alpha MEM (αMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) with 10% FBS and high glucose DMEM (HG‐DMEM; Invitrogen) with 5% FBS, respectively. Lewis lung carcinoma cells (LLC) were cultured in DMEM (Invitrogen) with 10% FBS.
ES in vitro differentiation culture system. In vitro differentiation of mock and mtPILSAP ES cells in methylcellulose was performed as previously described.( 9 ) Cells were harvested from the culture on day 8 for proteome analysis and immunoprecipitation followed by western blotting (IP‐Western).
Proteome analysis. Nuclear protein from day 8 mock and mtPILSAP EBs was purified, and the protein concentration was measured using a Bio‐Rad Dc Protein Assay Kit (Bio‐Rad Laboratories, Hercules, CA, USA). Proteome analysis using 20 μg each of nuclear protein was conducted by AMR (Tokyo, Japan).
IP‐Western. Polyclonal rabbit IgG against a synthetic peptide corresponding to amino acid residues 262–275 of mouse pigpen, including a cysteine linker (RDQGSRHDSEQDNSC), was prepared (anti‐mPigpen Ab). MSS31 cells were starved in αMEM with 0.1% BSA (BSA‐αMEM) for 24 h and incubated in BSA‐αMEM with 50 ng/mL VEGF (Sigma, St. Louis, MO, USA) or 20 ng/mL basic fibroblast growth factor (bFGF; BD Biosciences, San Jose, CA, USA) for 20 min. The cell extract (500 μg) was incubated with 10 μg antimouse PILSAP goat IgG (anti‐mPILSAP Ab; R&D Systems, Minneapolis, MN, USA) for 13 h at 4°C. Immune complexes were incubated with protein G‐sepharose (GE Healthcare Life Science, Uppsala, Sweden) for 2 h at 4°C. Immunoprecipitates were applied to a western blot with 2 μg/mL anti‐mPILSAP Ab or anti‐mPigpen Ab. The signals were detected with ECL plus western blotting detection reagents (GE Healthcare) and visualized using LAS1000 (Fuji, Tokyo, Japan).
Immunocytochemistry (ICC). MSS31 cells were starved in αMEM containing 0.1% FBS (0.1%αMEM) for 24 h and incubated in 0.1%αMEM with 50 ng/mL VEGF or 20 ng/mL bFGF for 20 min. Cells were fixed in 4% paraformaldehyde (PFA), incubated in blocking solution (BS: 2% BSA and 0.2% Triton X‐100 in PBS) at room temperature (RT:18–24°C) for 30 min, and treated with 10 μg/mL anti‐mouse PILSAP goat IgG or normal goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in 1/10 (v/v) BS (reaction buffer: RB) at 4°C overnight, followed by incubation with 4 μg/mL Alexa568‐conjugated donkey antigoat IgG (Invitrogen) in RB at RT for 1 h. Cells were then incubated firstly with 10 μg/mL antimouse pigpen rabbit IgG or normal rabbit IgG (Santa Cruz Biotechnology) in RB at RT for 1 h and then with 4 μg/mL Alexa488‐conjugated donkey antirabbit IgG (Invitrogen) in RB at RT for 1 h. Nuclei were stained with TO‐PRO‐3 (Invitrogen). Cells were washed with PBS at RT for 5 min twice between each steps. Images were photographed by a Zeiss LSM 510 confocal microscope system (Carl Zeiss, Oberkochen, Germany).
Inhibition of pigpen expression by siRNA. Control or siRNA oligonucleotides at a final concentration of 10 nm were transfected into MS1, MSS31, and LLC cells using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen). The sequences of siPigpen and scrPigpen were as follows: 5′‐GAACAGGAUAAUUCAGACATT‐3′ and 5′‐AAUCGAAUUAGCAGGAAACTT‐3′, respectively. The sequences of the other set, siPigpen‐A, siPigpen‐B, and nontargeting control siRNA (NC siRNA) as a negative control for these two siRNAs were as follows: 5′‐CAAACGACUAUACCCAACATT‐3′, 5′‐CAAUACCAUCUUCGUGCAATT‐3′, and 5′‐UCUUAAUCGCGUAUAAGGCTT‐3′, respectively. The efficacy of siRNAs for pigpen was evaluated by mRNA (data not shown) and protein levels.
Western blotting. The protein extracts (20 μg) from MSS31, MS1, and LLC cells transfected with scr‐ or siPigpen for 24 h were applied to a western blot with 2 μg/mL anti‐mPigpen Ab or anti‐mPILSAP Ab. The signals were detected with ECL plus western blotting detection reagents and visualized using LAS1000. Equal loading was confirmed by blotting with anti‐β‐actin Ab (Sigma).
EC proliferation assay. At 24 h after transfection of scr‐ or siPigpen into MSS31 or LLC cells (2000 cells/well in a 96‐well‐plate), cells were incubated in 0.1%αMEM with or without 50 ng/mL VEGF (MSS31) or 1 or 10% DMEM for another 24 h. In some experiment, cells were transfected with scr‐ or siPigpen, NC siPigpen, siPigpen‐A, or siPigpen‐B on the day before an assay. MS1 or MSS31 cells (20 000 or 10 000/well, respectively) were inoculated in a 96‐well‐plate and incubated in 0.1% HG‐DMEM or 0.1%αMEM. After 24‐h incubation, the medium of MS1 was changed to 100 μL/well of 0.1% HG‐DMEM with or without 20 ng/mL bFGF and MS1 cells were incubated for an additional 24 h. MSS31 cells were incubated for 48 h. Cell numbers were analyzed with a Cell Titer 96 Aqueous Assay (Promega, Madison, WI, USA).
EC wound migration assay. At 10 h after transfection of scr‐ or siPigpen into MS1 cells, medium was changed to growth medium. After 14‐h incubation, cells were replated into 12‐well plates at a density of 200 000/well. The next day, confluent monolayers were wounded with a yellow tip, and cells were incubated in 0.1% HG‐DMEM for an additional 24 h. Cells that had migrated into the denuded area were counted.
Transwell migration assay. MSS31 cells were transfected with scr‐ or siPigpen, NC siPigpen, siPigpen‐A, or siPigpen‐B. Next day cells were starved in 0.1%αMEM for 2 h and replated in 0.1%αMEM on a filter of 8‐μm pore size in the upper insert of 24‐well transwell chambers (Corning, Acton, MA, USA) at the density of 47 500 cells/95 μL/well. Six‐hundred microliter of 0.1%αMEM with or without 20 ng/mL bFGF was added into the lower chamber. After 7‐h incubation, unmigrated cells on the upper side of the filter were scraped off with a cotton swab and the filters were fixed and stained with 0.1% crystal violet in 70% ethanol for 30 min. Migration was quantified by counting cells in randomly selected high power fields (HPF).
EC network formation assay. MS1 cells treated as described above were inoculated onto 96‐well plates coated with growth factor‐reduced Matrigel (GFR‐Matrigel; BD Biosciences) at a density of 20 000 per well. Under these conditions, cells began to form tubes, and 4 h later, the tube length was measured with a BZ Analyzer (Keyence, Osaka, Japan).
Matrigel plug assay. GFR‐Matrigel (500 μL) containing 100 ng/mL bFGF (BD Biosciences), 32 U/mL heparin, and 200 nm scr‐ or siPigpen was subcutaneously injected into 5‐week‐old male C57BL/6 mice. On day 6, Matrigel was removed for immunohistochemistry (IHC) of vascular endothelial (VE)‐cadherin and hemoglobin (Hb) measurement. Hb content was measured using a hemoglobin B test (Wako, Osaka, Japan). The number of blood vessels positive for VE‐cadherin was counted under a fluorescent microscope (Biozero BZ‐8000, Keyence).
Syngenic tumor implantation model. A mixture of 150 μL GFR‐Matrigel and 150 μL of Hanks’ Balanced Salt Solution (HBSS) containing 500 000 LLC cells was subcutaneously injected into 5‐week‐old male C57BL/6 mice. On day 5, 100 pmol scr‐ or siPigpen was injected into the tumors. Every 3 days, the tumor volume was measured with calipers, and the injection of scr‐ or siPigpen was repeated. Tumor volume was estimated by using the formula of a (the major axis) × b (the minor axis)2/2. On day 14, tumors were removed for IHC for CD31, and the length of CD31‐positive tubes was measured with a BZ Analyzer (Keyence).
All protocols for experiments involving animals were approved by the Tokyo Medical and Dental University Bioethical and Animal Care Ethics Committees and conformed to the provisions of the Declaration of Helsinki in 1995 (as revised in Tokyo in 2004).
IHC analysis. Tissues from the Matrigel plug assay and the syngenic tumor implantation model were harvested, embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan), and snap‐frozen in liquid nitrogen. Thin sections (5–6 μm) were cut on a cryostat (Leica CM1850, Leica MZFLIII; Leica Microsystems, Wetzlar, Germany) and collected on glass slides. Specimens were dried for 30 min and fixed in 4% PFA in 0.1 M PBS for 10 min. After washing with PBS, specimens were incubated in BS for 1 h, and then incubated with 2 μg/mL anti‐VE‐cadherin (Alexis Biochemicals, San Diego, CA, USA) or 1/100 (v/v) antimouse CD31 monoclonal (Fitzgerald Industries International, Concord, MA, USA) and 10 μg/mL antimouse pigpen rat IgG in RB for 1 h. After three washes with PBS, specimens were incubated with 4 μg/mL Alexa Fluor 546 goat antirabbit and rat IgG (Invitrogen) for VE‐cadherin and CD31, respectively, or Alexa Fluor 488 goat antirabbit IgG (Invitrogen) for pigpen in RB for 1 h, washed with PBS three times, and incubated with Hoechst 33342 or TO‐PRO‐3 for nuclear staining. After a tap water rinse, VE‐cadherin, CD31, and pigpen signals in endothelial cells were observed under a fluorescent microscope (Biozero BZ‐8000, Keyence).
Statistical analysis. The statistical significance of differences was evaluated by anova, and P‐values were calculated using the Student’s t‐test. A value of P < 0.05 was considered statistically significant. The statistical calculation was done using JMP software (SAS, Cary, NC, USA).
Results
PILSAP activity regulated the expression of pigpen, a coiled body component protein. We previously showed that among vascular endothelial growth factor receptor 2 (VEGFR2)‐positive precursor cells, including vascular, hematopoietic, and muscle lineages, only ECs continued to express VEGFR2 until terminal differentiation in an in vitro mouse ES differentiation culture system.( 9 ) The time course of VEGFR2 expression in EBs showed two peaks at days 4 and 10, indicating expansion of VEGFR2‐positive mesodermal precursors and endothelial lineages, respectively.( 9 ) To clarify the mechanisms of how PILSAP plays a role in differentiation or maturation of ECs, we searched for molecules whose expression was correlated with the AP activity of PILSAP. On day 8, after day 7 when the expression of PILSAP was lowest and before day 10, the second peak when other EC markers including CD31 and Tie‐2 peaked as well,( 9 ) nuclear protein was extracted from mtPILSAP and mock EBs, and proteome analysis was performed. Pigpen was identified as a molecule whose expression was reduced to less than half in mtPILSAP EBs compared with mock EBs.
Binding of pigpen to PILSAP. The previous result that the AP activity of PILSAP regulates pigpen expression during EC differentiation does not necessarily indicate that pigpen directly interacts with PILSAP in ECs. Therefore, we examined whether pigpen could bind to PILSAP. Immunoprecipitation with anti‐PILSAP followed by western blotting of pigpen and PILSAP demonstrated binding between these two molecules in a mouse endothelial cell line, MSS31. Furthermore, the binding was induced by a 20‐min treatment with the representative angiogenic growth factors, VEGF and bFGF in ECs (Fig. 1a,b). Immunocytochemical analysis revealed that pigpen was localized in nuclei, whereas PILSAP was in cytosol and on nuclear membrane. VEGF and bFGF induced translocation of PILSAP from cytosol to nuclei (Fig. 1c). These results suggest that pigpen may be involved in angiogenesis in cooperation with PILSAP upon VEGF and bFGF stimuli.
Figure 1.
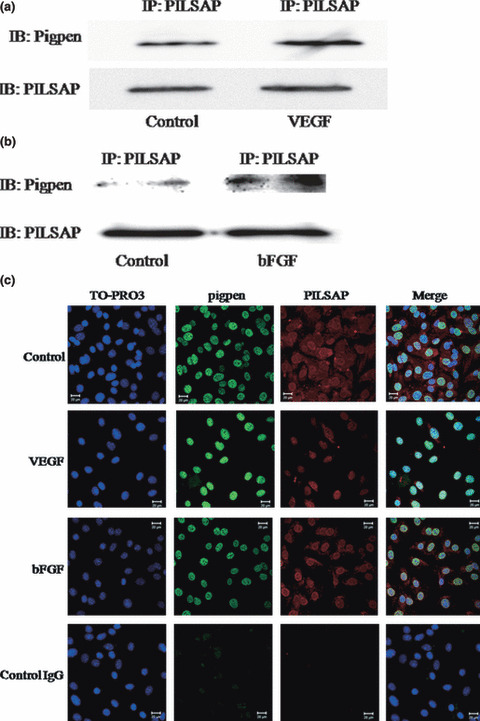
Immunopreciptation by anti‐puromycin‐insensitive leucyl‐specific aminopeptidase (PILSAP) Ab followed by western blotting of pigpen and PILSAP (a,b) and immunocytochemistry of pigpen (green) and PILSAP (red) (c) in MSS31 cells treated with vehicle, 50 ng/mL vascular endothelial growth factor (VEGF), or 20 ng/mL basic fibroblast growth factor (bFGF) for 20 min. Nuclei were counterstained with TO‐PRO‐3 (blue). Bars = 20 μm.
SiRNA for pigpen inhibited in vitro angiogenesis. We next investigated whether pigpen plays a role in angiogenesis. We synthesized siRNAs for pigpen and their controls (siPigpen and scrPigpen; NC siRNA, siPigpen‐A, and siPigpen‐B). SiPigpen strongly inhibited the expression of pigpen protein in mouse endothelial cell lines, MSS31 and MS1 (Fig. 2a) as well as pigpen mRNA (data not shown). The protein level of PILSAP was not altered by transfection of siRNAs for pigpen. Transfection of siPigpen into MSS31 or MS1 abrogated the increase in proliferation by VEGF or bFGF, respectively (Fig. 3a). The decrease in EC proliferation by inhibiting pigpen expression was confirmed by two other siRNAs, namely siPigpen‐A and siPigpen‐B (Fig. 3b). Moreover, siPigpen significantly inhibited MS1 migration and abrogated the increase by VEGF (Fig. 4a). Inhibition of EC migration by the transfection with three different siRNAs was observed in MSS31 (Fig. 4b). Furthermore, siPigpen significantly inhibited network formation on Matrigel (Fig. 5). These data demonstrate the positive effect of pigpen on EC proliferation, migration, and network formation.
Figure 2.
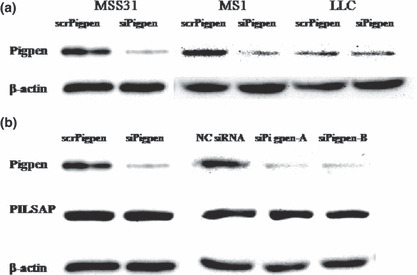
Western blot of pigpen in scr‐ or siPigpen‐transfected MSS31, MS1, and Lewis lung carcinoma (LLC) cells (a). Western blot of pigpen and PILSAP in MSS31 transfected with scr‐ or siPigpen, siPigpen‐A, siPigpen‐B, or nontargeting siRNA (NC siRNA) as a negative control for siPigpen‐A and ‐B (b). Equal loading was confirmed by β‐actin.
Figure 3.
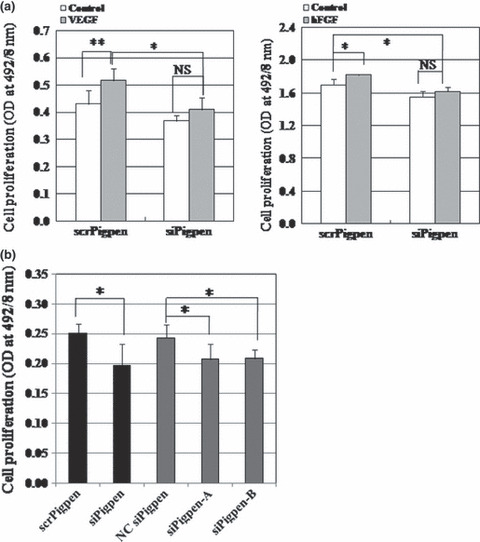
(a) Endothelial cell (EC) proliferation assay of MSS31 (left) and MS1 (right) cells transfected with scr‐ or siPigpen. MSS31 cells were treated with or without 50 ng/mL vascular endothelial growth factor (VEGF) for 24 h (n = 6). MS1 cells were treated with or without 20 ng/mL basic fibroblast growth factor (bFGF) for 24 h (n = 3). (b) EC proliferation assay of MSS31 cells transfected with scr‐ or siPigpen, nontargeting control siRNA (NC siRNA), siPigpen‐A, or siPigpen‐B. (n = 6 for scr‐ or siPigpen and n = 5 for NC siRNA, siPigpen‐A, or siPigpen‐B). Bars indicate SDs. *P < 0.05, **P < 0.01.
Figure 4.
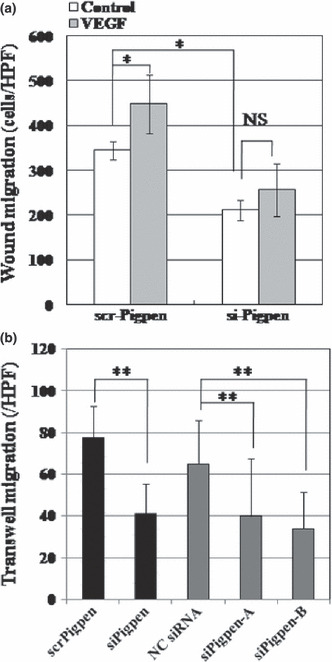
(a) Wound migration assay of scr‐ or siPigpen‐transfected MS1 cells with vehicle or 50 ng/mL vascular endothelial growth factor (VEGF) (n = 6). (b) Transwell migration assay of MSS31 cells transfected with scr‐ or siPigpen, nontargeting control siRNA (NC siRNA), siPigpen‐A, or siPigpen‐B (n = 12). Data indicate means and SDs. *P < 0.05, **P < 0.01.
Figure 5.
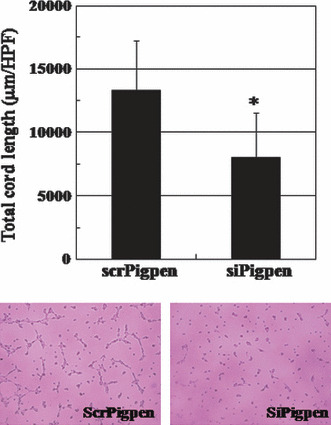
Network formation of MS1 cells transfected with scr‐ or siPigpen. Data indicate means and SDs, n = 6. *P < 0.05. Representative images are shown.
SiRNA for pigpen inhibited in vivo angiogenesis. Next, we performed a Matrigel plug assay to examine the effect of siPigpen on in vivo angiogenesis. GFR‐Matrigel containing 100 ng/mL bFGF, heparin, and 200 nm scr‐ or siPigpen was subcutaneously injected into 5‐week‐old mice. On day 6, Matrigel was removed, and we measured the Hb content and counted the number of blood vessels that were positive for VE‐cadherin in transplanted GFR‐Matrigel to analyze blood vessel formation and blood supply. Both the Hb content and blood vessel number were significantly increased by bFGF, and siPigpen abrogated this increase by bFGF (Fig. 6). Thus, pigpen appears to be involved in both in vitro and in vivo angiogenesis.
Figure 6.
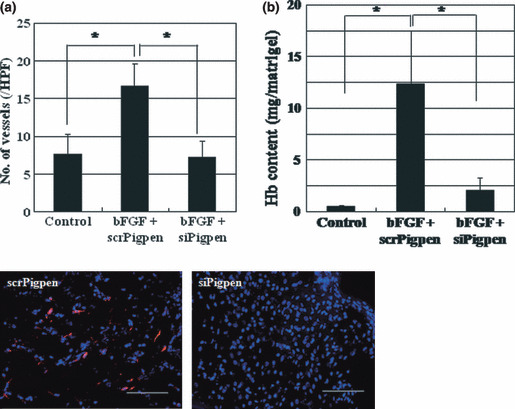
Matrigel plug assay was performed as described in the Materials and Methods. On day 6, blood vessels positive for vascular endothelial (VE)‐cadherin were counted (a) and hemoglobin content in the Matrigels was measured (b). Data indicate means and SEs (a) or SDs (b). n = 12 (Control) or 15 (basic fibroblast growth factor [bFGF] + scr‐ or siPigpen) in (a); n = 5 (Control and bFGF + siPigpen) or 6 (bFGF + siPigpen) in (b). *P < 0.05. Representative immunohistochemistry (IHC) of VE‐cadherin (red) and nuclei (blue) is shown. Scale bar = 100 μm. Hb, hemoglobin.
SiRNA for pigpen inhibited tumor growth as well as tumor angiogenesis. The result showing an inhibitory effect of siPigpen on in vivo angiogenesis prompted us to examine whether siPigpen could reduce tumor growth by inhibiting tumor angiogenesis. We employed a syngenic tumor implantation model of LLC. LLC cells express less pigpen than mouse ECs in vitro (Fig. 2a, scrPigpen) as well as in vivo (Fig. 7d, scrPigpen). Transfection of siPigpen into LLC cells did not alter the proliferation (Fig. 7a). LLC cells in GFR‐Matrigel were subcutaneously injected into C57BL/6 mice. On day 5 when tumors became palpable, scr‐ or siPigpen was injected into the tumor, and the injection was repeated every three days. Figure 7(b) shows the time course of tumor growth in both groups. Injection of siPigpen significantly reduced the tumor size compared with the scrPigpen group on days 10 and 14, after two and three injections, respectively. Immunohistochemical analysis of tumor on day 14 showed the efficient inhibition of pigpen expression by siPigpen (Fig. 7d). The total tube length of blood vessels that were positive for CD31 on day 14 was significantly reduced by siPigpen injection (Fig. 7c,d). These data indicate that pigpen may be a future target for blocking tumor angiogenesis.
Figure 7.
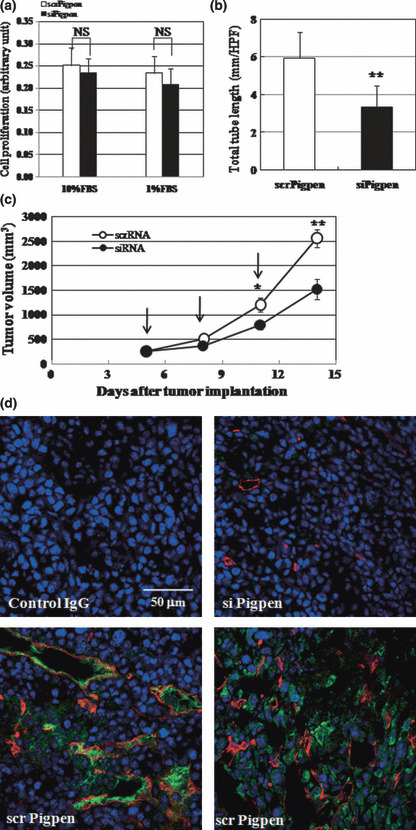
Syngenic tumor implantation model was performed as described in the Materials and Methods. (a) siPigpen did not inhibit Lewis lung carcinoma (LLC) cell proliferation in vitro. Data indicate means and SDs, n = 10. (b) siPigpen inhibited tumor growth of LLC. Data indicate means and SEMs, n = 10 and 11 for scr‐ and siPigpen, respectively (days 5, 8, 10) and n = 8 (day 14). (c) siPigpen inhibited tumor angiogenesis. Data indicate means and SDs, n = 15 (scrPigpen) or 10 (siPigpen). *P < 0.05, **P < 0.01. (d) Representative immunohistochemistry (IHC) of CD31 (red), pigpen (green), and nuclei (blue) is shown. Bar = 50 μm.
Discussion
We previously isolated PILSAP as a novel angiogenesis factor and showed that the expression of PILSAP is enhanced in mouse cells differentiating from ES cells to ECs.( 5 ) We also demonstrated that PILSAP is involved in postnatal angiogenesis.( 5 , 6 , 7 , 8 ) In this study, we searched for a molecule that is related to PILSAP and involved in endothelial differentiation, similar to PILSAP. Proteome analysis using mtPILSAP and mock EBs on day 8 of in vitro differentiation cultures revealed that the expression of pigpen, a nuclear coiled body component, was reduced to less than half in mtPILSAP EBs lacking the AP activity. This result suggests that AP activity of PILSAP may regulate pigpen expression, but does not indicate that pigpen directly interacts with PILSAP. However, we detected the complex of PILSAP and pigpen in the nuclear and membrane fractions of EBs and found that the binding was down‐regulated in mtPILSAP‐EBs (M. Abe, unpublished data, 2008). Pigpen has been suggested to be involved in angiogenesis, especially by inducing EC proliferation and differentiation.( 10 , 11 , 14 , 15 , 16 ) Therefore, we speculated that pigpen might play a role in angiogenesis in cooperation with PILSAP. First, we examined whether pigpen could bind to PILSAP using IP‐Western blotting of mouse EC protein extracts. Although it is known that PILSAP is a cytosolic enzyme( 5 ) and pigpen is a nuclear protein,( 11 ) western blotting using fractionated cell lysates of mouse EBs and ECs revealed that PILSAP localizes predominantly in the cytosol and membrane fractions, whereas pigpen localizes in the nuclear and membrane fractions (M. Abe, unpublished data, 2008). Moreover, ICC of PILSAP and pigpen in MSS31 showed their localization, namely cytosol/nuclear membrane and nucleus/nuclear membrane, respectively (Fig. 1c). The binding of these two molecules were augmented by VEGF and bFGF (Fig. 1a,b). ICC of these molecules demonstrated that VEGF and bFGF induced PILSAP translocation from cytosol to the nucleus/nuclear membrane where pigpen existed (Fig. 1c), although bFGF showed less effect compared with VEGF in these experiments with 20‐min incubation (Fig. 1).
Next, we investigated whether pigpen plays a role in postnatal angiogenesis as PILSAP. Three different siRNAs for pigpen inhibited proliferation and migration of MSS31 (3, 4). Although PILSAP regulated pigpen expression, pigpen did not alter PILSAP expression (Fig. 2b). Transfection of siPigpen, one of these siRNAs, inhibited proliferation and migration of two different cell types, MSS31 and MS1, and abrogated the increase in proliferation and migration by VEGF and bFGF (3, 4). Inhibition of pigpen expression resulted in suppression of angiogenesis both in vitro (3, 4, 5) and in vivo (Fig. 6). Furthermore, injection of siPigpen in syngenic tumors subcutaneously implanted into C57BL/6 mice inhibited tumor growth and tumor angiogenesis (Fig. 7). These findings suggest that the inhibition of pigpen may be useful for therapy of malignant tumors by inhibiting tumor angiogenesis.
We speculate that PILSAP–pigpen complex induced by VEGF and bFGF may play a role in angiogenesis. Further study is needed to clarify whether and how pigpen interacts with PILSAP to promote angiogenesis in vivo and whether pigpen is a substrate for PILSAP.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Scientific Research (no. 17590235) from the Ministry of Education, Science, Sports and Culture of Japan.
Disclosure Statement
The authors have no conflict of interest.
References
- 1. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 2. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–10. [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P, Ferreira V, Breier G et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380: 435–9. [DOI] [PubMed] [Google Scholar]
- 4. Ferrara N, Carver‐Moore K, Chen H et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380: 439–42. [DOI] [PubMed] [Google Scholar]
- 5. Miyashita H, Yamazaki T, Akada T et al. A mouse orthologue of puromycin‐insensitive leucyl‐specific aminopeptidase is expressed in endothelial cells and plays an important role in angiogenesis. Blood 2002; 99: 3241–9. [DOI] [PubMed] [Google Scholar]
- 6. Yamazaki T, Akada T, Niizeki O, Suzuki T, Miyashita H, Sato Y. Puromycin‐insensitive leucyl‐specific aminopeptidase (PILSAP) binds and catalyzes PDK1, allowing VEGF‐stimulated activation of S6K for endothelial cell proliferation and angiogenesis. Blood 2004; 104: 2345–52. [DOI] [PubMed] [Google Scholar]
- 7. Akada T, Yamazaki T, Miyashita H et al. Puromycin insensitive leucyl‐specific aminopeptidase (PILSAP) is involved in the activation of endothelial integrins. J Cell Physiol 2002; 193: 253–62. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T, Abe M, Miyashita H, Kobayashi T, Sato Y. Puromycin insensitive leucyl‐specific aminopeptidase (PILSAP) affects RhoA activation in endothelial cells. J Cell Physiol 2007; 211: 708–15. [DOI] [PubMed] [Google Scholar]
- 9. Abe M, Sato Y. Puromycin insensitive leucyl‐specific aminopeptidase (PILSAP) is required for the development of vascular as well as hematopoietic system in embryoid bodies. Genes Cells 2006; 11: 719–29. [DOI] [PubMed] [Google Scholar]
- 10. Alliegro MC, Alliegro MA. A nuclear protein regulated during the transition from active to quiescent phenotype in cultured endothelial cells. Dev Biol 1996; 174: 288–97. [DOI] [PubMed] [Google Scholar]
- 11. Alliegro MC, Alliegro MA. Identification of a new coiled body component. Exp Cell Res 1996; 227: 386–90. [DOI] [PubMed] [Google Scholar]
- 12. Alliegro MC, Alliegro MA. Protein heterogeneity in the coiled body compartment. Exp Cell Res 1998; 239: 60–8. [DOI] [PubMed] [Google Scholar]
- 13. Ogg SC, Lamond AI. Cajal bodies and coilin‐‐moving towards function. J Cell Biol 2002; 159: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blank M, Weinschenk T, Priemer M, Schluesener H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels. selective targeting of endothelial regulatory protein pigpen. J Biol Chem 2001; 276: 16464–8. [DOI] [PubMed] [Google Scholar]
- 15. Alliegro MC, Alliegro MA. Nuclear injection of anti‐pigpen antibodies inhibits endothelial cell division. J Biol Chem 2002; 277: 19037–41. [DOI] [PubMed] [Google Scholar]
- 16. Alliegro MC. Pigpen and endothelial cell differentiation. Cell Biol Int 2001; 25: 577–84. [DOI] [PubMed] [Google Scholar]


