Abstract
Malignant pleural mesothelioma (MPM) is difficult to diagnose at an early stage. The present study attempted to obtain a tumor‐specific antibody against MPM derived from tumor‐infiltrating B lymphocytes in MPM by using a xenotransplanted severe combined immunodeficiency (SCID) mouse model, and to identify the antigens recognized by the antibodies. Among the antigen–antibody relationships, the clinical usefulness of antibody titers in the sera was evaluated from the viewpoint of diagnosis of MPM and monitoring of therapeutic effects. Tumor tissue specimens from two patients with MPM were engrafted subcutaneously in SCID mice and blood samples were obtained and pooled every 2 weeks after xenotransplantation until 14 weeks when the mice were killed. A cDNA library was constructed from the mRNA of a MPM cell line (K921MSO). Immunoscreening of the libraries was carried out by serological identification of antigens by a recombinant expression cloning method (SEREX) and four antigens were identified as MPM‐associated antigens. Among them, antibody titers against two antigens, Gene‐X and thrombospondin‐2 (THBS‐2), were analyzed by phage plaque assay as the first step. ELISA systems correlated with the phage plaque assay to detect antibody titers against the two antigens were constructed using 20‐mer peptides of the antigen‐coding genes. The cut‐off value was decided by the average and standard deviation of normal healthy persons. Antibody against Gene‐X was detected in 10 out of 18 (55.6%) mesothelioma patients and antibody against THBS‐2 was detected in 16 out of 18 (88.9%) mesothelioma patients. No patients with lung cancer regardless of asbestos exposure exhibited positive antibody titer against the two antigens. Furthermore, the serum antibody titers decreased after surgical treatment of MPM and increased after recurrence of the disease. The titers of the antibodies against Gene‐X and THBS‐2 could be used as tumor markers for the diagnosis and follow up of patients with MPM. (Cancer Sci 2009; 100: 1326–1334)
Abbreviations:
- CT
threshold cycle number
- EPP
extrapleural pneumonectomy
- IFT‐88
intraflagellar transport 88 homolog
- MPM
malignant pleural mesothelioma
- SEREX
serological identification of antigens by a recombinant expression closing method
- STUB
stress‐induced‐phosphoprotein 1 homology and U‐box containing protein
- TIB
tumor‐infiltrating B lymphocytes
- THBS
thrombospondin
- TOPIIβ
topoisomerase IIβ
Malignant pleural mesothelioma (MPM) is an aggressive neoplasm arising from mesothelial cells and it is usually fatal. MPM is thought to be associated with previous exposure to asbestos fibers. Due to the widespread use of asbestos fibers in the latter half of the 20th century, the incidence of MPM is predicted to rise sharply in industrialized countries in the next few decades after 30–40 years of a latent period.( 1 ) The number of new cases in Europe is expected to double between 1998 and 2018.( 2 ) Based on the prevalence in USA and Europe, Japan is also facing an epidemic of MPM belatedly. In Japan, 500 patients with MPM died in 1995 and the number of deaths increased to approximately 900 patients in 2003.( 3 ) Not only occupational exposure, but also environmental exposure becomes a public health problem, because asbestos was used widely in buildings as a construction material and asbestos cement pipes.( 4 , 5 )
Malignant pleural mesothelioma is difficult to diagnose radiographically at an early stage and often progresses to an advanced stage without symptoms. Although a new chemotherapy regimen using cis‐diamminedichloroplatinum (CDDP) plus pemetrexed has shown some improvement, the median survival is generally only 9–12 months.( 6 , 7 , 8 , 9 ) The potentially curative approach to MPM seems to be extrapleural pheumonectomy (EPP) at an early stage, followed by chemotherapy and radiotherapy (trimodality treatment). Sugarbaker et al. reported that the median survival after EPP is 19 months and the 2‐ and 5‐year survival rates are 38 and 15% respectively.( 9 ) However, the perioperative mortality of EPP has been reported to be as high as 11%.( 9 ) No randomized controlled study between EPP and an alternative treatment has been carried out because of the limited number of MPM patients at stages I and II.
Tumor‐infiltrating B lymphocytes (TIB) in lung cancer produce antibodies against tumor‐associated antigens and the antibody titer against certain antigens is useful for evaluation of the outcome of anticancer treatments.( 10 , 11 , 12 ) Therefore, the present study focused on the roles of TIB in MPM. The present study was designed to obtain tumor‐specific antibodies derived from TIB of malignant mesothelioma by using a xenotransplanted SCID mouse model.( 12 , 13 ) TIB‐derived IgG was applied to the serological identification of antigens using the recombinant expression cloning method (SEREX) in order to identify tumor antigens associated with MPM and to discover “diagnostic biomarkers” useful for diagnosis and/or evaluation of the effect of treatment for MPM.
Materials and Methods
The study protocol was approved by the Human and Animal Ethics Review Committee of the University of Occupational and Environmental Health, Japan, and written informed consent was obtained from each patient regarding the usage of surgical specimens and sera.
Patients. Eighteen patients with MPM listed in Table 1 were treated in our hospital. Among the 18 patients with MPM, 17 patients were male and one was female. The average age was 65.9 years (range 54–82 years). According to the histological classification, eight patients were epithelioid type, six were sarcomatoid type, and four were biphasic type. According to the international MPM staging system,( 14 ) three patients were at stage I, four were at stage II, eight were at stage III, and three were at stage IV. The sera of the 18 patients with MPM were obtained before any treatment. Among them, nine patients were subjected to EPP. The sera of 47 patients with lung cancer were also collected and stored at –20°C until use. Among them, 27 had a history of asbestos exposure in the work place and 20 did not. The sera of 16 patients with breast cancer were also collected. Healthy donor sera were collected from 25 volunteers aged from 26 to 44 years as a control.
Table 1.
Characteristics of patients with malignant pleural mesothelioma
| Patient | Age (years) | Sex | Stage | Treatment | Histology | Autoimmune |
|---|---|---|---|---|---|---|
| M1 | 56 | Male | I | EPP | Sarcomatoid | – |
| M2 | 68 | Male | III | EPP | Epithelioid | Membranous nephropathy |
| M3 | 57 | Male | IV | SC | Epithelioid | ITP, hyperγglobulinemia |
| M4 | 64 | Male | III | SC | Sarcomatoid | – |
| M5 | 76 | Male | III | Chemotherapy | Sarcomatoid | – |
| M6 | 75 | Male | II | EPP | Sarcomatoid | – |
| M7 | 61 | Male | II | EPP | Epithelioid | – |
| M8 | 54 | Male | III | EPP | Biphasic | – |
| M9 | 78 | Male | I | Chemotherapy | Epithelioid | – |
| M10 | 65 | Female | II | EPP | Epithelioid | – |
| M11 | 82 | Male | III | SC | unknown | Unknown |
| M12 | 80 | Male | III | SC | Epithelioid | Unknown |
| M13 | 55 | Male | I | EPP | Epithelioid | – |
| M14 | 69 | Male | II | SC | Epithelioid | – |
| M15 | 67 | Male | III | EPP | Biphasic | – |
| M16 | 56 | Male | IV | SC | Biphasic | – |
| M17 | 56 | Male | IV | Chemotherapy | Sarcomatoid | – |
| M18 | 67 | Male | III | EPP | Biphasic | – |
M1 and M2 patients with malignant mesothelioma engrafted in SCID mice were studied.
M1–M18 patients had blood samples taken in order to analyze their antibody titers against the identified antigens.
EPP, extrapleural pneumonectomy; ITP, idiopathic thrombocytopenic purpura; SC, supportive care.
Engraftment of human mesothelioma tissue into SCID mice. Female SCID mice (6 weeks old) were purchased from Charles River Japan (Tokyo, Japan) and were maintained in specific pathogen‐free conditions throughout the study. Approximately 5 × 5 × 5 mm of the fresh mesothelioma tissue specimens of patient no. 1 and 2 were engrafted subcutaneously into the lateral flank of the SCID mice. Blood from the SCID mice was obtained by retro‐orbital venipuncture and the tumor volume was measured every 2 weeks. The serum was collected and pooled at –20°C after being centrifuged at 1500 g for 10 min at 20°C. The SCID mice were killed at 14 weeks after engraftment and whole blood was then collected to obtain the serum.
Tumor tissue specimens and cell lines. The two mesothelioma tissue specimens (M1 and M2) engrafted into SCID mice were grown in SCID mice. One mesothelioma cell line designated as K921MSO was established from patient M4. The method for establishing the tumor cell line has been described previously.( 15 ) This cell line was cultured in complete culture medium consisting of RPMI‐1640 (Gibco‐BRL, Grand Island, NY, USA) supplemented with 10% heat‐inactivated fetal calf serum (Equitech‐Bio, Ingram, TX, USA), 10 mmol/L HEPES, 100 units/mL penicillin G, and 100 mg/mL streptomycin sulfate.
Immunohistochemical staining for B lymphocytes. Immunohistochemical staining of B lymphocytes infiltrating into the tumor tissue was carried out using peroxidase‐conjugated mouse monoclonal antihuman CD20 antibody (L26; Dako Japan, Tokyo, Japan), as described previously.( 10 ) Briefly, the formalin‐fixed paraffin‐embedded tissue sections were deparaffinized, dehydrated, and incubated with 3% hydrogen peroxidase in distilled water for 5 min. They were rinsed with distilled water and placed in TBS for 5 min, incubated with L26 diluted with TBS 1 : 50 for 60 min at room temperature, and then the labeled Streptavidin Biotin Kit (Dako Japan) was used. Thereafter, the slides were washed with water, dehydrated, mounted, and examined microscopically.
RNA extraction and construction of the cDNA library. mRNA from the mesothelioma tissue from patient M1 and the mesothelioma cell line K921MSO were prepared using the Fast Track mRNA Extraction Kit (Invitrogen, Carlsbad, CA, USA). A cDNA library was constructed in a λ‐ZAP Express vector, using a cDNA library kit (Stratagene, La Jolla, CA, USA). Wild‐type λ‐ZAP Express bacteriophage were amplified in Escherichia coli XL1 Blue MRF (Stratagene) by overnight propagation of 5000 pfu on NZY agar plates (ForMedium, Norfolk, UK). A 12‐mL aliquot of sphingomyelin buffer (5.8 g NaCl, 2 g MgSO4–7H2O, 20 mM Tris‐HCl [pH 7.5], 0.01% gelatin) was then added to the plates, which were gently agitated at 4°C overnight in a Bio‐Shaker (Taitec, Saitama, Japan). The resultant suspension was collected and centrifuged at 500 g for 10 min at 20°C, and the supernatants were collected and stored at 4°C. The established libraries contained 5 × 105 recombinant cDNA each, and were screened for the existence of antibody by SEREX.
SEREX method. In order to identify the antigens recognized by the IgG from TIB, the amplified cDNA expression library was screened using the sera from the SCID mice with engrafted tumors from M1 and M2 patients. The serum was incubated overnight at 4°C with nitrocellulose membranes containing phage plaques at a density of 10 000 pfu per plate. After washing, the membranes were incubated with a 1 : 1000 dilution of peroxidase‐conjugated, Fc fragment‐specific goat antihuman IgG (Jackson Immunoresearch, West Grove, PA, USA) for 1 h at room temperature and the reactive phage plaques were then visualized using 3‐3′‐diaminobenzidine.
Sequence analysis of the reactive cDNA clones. Immunoreactive cDNA was subcloned, purified, and excised to the pBK‐CMV Phagemid Vector (Stratagene). Plasmid DNA was prepared using the Wizard Miniprep DNA purification system (Promega, Madison, WI, USA). The inserted DNA was evaluated by EcoRI–XhoI restriction mapping and each cDNA insert was sequenced. The sequencing reactions were carried out using ABI PRISM 3100 (PE Biosystems, Tokyo, Japan). DNA sequence alignments were carried out using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) on the EMBL and GenBank databases.
Reverse transcription of the antigen‐coding gene in mesothelioma and normal tissues. Individual total RNA from the frozen malignant mesothelioma tissue specimens from patients (M1–M5, M7, M10, M14, M15) and K921MSO and L324MSO cell lines was obtained using the RNeasy plus mini kit (Qiagen Science, German Town, MD, USA). The commercially available panel of total RNA obtained from the 20 normal tissues including adrenal gland, bone marrow, brain, fetal brain, fetal liver, heart, kidney, liver, lung, placenta, prostate, salivary gland, skeletal muscle, testis, thymus, thyroid gland, trachea, uterus, colon, and spinal cord (Clontech Laboratories, Palo Alto, CA, USA) was used as normal controls. RNA was converted to cDNA using a First Strand cDNA Synthesis Kit (Amersham Pharmacia Biotech, Tokyo, Japan). The cDNA were used as templates for PCR amplification. Gene‐specific oligonucleotide primers were designed for each DNA segment at the estimated proper melting temperature. Gene‐specific primers were synthesized commercially by Hokkaido System Science (Sapporo, Japan). Four antigens were identified by the SEREX method as shown in Table 2. All primers for the four antigens detected were designed with Primer Express (Applied Biosystems, Foster, CA, USA) as shown in Table 3. The expected sizes of the PCR products were 251, 851, 851, and 500 bp for BAC clone CIT987SK‐A‐328A3 (AC002301) (designated as Gene‐X by us; clone 1), THBS‐2 (clone 2), STUB‐1 (clone 3), and IFT‐88 (clone 4) respectively. Each PCR amplification contained 2.5 µL cDNA supplemented with 2.5 µL of 10× PCR buffer (Takara Bio, Shiga, Japan), 1 µL of 10 mmol/L dNTP, 0.5 µL each of a 20‐mmol/L solution of primers, 1 unit of Taq DNA polymerase, and water added to a total volume of 25 µL. The mixture was heated to 94°C for 5 min. Amplification was then carried out in a thermal cycler (Biometra, Göttingen, Germany) with 30 cycles for all antigens. The cycles of amplification for clones 1, 2, and 4 were: 1 min at 94°C, 1 min at 65°C, and 1 min at 72°C; and for clone 3, 1 min at 94°C, 1 min at 63°C, and 1 min at 72°C. The PCR products were extended in a final step of 10 min at 72°C. The integrity and quantity of the cDNA were determined by comparison with β‐actin levels. The PCR products were analyzed by 1.5% gel electrophoresis and ethidium bromide visualization.
Table 2.
Antigen‐coding genes identified by IgG derived from tumor‐infiltrating B lymphocytes from xenotransplanted SCID mice
| cDNA clone | MPM patient | Homology search | Function of the gene |
|---|---|---|---|
| Clone‐1 | M1 | AC002301 (Gene‐X) | Unknown |
| Clone‐2 | M1 | Thrombospondin‐2 (THBS‐2) | Antiangiogenesis |
| Clone‐3 | M2 | STIP1 homology and U‐box containing protein 1 (STUB1) | Ubiquitin‐protein ligase activity |
| Clone‐4 | M2 | Intraflagellar transport 88 homolog (IFT‐88) | Unknown |
Table 3.
Specific primers of identified antigens for RT‐PCR
| Gene | Primers | Size (bp) | Annealing (°C) |
|---|---|---|---|
| RT‐PCR | |||
| Gene‐X | 5′‐GAG GCA AGA GAA TCA CAT GAA TCC‐3′ | 251 | 65 |
| 5′‐GCT GTA CTG ATC CTT TTC TCC TGA A‐3′ | |||
| THBS‐2 | 5′‐GGC GCA TCT AAC GCG TAT CT‐3′ | 851 | 65 |
| 5′‐CAG CTC CAC ACG CAA AAA AG‐3′ | |||
| STUB‐1 | 5′‐TAC ACC AAC CGG GCC TTG T‐3′ | 851 | 63 |
| 5′‐CCA GAG TCC AAC AGC AGA ACT TG‐3′ | |||
| IFT88 | 5′‐CGG CTA GAT GAG GCT TTG GA‐3′ | 500 | 65 |
| 5′‐TGT GCA GAG ACG AAC TAA GAA ACG‐3′ | |||
| Quantitative RT‐PCR | |||
| Gene‐X | 5′‐AGT CCA GGG CTC CTG CTG AA‐3′ | 103 | 60 |
| 5′‐TGG AAG CTC CGA CCG ACA T‐3′ | |||
| THBS‐2 | 5′‐CTT TGA GGT TGA TCG TTG TGT TGT‐3′ | 82 | 60 |
| 5′‐TTC GTT GGT CTC GGG AAT ACA‐3′ | |||
| STUB‐1 | 5′‐GGC CAA GCA CGA CAA GTA CAT‐3′ | 106 | 60 |
| 5′‐GCT GAT CTT GCC ACA CAG GTA GT‐3′ | |||
| IFT88 | 5′‐GAA ACT TCA CGC AAT CCT ACG A‐3′ | 111 | 60 |
| 5′‐ACCACCTGCATTAGCCATTCA‐3′ | |||
Quantitative RT‐PCR of the antigen‐coding gene. Quantitative RT‐PCR was carried out using the ABI PRISM 7000 (Applied Biosystems). The relative amounts of Gene‐X, THBS‐2, STUB‐1, and IFT‐88 mRNA were measured by means of detection of intercalated SYBR green. PCR was done with 10 µL of SYBR Green PCR Master Mix (Applied Biosystems), 1 µL of cDNA, and each primer set described below in a total volume of 20 µL. The PCR cycles were 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. The primer sequences of the four genes for quantitative RT‐PCR are shown in Table 3. The quantitative PCR primer sequences of β‐actin were GGCATCGTGATGGACTCCG and GCTGGAAGGTGGACAGCGA. The concentration of each primer set was 200 nmol/L for the identified gene and β‐actin. The CT was defined as the fractional cycle number at which the amount of amplified target product reached a fixed threshold. ΔCT was calculated by comparing the proportion of the CT between the identified antigen and β‐actin in the same amount of each templete. Relative quantification was achieved by comparisons with the ΔCT of normal lung. The relative expression was calculated using the following formula: relative expression = 2 – (ΔCT sample – ΔCT normal lung).
Analysis of the antibody titer against antigens detected in the sera of patients. To determine the antibody titer against immunoreactive clones, a phage plaque assay was carried out as described previously.( 10 , 13 ) Briefly, the phage from a positive clone were mixed with irrelevant phage as an internal negative control at a ratio 1:1 and then transfected into E. coli. Plaques were blotted onto nitrocellulose membranes and washed. Next, they were incubated with sera at 1:200, 1:1000, 1:5000, and 1:25 000 dilutions with TBS containing 0.02% NaN3, which had been incubated at 4°C over 16 h. Before these assays, patients’ sera were incubated with membranes blotted with irrelevant phage plaques at 4°C over 16 h in order to remove IgG against E. coli. The spots were evaluated as positive only if the tested clones were clearly distinguishable from the negative phage.
Establishment of ELISA for antibodies against Gene‐X and THBS‐2. Three 20‐mer peptides were predicted with a computed algorithm system on the basis of their hydrophilicity, probability of surface exposure, flexibility, and antigenic index from the base sequence of the open reading frame of Gene‐X and THBS‐2, each peptide synthesized with over 95% purity was purchased from BEX Co., Ltd. (Tokyo, Japan) as shown in Figure 1. Each synthesized peptide of Gene‐X and THBS‐2 was diluted in PBS and coated onto a flat‐bottomed 96‐well ELISA plate (Iwaki, Asahi Techno Glass, Chiba, Japan) at 4°C overnight. After serial dilution of the peptides, the optimal peptide concentrations of Gene‐X and THBS‐2 were decided to be 1 µg/mL and 100 ng/mL respectively. The plates were washed with PBS in Tween and blocked with 1% BSA in PBS at room temperature for 1 h. After washing three times, the patient sera (diluted 1/100, 1/500, and 1/2500 in 1% BSA in PBS) were added and incubated at 4°C overnight to assure the correlation of the antibody titers between the phage plaque assay and ELISA. The plates were washed and incubated with the secondary antibody, horseradish peroxidase‐conjugated goat antihuman IgG (H + L‐chain) (Medical and Biological Laboratories, Nagoya, Japan) at 1/4000 dilution for 5 h at 4°C. The plates were washed and incubated with tetramethylbenzidine substrate solution (1,2‐phenylenediamine dihydrochloride) (Thermo Fisher Scientific, Waltham, MA, USA) for 20 min at room temperature. After the addition of 0.18 M H2SO4 (100 µL), the absorbance was determined with an iMark Microplate Absorbance Reader (Bio‐Rad Laboratories, Hercules, CA, USA) at 450 nm.
Figure 1.

Three 20‐mer peptides were predicted with a computed algorithm system on the basis of hydrophilicity, probability of surface exposure, flexibility, and antigenic index from the base sequence of the ORF of Gene‐X and thrombospondin (THBS)‐2, and synthesized and purified commercially to over 95%. The identified antigen in this study was located downstream of the registered ORF of THBS‐2. The identified second ORF of THBS‐2 was located from 4506 to 4719 bp, and the prediction of antigenic peptide of THBS‐2 was carried out in the second ORF.
Results
Immunohistochemical detection of B cells in the tumor specimens. The tumor specimens from two patients with malignant mesothelioma were engrafted into SCID mice. The mesothelioma from the M1 patient was diagnosed pathologically as sarcomatoid type and M2 as epithelioid type. To confirm the infiltration of B lymphocytes, the mesothelioma specimens were stained with anti‐CD20 antibody. Photomicrographs of the infiltration of CD20‐positive B lymphocytes are shown in Figure 2.
Figure 2.
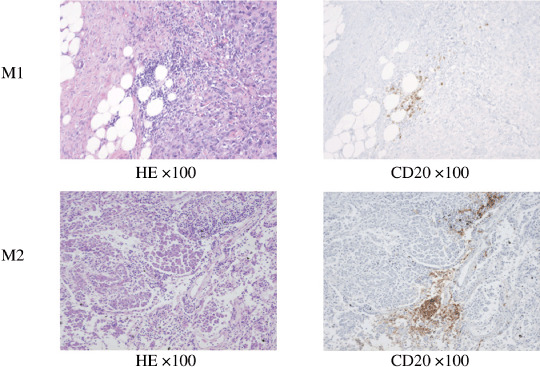
Photomicrograph of malignant mesothelioma specimens obtained from patients M1 and M2. B lymphocytes were infiltrated in tumor tissues. Left, hematoxylin–eosin staining; right, immunohistochemical staining with anti‐CD20 antibody.
Human IgG production in the SCID mice transplanted with tumor tissue. The human IgG level in the sera of SCID mice and volume of the engrafted tumors were measured every 2 weeks. The human IgG levels increased gradually and reached a peak level at 4 and 8 weeks after transplantation of M2 and M1, respectively, but declined gradually thereafter. The peak level of human IgG of M1 and M2 were 570 and 660 µg/mL respectively. The transplanted tumors grew up on the lateral flanks of the mice.
Identification of immunoreactive cDNA clones by SEREX. The cDNA libraries of the mesothelioma tissue (M1) were screened by the sera from the SCID mice xenotransplanted with M1 and the cDNA libraries of the mesothelioma cell line K921MSO were screened by the sera from the SCID mice xenotransplanted with M2 tissue (dilution 1:200). Four positive cDNA clones were identified. On the basis of the DNA sequence analysis, DNA homology search of these genes was carried out using BLAST on the EMBL and GenBank databases and the four genes were identical to Gene‐X, THBS‐2, STUB‐1, and IFT‐88. Gene‐X and THBS‐2 were identified from the serum of SCID mice xenotransplanted with the mesothelioma tissue from patient M1. STUB‐1 and IFT‐88 were identified from the serum of SCID mice xenotransplanted with the mesothelioma tissue from patient M2 (Table 2). THBS‐2 is known as a potent inhibitor of tumor growth and angiogenesis and STUB‐1 is as a ubiquitin‐protein ligase. The function of the other two genes (Gene‐X and IFT‐88) is unknown. Expression of the four genes was examined by RT‐PCR in the five mesothelioma tissues and two mesothelioma cell lines. Gene‐X was expressed in the autologous M1 and the allogeneic M2 mesothelioma, and STUB‐1 was expressed in the autologous M2 and the allogeneic M1, M4, and M5 mesotheliomas and the two cell lines. THBS‐2 and IFT‐88 were also expressed in all of five allogeneic mesothelioma and the two cell lines. (Fig. 3a). The expression of these antigen‐coding genes in normal tissues is shown in Figure 3(b). Gene‐X was expressed in the liver, testis, thyroid gland, and colon. THBS‐2 was expressed in the brain, heart, and spinal cord. STUB‐1 was expressed in the adrenal gland, brain, fetal brain, heart, kidney, and skeletal muscle. IFT‐88 was expressed in the heart, kidney, testis, and thyroid gland. Quantitative RT‐PCR data of the four genes are shown in Figure 3(c), when the expression level of the normal lung was estimated as 1.0. Gene‐X was expressed in three of nine MPM tissues over 15.7‐fold of normal testis, which was highest expressed in normal tissues. THBS‐2 was expressed in seven of nine MPM tissues over 10.1‐fold of normal heart, which was highest expressed in normal tissues. However, STUB‐1 and IFT‐88 were not expressed in any MPM over 7.8‐fold of normal heart or 8.8‐fold of normal testis, respectively, which were highest expressed in normal tissues.
Figure 3.
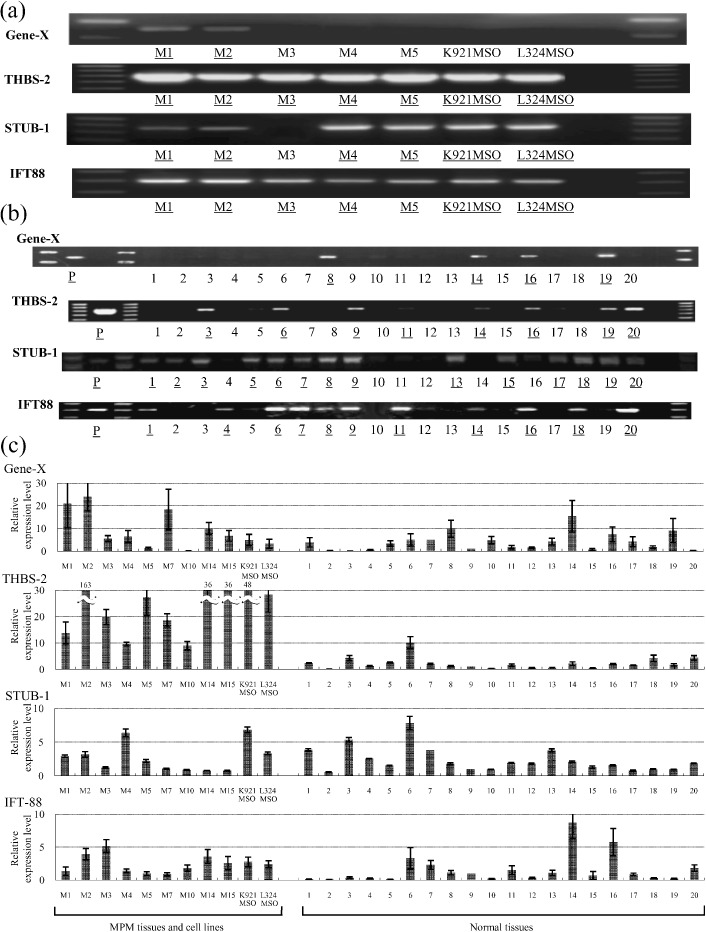
RT‐PCR expression of the four antigens in mesothelioma and normal tissues. (a) All four antigens were expressed in autologous mesothelioma tissue. Gene‐X was expressed in allogeneic M2 mesothelioma tissue, and stress‐induced‐phosphoprotein 1 homology and U‐box containing protein (STUB)‐1 was expressed in allogeneic M1, M4, and M5 mesothelioma tissues. Thrombospondin (THBS)‐2 and intraflagellar transport 88 homolog (IFT‐88) were also expressed in all allogeneic mesothelioma tissues. Underlines are positive bands. (b) RT‐PCR expression of the four antigens in the panel of normal tissues. Lane 1, adrenal gland; lane 2, bone marrow; lane 3, brain; lane 4, fetal brain; lane 5, fetal liver; lane 6, heart; lane 7, kidney; lane 8, liver; lane 9, lung; lane 10, placenta; lane 11, prostate; lane 12, salivary gland; lane 13, skeletal muscle; lane 14, testis; lane 15, thymus; lane 16, thyroid gland; lane 17, trachea; lane 18, uterus; lane 19, colon; lane 20, spinal cord; P, positive control. Underlines are positive bands. (c) Quantitative RT‐PCR expression of the four antigens in mesothelioma tissue and normal tissues. The bars are fold expression compared to normal lung tissues. Gene‐X was expressed in three of nine malignant pleural mesothelioma (MPM) tissues over 15.7‐fold of normal testis, which was highest expressed in normal tissues. THBS‐2 was expressed in seven of nine MPM tissues over 10.1‐fold of normal heart, which was highest expressed in normal tissues. However, STUB‐1 and IFT‐88 were not expressed in any MPM over 7.8‐fold of normal heart and 8.8‐fold of normal testis, respectively, which were highest expressed in normal tissues. M1–M15, MPM tissues. K921MSO and L324MSO are MPM cell lines. Line 1–20 are normal tissues corresponding to the panel used in Figure 3(b).
Antibody titers of Gene‐X and THBS‐2 among patients with mesothelioma, lung cancer, and breast cancer. The titer of antibody against the four antigens was evaluated by a phage plaque assay. Detection rates of antibodies against the four antigens evaluated by phage plaque assay are shown in Table 4. Among the four antigens, antibodies were frequently detected against Gene‐X and THBS‐2. Therefore, ELISA systems to detect antibodies against these two genes were constructed. As shown in Figure 4, the antibody titers the antibody titers were examined by the ELISA system against 6 synthesized peptides of Gene‐X and THBS‐2. As shown in Figure 4, the antibody titers of Gene‐X40–59 and THBS‐226–45 were well correlated with the results by phage plaque assay. Therefore, antibody titers against Gene‐X40–59 and THBS‐226–45 were evaluated quantitatively in 25 healthy persons. The cut‐off values of antibody titers were decided on the basis of the average plus two times the standard deviation of the healthy persons. The estimated cut‐off value of Gene‐X40–59 was 0.414 and the value of THBS‐226–45 was 0.079. On the basis of these cut‐off values, the antibody against Gene‐X was decided as positive in 10 of 18 (55.6%) MPM patients and 0 of 25 (0%) healthy donors. The antibody against THBS‐226–45 was decided as positive in 16 of 18 (88.9%) MPM patients and 2 of 25 (8.0%) healthy donors (Table 5). The titers of antibodies against Gene‐X40–59 and THBS‐226–45 were analyzed in lung cancer patients with or without asbestos exposure and breast cancer patients (Fig. 5a,b). Among the 47 patients with lung cancer and 16 patients with breast cancer, all patients showed negative levels of the antibodies against Gene‐X40–59 and THBS‐226–45. In order to evaluate the effect of surgical resection of MPM on the antibody titers, postoperative sera of patients M15 and M18 were subjected to the analyses. The titers of the M15 and M18 patients’ antibodies against Gene‐X40–59 and THBS‐226–45 decreased 2–4 months after surgical resection. The M15 and M18 patients encountered recurrence of the disease 10 and 9 months after the operation respectively. The titers of antibody against THBS‐2 were increased at the time of recurrence (Fig. 5c).
Table 4.
Detection of antibodies against the identified antigens in sera of mesothelioma patients and healthy donors by phage plaque assay
| Antigen | MPM patient | Healthy donors |
|---|---|---|
| Gene‐X† | 13/18 (72.2%) | 0/25 (0%) |
| THBS‐2‡ | 17/18 (94.4%) | 2/25 (8.0%) |
| STUB‐1§ | 1/18 (5.6%) | 0/25 (0%) |
| IFT‐88§ | 1/18 (5.6%) | 2/25 (8.0%) |
Judged at †1/1500 dilution, ‡1/1000 dilution, §1/200 dilution.
Figure 4.

The correlation of antibody titer against (a) Gene‐X and (b) thrombospondin (THBS)‐2 between phage plaque assay and ELISA. The antibody titers against Gene‐X40–59 and THBS‐226–45 were well correlated with the results by phage plaque assay. R = coefficient of correlation. A significant correlation was considered between phage plaque assay and ELISA when the P‐value was less than 0.05.
Table 5.
Detection of antibodies against the identified antigens in sera of mesothelioma patients and healthy donors by ELISA
| Antigen | MPM patient | Healthy donors |
|---|---|---|
| Gene‐X | 10/18 (55.6%) | 0/25 (0%) |
| THBS‐2 | 16/18 (88.9%) | 2/25 (8.0%) |
Figure 5.
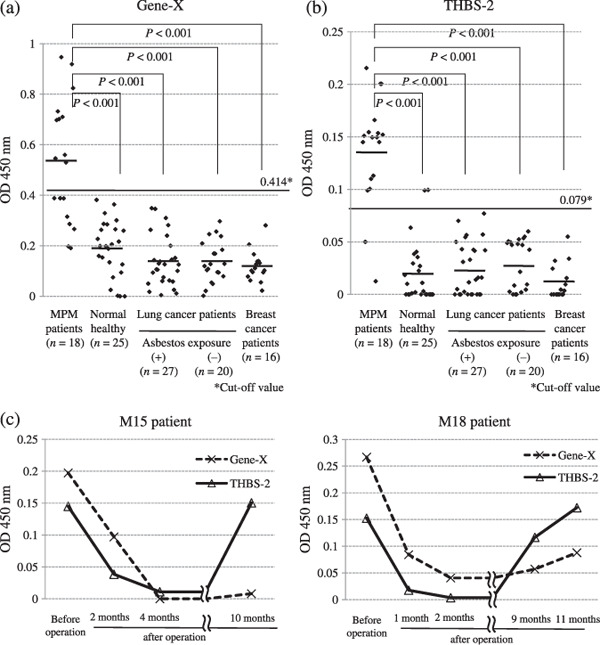
The antibody titer against (a) Gene‐X and (b) thrombospondin (THBS)‐2 according to malignant pleural mesothelioma (MPM) patients, normal healthy persons, lung cancer patients with or without asbestos exposure, and breast cancer patients. To determine the clinical significance of these antigens, a comparison of antibody titers was carried out by ELISA. These data were analyzed with 1/100 dilutions of the sera, and the optical density at 450 nm is shown. The cut‐off values of Gene‐X and THBS‐2 were determined to be 0.414 and 0.079 respectively. (c) Postoperative time course of antibody titers against Gene‐X and THBS‐2 in patients M15 and M18. To determine the therapeutic effect before and after the operation, a comparison of antibody titers was carried out in patients M15 and M18 by ELISA. The optical densities at 450 nm were analyzed in the two patients. In both patients, the titers of antibodies against Gene‐X and THBS‐2 decreased from 2 to 4 months after surgical resection. The titers of antibody against THBS‐2 were increased at the time of recurrence.
Discussion
The humoral immune response against tumor antigens has been investigated since the 1970s.( 16 , 17 , 18 , 19 ) The present study focused on the role of TIB from the viewpoint of the tumor‐specific immune response. Previous studies demonstrated that TIB produce IgG in SCID mice xenotransplanted with human lung cancer tissue.( 10 , 11 , 12 , 13 , 20 ) TIB‐derived IgG was analyzed by the SEREX method and 22 antigens were identified in a patient with lung cancer. A mutated p53 was one of the identified antigens and the change in the antibody titer against mutated p53 was correlated with the clinical course of the patient.( 13 ) In another patient with pleomorphic carcinoma of the lung, antibodies against SEREX defined antigens (MAGE‐B2 and two function unknown antigens) that could be used as tumor markers reflecting the clinical course.( 10 ) These findings indicated that TIB plays an important role in the humoral responses against tumor‐associated antigens.
By using these IgG antibodies derived from TIB, four antigens (Gene‐X, THBS‐2, STUB‐1, and IFT88) were identified. Gene‐X has 100% homology to the registered gene in GenBank (accession no. AC002301) that is located on chromosome 16p11 and encodes 251 amino acids. The function of this gene is not yet known. It was reported that a 26.5‐kb gene rich duplication is shared by human Xq28 and 16p11.( 21 ) The overall nucleotide similarity within the interchromosomal duplication was found to be 94–97%, on the basis of a genomic DNA sequence analysis.( 22 )
Thrombospondin‐2 is located on the long arm of chromosome 6 and belongs to the thrombospondin family. It is a disulfide‐linked homotrimeric glycoprotein located at the cell membrane( 23 , 24 ) that mediates cell‐to‐cell and cell‐to‐matrix interactions. This protein is known to be a potent inhibitor of tumor growth and angiogenesis. However, the thrombospondin family, in particular THBS‐2, is reported to be pleiotrophic in function.( 25 ) The pericellular levels of MMP2 are controlled to a large extent by THBS‐2 (and potentially also by THBS‐1) and the elevated levels of MMP2 are likely to play a role in reduction of cellular adhesion with abnormal collagen fibril structure and augmentation of the proliferation of vascular endothelial cells.( 25 ) Therefore, THBS‐2 is intimately involved in the promotion of invasion and metastasis of cancer cells. The open reading frame (3510 bp) encodes a protein of 1170 amino acids with known features of the THBS‐2 subunit.( 26 ) The THBS mRNA 3′‐untranslated region has a role in the regulation of THBS mRNA and the mRNA stability was observed in cultured cells in response to platelet‐derived growth factor.( 27 , 28 ) The identified antigen in the present study was located downstream of the registered ORF of THBS‐2. The ORF of THBS‐2 was located from 249 to 3767 bp. The identified second ORF of THBS‐2 was located from 4506 to 4719 bp. It is not known why this small protein is translated in MPM patients. However, Oyama et al. reported that the small protein was unexpectedly translated from upstream or downstream of the registered ORF in a proteomic analysis of an erythroleukemia cell line K562.( 29 ) Mechanisms including leaky scanning or sequence such as an internal ribosome entry site may be associated with the transcription of the variants.( 29 , 30 ) There are some reports showing overexpression of THBS‐1 in bladder, breast, and lung cancers.( 31 , 32 , 33 , 34 ) THBS‐2 protein has a homologous structure to THBS‐1; therefore, the antibody against THBS‐2 may show immunological cross reaction against THBS‐1. However, in the present study, we detected antibody against the second ORF of THBS‐2; therefore, the antibody against the second ORF of THBS‐2 may not cross react with THBS‐1. Futhermore, the antibody titer against THBS‐2 was significantly higher in MPM patients than in lung cancer patients. Because MPM is sometimes difficult to distinguish from lung cancer, the antibody titer against THBS‐2 might become a useful tumor marker for making a differential diagnosis.
Stress‐induced‐phosphoprotein 1 homology and U‐box containing protein‐1 is located on the short arm of chromosome 16. This protein had been identified using the SEREX method in patients with colon cancer.( 35 ) STUB‐1 protein is also designated carboxyl terminus of Hsp70‐interacting protein (CHIP). Forced expression of STUB‐1 in fibroblasts by transduction of the STUB‐1 gene increased the refolding of proteins under stress conditions such as thermal denaturation, and inhibition of Hsp70 chaperone activity by ATP depletion abrogated the effects of STUB‐1 on protein folding, indicating that the STUB‐1‐mediated events were Hsp70 dependent.( 36 ) STUB‐1 is also reported to be a direct chaperone of wild‐type p53 and to maintain p53 in wild‐type conformation under physiological conditions.( 37 )
Intraflagellar transport 88 homolog is located on the long arm of chromosome 13 and the function of this gene remains unknown. This gene belongs to the tetratricopeptide repeat family. It was reported that mutations of a similar gene in mouse could cause polycystic kidney disease.( 38 ) Polycystic disease of the kidney is known to be associated with nephrotic syndrome with membranous syndrome.( 39 ) IFT‐88 was identified by using the sera of SCID mice engrafted with tumor tissue derived from patient M2. The M2 patient suffered from membranous nephropathy and the membranous nephropathy was improved after an extrapleural pneumonectomy for MPM. Moreover, membranous nephropathy had been reported to be associated with MPM.( 40 , 41 ) The causal relationship between IFT‐88 and membranous nephropathy should therefore be elucidated in a further study.
Among the four genes, Gene‐X and THBS‐2 were overexpressed frequently in MPM. Furthermore, antibodies against Gene‐X and THBS‐2 were detected in 55.6 and 88.9%, respectively, of sera of patients with MPM. The antibody titer against these antigens decreased after surgical resection of MPM. Therefore, these antibody titers could be useful not only for diagnosis of MPM but also evaluation of the outcome of therapy.
Mesothelin has been reported to be a new mesothelioma‐associated antigen.( 42 ) It is highly expressed in several cancers including pancreatic, ovarian, pulmonary adenocarcinoma, and mesothelioma. Several investigators have reported that a high level of serum soluble mesothelin is associated with MPM and it is a promising marker for the diagnosis and clinical monitoring of MPM.( 43 ) The antibody against mesothelin detected by ELISA is the IgG isotype in MPM patients, implying that cognate helper T‐cell immunity might be present and activated in the patients with a respective B‐cell response.( 44 ) Mesothelin is a glycosylphosphatidylinositol‐linked membrane protein overexpressed on the cell surface. Therefore, mesothelin could be a potential target for antibody‐based immunotherapy. A clinical trial using monoclonal antibodies against mesothelin is underway for MPM patients.( 45 )
Robinson et al. have reported eight antigens identified by the SEREX method using sera of patients with MPM. Among them TOPIIβ was detected in 13 out of 14 patients with MPM.( 46 ) Interestingly, the antibody titer against TOPIIβ in patients with MPM was correlated with poor survival of MPM patients.
In conclusion, the B cells infiltrated in MPM are sensitized and produce antibody against tumor‐associated antigens. Among the SEREX‐defined antigens, the titers of antibodies against Gene‐X and THBS‐2 could be used as tumor markers for the diagnosis of patients with malignant mesothelioma.
Acknowledgments
This study was supported in part by a University of Occupational and Environmental Health Research Grant for the Promotion of Occupational Health and Grant‐in‐Aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. We thank Yukari Oshibuchi, Misako Fukumoto, Yuki Goto, and Aya Katayama for their expert technical help.
References
- 1. Peto J, Hodgson JT, Matthews FE et al . Continuing increase in mesothelioma mortality in Britain. Lancet 1995; 345: 535–9. [DOI] [PubMed] [Google Scholar]
- 2. Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer 1999; 79: 666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Statistics and Information Department Labour and Welfare of Japan . Vital Statistics of Japan 2003. Tokyo: Health and Welfare, Statistics Association, 2003. [Google Scholar]
- 4. Marinaccio A, Scarselli A, Binazzi A et al . Asbestos related diseases in Italy: an integrated approach to identify unexpected professional or environmental exposure risks at municipal level. Int Arch Occup Environ Health 2008; 81: 993–1001. [DOI] [PubMed] [Google Scholar]
- 5. Maule MM, Magnani C, Dalmasso P, Mirabelli D, Merletti F, Biggeri A. Modeling mesothelioma risk associated with environmental asbestos exposure. Environ Health Perspect 2007; 115: 1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruffie P, Feld R, Minkin S et al . Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: a retrospective study of 332 patients. J Clin Oncol 1989; 7: 1157–68. [DOI] [PubMed] [Google Scholar]
- 7. Chailleux E, Dabouis G, Pioche D et al . Prognostic factors in diffuse malignant pleural mesothelioma. A study of 167 patients. Chest 1988; 93: 159–62. [DOI] [PubMed] [Google Scholar]
- 8. Adams VI, Unni KK, Muhm JR et al . Diffuse malignant mesothelioma of pleura. Diagnosis and survival in 92 cases. Cancer 1986; 58: 1540–51. [DOI] [PubMed] [Google Scholar]
- 9. Sugarbaker DJ, Flores RM, Jaklitsch MT et al . Resection margins, extrapleural nodal status, and cell type determine postoperative long‐term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–65. [DOI] [PubMed] [Google Scholar]
- 10. Mizukami M, Hanagiri T, Baba T et al . Identification of tumor associated antigens recognized by IgG from tumor‐infiltrating B cells of lung cancer: correlation between Ab titer of the patient's sera and the clinical course. Cancer Sci 2005; 96: 882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mizukami M, Hanagiri T, Shigematsu Y et al . Effect of IgG produced by tumor‐infiltrating B lymphocytes on lung tumor growth. Anticancer Res 2006; 26: 1827–32. [PubMed] [Google Scholar]
- 12. Mizukami M, Hanagiri T, Yasuda M et al . Antitumor effect of antibody against a SEREX‐defined antigen (UOEH‐LC‐1) on lung cancer xenotransplanted into severe combined immunodeficiency mice. Cancer Res 2007; 1: 8351–7. [DOI] [PubMed] [Google Scholar]
- 13. Yasuda M, Takenoyama M, Obata Y et al . Tumor‐infiltrating B lymphocytes as a potential source of identifying tumor antigen in human lung cancer. Cancer Res 2002; 62: 1751–6. [PubMed] [Google Scholar]
- 14. Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995; 108: 895–7. [DOI] [PubMed] [Google Scholar]
- 15. Sugaya M, Takenoyama M, Osaki T et al . Establishment of 15 cancer cell lines from patients with lung cancer and the potential tools for immunotherapy. Chest 2002; 122: 1282–288. [DOI] [PubMed] [Google Scholar]
- 16. Yasumoto K, Manabe H, Nomoto K, Inokuchi K. Antibody specific for lung cancer cells detected in sera of patients with bronchogenic carcinoma. Gann 1983; 74: 595–601. [PubMed] [Google Scholar]
- 17. Sikora K, Alderson T, Ellis J et al . Human hybridomas from patients with malignant disease. Br J Cancer 1983; 47: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Old LJ, Chen YT. New paths in human cancer serology. J Exp Med 1998; 187: 1163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Punt CJ, Barbuto JA, Zhang H et al . Anti‐tumor antibody produced by human tumor‐infiltrating and peripheral blood B lymphocytes. Cancer Immunol Immunother 1994; 38: 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Imahayashi S, Ichiyoshi Y, Yoshino I et al . Tumor‐infiltrating B‐cell derived IgG recognizes tumor components in human lung cancer. Cancer Invest 2000; 18: 530–6. [DOI] [PubMed] [Google Scholar]
- 21. Eichler EE, Lu F, Shen Y et al . Duplication of a gene‐rich cluster between 16p11.1 and Xq28: a novel pericentromeric‐directed mechanism for paralogous genome evolution. Hum Mol Genet 1996; 5: 899–912. [DOI] [PubMed] [Google Scholar]
- 22. Horvath JE, Viggiano L, Loftus BJ et al . Molecular structure and evolution of an alpha satellite/non‐alpha satellite junction at 16p11. Hum Mol Genet 2000; 9: 113–23. [DOI] [PubMed] [Google Scholar]
- 23. Lawler J, Detmar M. Tumor progression: the effects of thrombospondin‐1 and ‐2. Int J Biochem Cell Biol 2004; 36: 1038–45. [DOI] [PubMed] [Google Scholar]
- 24. Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol 2004; 36: 961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell–matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol 2004; 36: 1115–25. [DOI] [PubMed] [Google Scholar]
- 26. Frazier WA. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol 1987; 105: 625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hennessy SW, Frazier BA, Kim DD et al . Complete thrombospondin mRNA sequence includes potential regulatory sites in the 3′ untranslated region. J Cell Biol 1989; 108: 729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LaBell TL, Byers PH. Sequence and characterization of the complete human thrombospondin 2 cDNA: potential regulatory role for the 3′ untranslated region. Genomics 1993; 17: 225–9. [DOI] [PubMed] [Google Scholar]
- 29. Oyama M, Kozuka‐Hata H, Suzuki Y, Semba K, Yamamoto T, Sugano S. Diversity of translation start sites may define increased complexity of the human short ORFeome. Mol Cell Proteomics 2007; 6: 1000–6. [DOI] [PubMed] [Google Scholar]
- 30. Stoneley M, Willis AE. Cellular internal ribosome entry segments: structures, trans‐acting factors and regulation of gene expression. Oncogene 2004; 23: 3200–7. [DOI] [PubMed] [Google Scholar]
- 31. Grossfeld GD, Ginsberg DA, Stein JP et al . Thrombospondin‐1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst 1997; 89: 219–27. [DOI] [PubMed] [Google Scholar]
- 32. Roth JJ, Reiver DM, Granick MS, Rothman VL, Nicosia RF, Tuszynski GP. Histopathology and clinical assessment correlate with the cysteine‐serine‐valine‐threonine‐cysteineglycine (CSVTCG) receptor of thrombospondin‐1 in breast tumors. Histol Histopathol 1997; 12: 1013–18. [PubMed] [Google Scholar]
- 33. Toi M, Hoshina S, Takayanagi T, Tominaga T. Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res 1994; 85: 1045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohta Y, Endo Y, Tanaka M et al . Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res 1996; 2: 1411–16. [PubMed] [Google Scholar]
- 35. Scanlan MJ, Chen YT, Williamson B et al . Characterization of human colon cancer antigens recognized by autologous antibodies. Int J Cancer 1998; 29: 652–8. [DOI] [PubMed] [Google Scholar]
- 36. Kampinga HH, Kanon B, Salomons FA, Kabakov AE, Patterson C. Overexpression of the cochaperone CHIP enhances Hsp70‐dependent folding activity in mammalian cells. Mol Cell Biol 2003; 23: 4948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veenu T, Amjad A, Rajiv B, Uttam P. CHIP chaperones wild type p53 tumor suppressor protein. J Biol Chem 2007; 28: 28 441–54. [DOI] [PubMed] [Google Scholar]
- 38. Schrick JJ, Onuchic LF, Reeders ST et al . Characterization of the human homologue of the mouse Tg737 candidate polycystic kidney disease gene. Hum Mol Genet 1995; 4: 559–67. [DOI] [PubMed] [Google Scholar]
- 39. Contreras G, Mercado A, Pardo V, Vaamonde CA. Nephrotic syndrome in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1995; 6: 1354–9. [DOI] [PubMed] [Google Scholar]
- 40. Sakamoto K, Suzuki H, Jojima T et al . Membranous glomerulonephritis associated with diffuse malignant pleural mesothelioma: report of a case. Surg Today 2000; 30: 1124–6. [DOI] [PubMed] [Google Scholar]
- 41. Galesic K, Bozic B, Heinzl R et al . Pleural mesothelioma and membranous nephropathy. Nephron 2000; 84: 71–4. [DOI] [PubMed] [Google Scholar]
- 42. Ho M, Hassan R, Zhang J et al . Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res 2005; 11: 3814–20. [DOI] [PubMed] [Google Scholar]
- 43. Ordóñez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol 2003; 16: 192–7. [DOI] [PubMed] [Google Scholar]
- 44. Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer 2008; 44: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hassan R, Ebel W, Routhier EL et al . Preclinical evaluation of MORAb‐009, a chimeric antibody targeting tumor‐associated mesothelin. Cancer Immun 2007; 19: 20–9. [PMC free article] [PubMed] [Google Scholar]
- 46. Robinson C, Callow M, Stevenson S et al . Serologic responses in patients with malignant mesothelioma evidence for both public and private specificities. Am J Respir Cell Mol Biol 2000; 22: 550–6. [DOI] [PubMed] [Google Scholar]


