Abstract
Immunization with xenogeneic antigens is an attractive approach to induce cross‐reactive humoral and cellular immunity to inhibit tumor growth or angiogenesis. To identify novel xenogenic targets for immunotherapy, we have developed a modified serological expression cloning (SEREX) strategy, termed Cross‐reactive SEREX (CR‐SEREX). Among 78 positive clones identified by CR‐SEREX, Xenopus receptor for hyaluronic‐acid‐mediated motility (xRHAMM) was most frequently identified (18 times), indicating the strongest immunogenic potential for xenogenic immunotherapy. A DNA vaccine based on xRHAMM effectively induced a protective antitumor immunity against local tumor and lung metastasis in B16 melanoma mouse models. Angiogenesis was inhibited and cell apoptosis was increased within tumors. Antitumor activity of xRHAMM worked through stimulation of an antigen‐specific cellular response as well as through a specific humoral response against RHAMM, as confirmed by the depletion of immune cell subsets in vivo. Thus, a xenogenic vaccine based on xRHAMM induced an effective immunity against B16 melanoma cells and endothelial cells. (Cancer Sci 2010; 101: 862–868)
Immunotherapy based on cancer or vascular endothelial cell antigens represents a valuable strategy for cancer therapy.( 1 ) Overcoming immune tolerance is a prerequisite for the generation of effective antitumor immunity; however, central and peripheral immune tolerance to tumors is a major obstacle in cancer immunotherapy.( 1 , 2 ) Accumulating evidence shows that immunization with xenogeneic molecules, such as survivin and human epidermal growth factor receptor 2 (HER2), can induce an effective immune response to inhibit tumor growth.( 3 , 4 , 5 , 6 , 7 , 8 , 9 ) In addition to these tumor cell antigenic molecules, many angiogenesis‐associated antigens, such as basic fibroblast growth factor receptor 1 (bFGFR‐1), vascular endothelial growth factor (VEGF), and VEGF receptor 2 (VEGFR2) can induce a cytotoxic CD8+ T‐cell immune response or a specific neutralizing antibody to destroy tumor endothelial cells.( 10 , 11 , 12 , 13 , 14 )
Serological expression cloning (SEREX) is an effective immunomic strategy to identify tumor antigen molecules, which is based on the ability of human tumor antigens to induce effective immune responses and to produce high titers of IgG against identified antigens.( 15 , 16 , 17 ) The antibody titer in human sera is often low; however, the xenogenic cross‐immune reaction is always weaker than the homogenic reaction. To identify novel xenogenic molecules, we have developed a modified SEREX strategy, termed cross‐reactive SEREX (CR‐SEREX), which is similar to but different from conventional SEREX.( 18 ) Using CR‐SEREX, we have previously screened a rat testicular cDNA library with rabbit antiserum against breast cancer cells. The leading candidate gene, rat testicular antigenic protein 2a (RTAP2a), encodes a novel protein that is selectively expressed in the adult testis and in tumor cells.( 18 ) Knockdown of the expression of the mouse RTAP2a ortholog, Biot2, in mouse cancer cells inhibited tumor proliferation and growth in vitro and in vivo.( 19 )
To identify novel xenogenic tumor‐ and angiogenesis‐associated antigens, using the CR‐SEREX strategy, we prepared an antibody pool against human umbilical vein endothelial cells (HUVECs) providing the possibility of obtaining angiogenesis‐associated antigens. We used a xenogenic cDNA expression library derived from oocytes of Xenopus laevis for two reasons: (1) In previous studies, we have shown that xenogenic tumor vaccines based on Xenopus molecules (bFGFR‐1 and VEGF) can significantly inhibit tumor angiogenesis;( 12 , 13 ) and (2) no cDNA library of Xenopus cancer is available, but many significant characteristics are shared by tumor cells and embryonic cells, including potent proliferation, immune escaping, the similarity between invasion and implantation, and common gene expression.( 20 , 21 , 22 , 23 ) Thus, theoretically, we should identify novel xenogenic angiogenesis‐ or tumor‐associated antigens. Among 78 CR‐SEREX positive clones, Xenopus receptor for hyaluronic‐acid‐mediated motility (xRHAMM) was identified most frequently (18 times), indicating the strongest immunogenic potential for xenogenic immunotherapy. A xenogenic vaccine based on xRHAMM induced an effective immunity that inhibited the development of local tumor and lung metastasis in a mouse B16 melanoma model.
Materials and Methods
Cell culture.
Endothelial cells were isolated from human umbilical veins as previously described,( 24 ) and were incubated in M199 medium (20% heat inactivated fetal calf serum, 100 UI/mL penicillin, 100 mg/mL streptomycin, and 2 mm L‐glutamin, 10 ng/mL bFGF) in a flask coated with 1% gelatin. The mouse melanoma cell line B16 and mouse endothelial cell line MS1 were cultured in RPMI‐1640 culture medium supplemented with 10% fetal calf serum, 2 mm L‐glutamine 100 UI/mL penicillin, and 100 mg/mL streptomycin. All cells were incubated at 37°C under 5% CO2 humid atmosphere.
Preparation of immune serum and CR‐SEREX analysis.
Exponentially growing HUVECs were collected and mixed evenly with adjuvant to produce polyclonal antibody. Each rabbit was injected with 1.5 × 10 7 HUVECs each time. After five immunizations, blood was taken from the rabbit’s heart, sera were isolated, and sera titers were evaluated. Aliquots of sera at were stored at −80°C for later use.
A Xenopus oocytes cDNA library (Clontech, Mountain View, CA, USA) was screened as protocol with little modification. The rabbits’ sera diluted to 1:2000 had been shown to provide a low signal to noise ratio (data not shown). The normal rabbit sera were used as a control to remove the false‐positive reactive clones.
Immunization and tumor models.
Mice at 6–8 weeks of age were immunized (six per group) by intramuscular injection in both later quadriceps once weekly for 6 continuous weeks with 50 μg pcDNA3.1‐xRHAMM, pcDNA3.1‐mRHAMM, and pcDNA3.1 vector, respectively. The DNA vaccine was encapsulated with cationic liposome (plasmid/nanoliposome, 1:3). Additional control mice were injected with normal saline (n.s.; nonimmunized mice). For the investigation of the protective effect against local tumors, 7 days after the last immunization, the mice were challenged subcutaneously with 2 × 105 B16 melanoma cells. Tumor dimensions were measured every 3 days with calipers, and tumor volumes were calculated according to the following formula: 0.52 × length × width2. To test the efficacy of vaccines to the tumor lung metastasis, 2 × 105 B16 melanoma cells were injected into the tail vein of each mouse on day 7 after the last immunization. Two weeks later, the mice were killed to count lung metastasis nodules and to measure the weight of the lungs.
Tumor histochemical examination and quantization of tumor composition.
Paraffin‐embedded tumor specimens were sectioned to 5 μm thickness, and hematoxylin–eosin (H&E) staining was performed. Sections from peripheral and central regions of a tumor were imaged, and areas of viable tumor tissue and necrosis were evaluated using the Zeiss Axiovision software program (Carl Zeiss, Jena, Germany) as previously described.( 25 ) For these analyses, six different tumor samples were evaluated per experimental condition.
TUNEL analysis.
Frozen sections were prepared and analyzed with a DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) according to the manufacturer’s guidelines, which is based on the enzymatic addition of digoxigenin‐nucleotide to the nicked DNA by terminal deoxynucleotidyl transferase.
Immunohistochemistry.
To explore whether the antitumor immunity involved the inhibition of angiogenesis, frozen sections of tumor tissues were used to determine vessel density with an anti‐CD31 antibody. Blood vessel density was determined by counting the number of CD31‐positive microvessels per high‐power field across the whole section of tumors.
In vivo sponge angiogenesis assay.
The sponge angiogenesis assay was done as previously described with modification.( 26 ) Briefly, C57BL/6J mice were immunized six times as above with n.s or plasmid/liposome complex. bFGF (PeproTech, Rocky Hill, NJ, USA) was admixed with Matrigel (Becton Dickinson, Bedford, MA) before being adding to surgical sponges (4 × 4 × 2 mm). Each sponge was implanted s.c. on the abdomen of immunized or control mice (five per group). Two weeks later, sponges were harvested and homogenized in 0.1 mL ddH2O. Hemoglobin contents in lysates were quantified by measuring the colorimetric change following addition of tetramethylbenzidine.
Adoptive therapy for local tumor and lung metastasis.
To assess the efficacy of humoral and cellular immunity, T cells were isolated and immunoglobulins were purified for adoptive therapy to local tumor xenograft and lung metastasis models in vivo. 2 × 105 B16 cells were injected subcutaneously to establish local tumor xenografts, and injected intravenously for lung metastasis modeling. After the last immunization as above, T cells were isolated from mice spleen and immunoglobulins were purified from the pooled sera. Freshly isolated T lymphocytes (1 × 107) were injected into recipient mice through the tail vein on the second day after B16 challenge. Purified immunoglobulins were intravenously transferred (200 mg/kg per mouse) 1 day before the mice were challenged with B16 cells and then were administered once every 3 days.
51Cr‐release CTL assay.
The possible RHAMM‐specific cytotoxicity mediated by CTLs was determined by 51Cr release assay. Briefly, C57BL/6J mice were immunized as described above. Spleens were collected on day 7 after the last vaccination. T lymphocytes were isolated from single‐cell suspensions with Nylon Fiber Column T (L‐Type; Wako, Osaka, Japan) as CTL effector cells. mRHAMM‐positive cells, mouse B16 melanoma cells and MS1 endothelial cells, and mRHAMM‐negative COS7 cells (African green monkey SV40‐transfed kidney fibroblast cell line) were used as target cells in these experiments. Effector and target cells were seeded into the 96‐well microtiter plate at various effector/target ratios. The percentage of specific 51Cr release was calculated as follows:
Depletion of immune cell subsets in vivo.
Mice were injected i.p. with anti‐CD4 (clone GK1.5, rat IgG), anti‐CD8 (clone 2.43, rat IgG), anti‐natural killer (clone PK136) mAb, or isotype controls at 500 mg/kg per mouse 1 day before the immunization and then immunized with 50 μg of plasmids. Mice were challenged with B16 cells (2 × 105) on day 7 after the sixth immunization. Tumor size was measured 21 days after injection.
Statistical analysis.
The statistical differences between the experimental groups were evaluated by Student’s t‐test; differences were considered significant when P‐values were below 0.05.
Results
CR‐SEREX analysis.
In present study, to identify novel xenogenic angiogenesis‐ or tumor‐associated antigens, we prepared an antibody pool against HUVEC antigens to screen a target cDNA library from Xenopus oocytes (Fig. 1). Approximately 1.5 × 106 cDNA library recombinants were screened with rabbit serum that had been raised by immunization with HUVECs (Fig. 1). Among 78 positive clones, representing 51 distinct genes, RHAMM was identified most frequently (18 times), indicating the strongest immunogenic potential for xenogenic immunotherapy.
Figure 1.
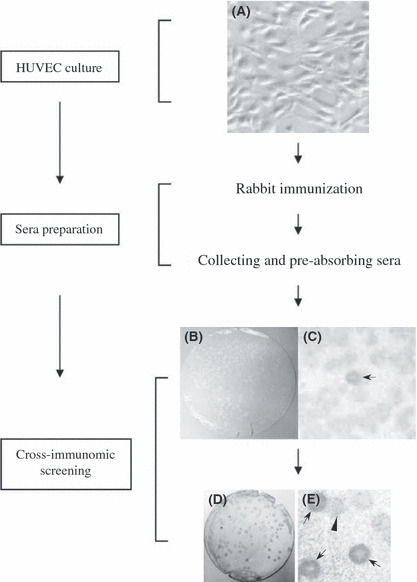
Diagrams represent the flowchart of CR‐SEREX (cross‐reactive serological expression cloning) strategy. (A) Human umbilical vein endothelial cells (HUVEC); (B–E) CR‐SEREX screening results, positive clones were indicated by arrows, the negative clone was indicated by arrowhead; (B) first cycle screening results; (D) second cycle screening results; (C,E) the larger view of (B) and (D).
DNA vaccine based on xRHAMM elicits protective antitumor immunity.
DNA‐based immunization for cancer immunotherapy applications can offer some advantages compared with other forms of immunization.( 27 ) We first investigated whether DNA vaccination with pcDNA3.1‐xRHAMM could induce an effective immune response in vivo. Mice were immunized six times as described above and challenged subcutaneously with B16 melanoma cells. As expected, a more significant inhibition of tumor growth was observed in mice immunized with pcDNA3.1‐xRHAMM compared with mice immunized with pcDNA3.1‐mRHAMM. In contrast, no obvious antitumor immunity was detected in mice immunized with pcDNA3.1 vector (Fig. 2A). To explore the efficacy of pcDNA3.1‐xRHAMM immunization in a lung metastasis model, immunized mice were challenged through tail‐vein injection of B16 melanoma cells. Treatment with pcDNA3.1‐xRHAMM and pcDNA3.1‐mRHAMM vaccine both suppressed the formation and growth of lung metastasis, and fewer surface metastases and lower lung weight were observed compared with other groups (Fig. 2B). However, the inhibition effects induced by xRHAMM were more potent than mRHAMM (Fig. 2B). Therefore, these data suggest that the DNA vaccine based on xenogenic xRHAMM can elicit a more potent protective antitumor immunity than the corresponding autologous homology.
Figure 2.
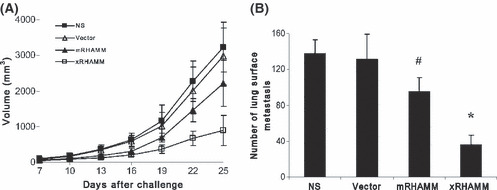
Induction of protective antitumor immunity. (A) There were apparent differences in tumor volume between pcDNA3.1‐Xenopus receptor for hyaluronic‐acid‐mediated motility (xRHAMM)‐immunized and control groups. Tumor growth was monitored once every 3 days and volume was calculated as 0.52 × length × width2. (B) pcDNA3.1‐xRHAMM vaccine significantly decreased the number of lung surface metastases. NS, normal saline. #P < 0.05, *P < 0.01.
Apoptosis in tumors.
Pathological analysis, through H&E staining, was performed to investigate morphological changes in local tumor tissues. Immunization with pcDNA3.1‐xRHAMM and ‐mRHAMM both produced pronounced effects on increasing the level of tumor cell necrosis (Fig. 3A). The necrosis in xRHAMM‐immunized tumors was most significant, comprising 62% of total tumor area (Fig 3A). Necrosis was characterized by the presence of fragmented nuclear and cytoplasmic debris and a nearly complete lack of intact cells (Fig. 3A). In contrast, tumors from other groups had only sparse patches of less advanced necrosis (Fig. 3A). To evaluate whether the observed growth retardation and tumor necrosis were associated with an increased rate of programmed cell death, tumors tissues were stained with the TUNEL method to detect DNA fragmentation. The most obvious elevation in the rate of apoptosis in tumors immunized with pcDNA3.1‐xRHAMM, compared with tumors immunized with pcDNA3.1‐mRHAMM and other groups, was observed (Fig. 3B).
Figure 3.
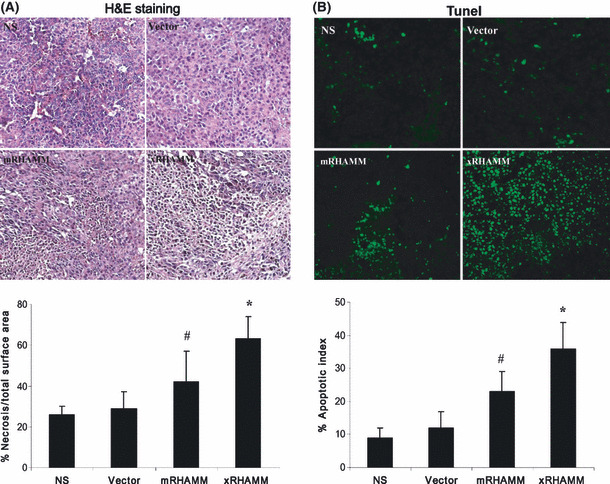
Pathological analyses of tumor tissues. (A) Fixed sections of tumors were stained with H&E. The surface area of necrotic tissue relative to total surface area of the field of view was quantified by morphometric analysis. (B) Apoptosis in xenograft tumors was determined by TUNEL analysis. SDs are indicated by error bars. #P < 0.05, *P < 0.01. NS, normal saline.
Adaptive immunity through T‐cell or immunoglobulin adoptive transfer.
To explore the mechanism by which the antitumor activity was elicited by pcDNA3.1‐xRHAMM, T cells isolated from immunized mice were transferred intravenously into recipient mice, which had been subcutaneously inoculated with B16 cells 1 day before. Tumor growth was significantly slower in the group that received T cells from pcDNA3.1‐xRHAMM‐immunized mice, but was slightly slower in mice that received T cells from mRHAMM‐immunized mice (Fig. 4A). The adoptive transfer of purified immunoglobulins, containing specific antibodies against mRHAMM (Fig. S1), also resulted in the inhibition of tumor growth (Fig. 4B). In the lung metastasis model, the adoptive transfer of T cells or of purified immunoglobulins also produced inhibition effects (Fig. 4C,D).
Figure 4.
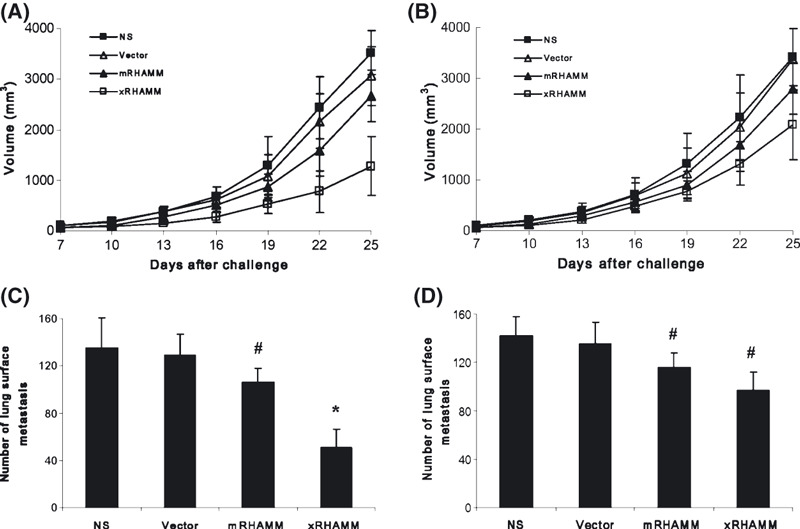
The antitumor effect by the adoptive transfer of T cells and immunoglobulins in vivo. (A,C) Treatment with T cells isolated from pcDNA3.1‐Xenopus receptor for hyaluronic‐acid‐mediated motility (xRHAMM)‐immunized mice showed apparent protective antitumor effect, compared with controls, to local tumors and lung metastasis. However, there was mild protective effect for the adoptive transfer of immunoglobulins in vivo (B,D). (A,B) The subcutaneous local tumor model; (C,D) lung metastasis model. SDs are indicated by error bars. #P < 0.05, *P < 0.01. NS, normal saline.
Inhibition of angiogenesis.
Immunohistochemical staining of tumor tissue from pcDNA3.1‐xRHAMM‐ and ‐mRHAMM immunized mice with anti‐CD31 showed significantly decreased microvessel density compared other control groups. The anti‐angiogenesis effect induced by xRHAMM was stronger than mRHAMM (Fig. 5A). The inhibition of angiogenesis was further confirmed by the sponge angiogenesis assay. The protective immunity elicited by pcDNA3.1‐xRHAMM significantly inhibited bFGF‐induced angiogenesis relative to mRHAMM‐immunized and other groups (Fig. 5B).
Figure 5.
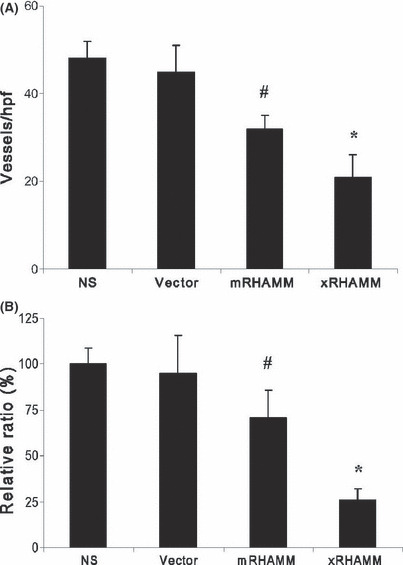
(A) Vessels were stained with CD31 antibody and vessel density was determined by counting the number of the microvessels per high‐power field. (B) In vivo evaluation of anti‐angiogenesis using the sponge model was quantified by measuring the colorimetric change. SDs are indicated by error bars. #P < 0.05, *P < 0.01. NS, normal saline.
Function of T‐cell subsets in antitumor activity.
Specific CTL activity was tested by the 51Cr release assay in vitro. Compared with T lymphocytes from control groups, although T lymphocytes from mice immunized with pcDNA3.1‐xRHAMM and ‐mRHAMM showed increased cytotoxicity against RHAMM‐positive target cells (Fig. S2 and Fig. 6A,B), they showed no cytotoxicity against PHAMM‐negative target cells (Fig. 6A,C). T cells from xRHAMM‐immunized mice exhibited more potent cytotoxicity than those from mRHAMM‐immunized mice to both B16 melanoma cells (Fig. 6A) and mouse endothelial cells (Fig. 6B).
Figure 6.
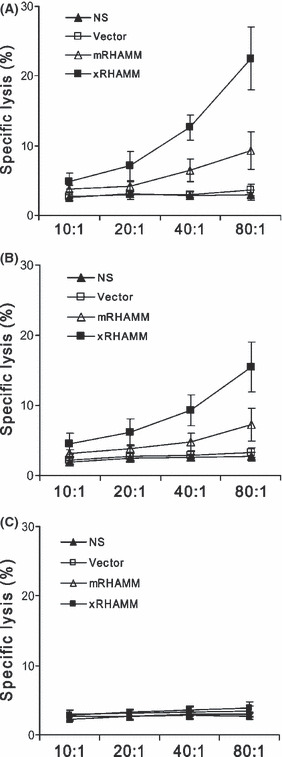
Representative experiment of CTL‐mediated cytotoxicity in vitro. T cells derived from pcDNA3.1‐Xenopus receptor for hyaluronic‐acid‐mediated motility (xRHAMM)‐immunized mice were tested against mRHAMM‐positive cells, mouse B16melanoma cells (A) and MS1 endothelial cells (B), and mRHAMM‐negative COS7 cells (C), at different effector–target ratios by a standard 4‐h 51Cr release assay. SDs are indicated by error bars.
To further confirm the roles of immune cell subsets in the antitumor activity elicited by xRHAMM, CD4+ or CD8+ T lymphocytes or natural killer cells were depleted with neutralization antibody. Mice depleted of CD4+ or CD8+ T lymphocytes and vaccinated with xRHAMM showed reduced or no protection to B16 challenge. Depletion of CD8+ T lymphocytes almost completely suppressed the protective effects. In contrast, the depletion of natural killer cells had no obvious effect on the antitumor activity (Fig. 7).
Figure 7.
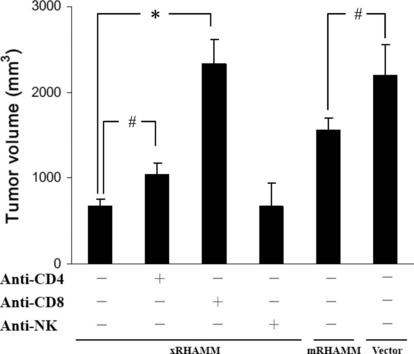
Abrogation of antibodies production and antitumor activity by the depletion of immune cell subsets. Depletion of CD4 and CD8 T lymphocytes showed partial or almost complete abrogation of the antitumor activity. SDs are indicated by error bars. #P < 0.05, *P < 0.01.
Discussion
The use of an anti‐HUVEC antibody pool provided the possibility of obtaining angiogenesis‐associated antigens. Furthermore, the target cDNA library from oocytes provided the possibility of obtaining tumor‐associated antigens. Thus, the genes identified here by CR‐SEREX would theoretically function both in cancer cells and endothelial cells.
Among 78 positive clones, representing 51 distinct genes, RHAMM was identified most frequently (18 times), indicating the strongest immunogenic potential for xenogenic immunotherapy. RHAMM expression is restricted to the testis, thymus, placenta, vascular endothelial cells, and various cancer cells.( 28 , 29 , 30 , 31 , 32 ) Such an expression pattern is considered suitable for immunotherapy targeted against RHAMM in tumors. RHAMM exhibits various functions, such as in vascular endothelial cell migration, angiogenesis, and mediation of hyaluronic‐acid‐induced cell motility.( 30 , 31 , 32 ) The overexpression of RHAMM causes cellular transformation( 33 ) and promotes tumor progression and metastasis.( 34 ) RHAMM is also an immunogenic antigen in leukemias and in several solid tumors.( 35 ) A dendritic cell vaccine produced from cells transfected with RHAMM mRNA markedly inhibited tumor growth in mouse tumor models.( 32 ) Taken together, we regarded xRHAMM as a strong candidate for immune gene therapy targeting both tumor cells and endothelial cells.
Xenogeneic antigens have been shown to be more effective, in clinical trials as well as in animal models, at inducing an antitumor immune response than the corresponding autologous antigens.( 3 , 4 , 5 , 6 , 7 , 8 , 9 ) Our results suggested that a xenogenic DNA vaccine based on xRHAMM induced an effective humoral and cellular immune response against both tumor and endothelial cells. This immunity was potent enough to directly cause the apoptosis of tumor cells and to inhibit angiogenesis in tumors. The inhibition of angiogenesis would enhance the apoptosis of tumor cells. The depletion of immune cell subsets in vivo indicated that CD8+ T cells played a major role in this antitumor immune response. Also, CD4+ T cells contributed to this process, mainly by eliciting the production of autoantibody against RHAMM. Previous studies in mice have shown that anti‐RHAMM antibody can not only block the migration of endothelial cells through the basement membrane substrate in an in vitro model, but can also block basic FGF‐induced neovascularization.( 36 )
RHAMM is a well‐conserved protein showing more than 80% amino acid sequence identity between mice and humans,( 37 ) which may be the reason why antitumor activities in mice between two DC vaccines based on mouse and human RHAMM were comparable.( 35 ) Xenopus, mouse, and human RHAMM proteins are particularly homologous at their N and C termini, but xRHAMM is about 45% and 65% identical to human and mouse RHAMM, respectively.( 38 ) This difference in sequence identity may underlie the superior efficacy of the xRHAMM vaccine.
In conclusion, we identified xRHAMM, through CR‐SEREX, as a novel xenogenic target for antitumor or anti‐angiogenesis immunotherapy. The xenogenic vaccine based on xRHAMM induced an effective humoral and cellular immune response against local tumor and lung metastases.
Supporting information
Fig. S1. Specificity of purified immunoglobulin to RHAMM. The specificity of purified immunoglobulin to RHAMM was detected with western‐blot. pcDNA3.1‐mRHAMM plasmids were transfected into RHAMM‐negative COS7 cells. Purified immunoglobulin from control or immunized mice was used to detect the RHAMM in control and transfected COS7 cells. The results indicated that immunized immunoglobulin could specifically recognize RHAMM.
Fig. S2. Expression of RHAMM in B16 and MS1 cells. The expression of RHAMM in B16 and MS1 cells were detected with RT‐PCR. The results indicated that RHAMM was expressed both in B16 and MS1 cells.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgment
This work was supported by the National Key Basic Research Program of China (2010CB529900).
To whom correspondence should be addressed. E‐mail: yhansh@scu.edu.cn
References
- 1. Rosenberg SA. Progress in human tumor immunology and immunotherapy. Nature 2001; 411: 380–4. [DOI] [PubMed] [Google Scholar]
- 2. Marincola FM, Jaffe EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000; 74: 181–273. [DOI] [PubMed] [Google Scholar]
- 3. Bergman PJ, McKnight J, Novosad A et al. Long‐term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res 2003; 9: 1284–90. [PubMed] [Google Scholar]
- 4. Srinivasan R, Wolchok JD. Tumor antigens for cancer immunotherapy: therapeutic potential of xenogeneic DNA vaccines. J Transl Med 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pupa SM, Iezzi M, Di Carlo E et al. Inhibition of mammary carcinoma development in HER‐2/neu transgenic mice through induction of autoimmunity by xenogeneic DNA vaccination. Cancer Res 2005; 65: 1071–8. [PubMed] [Google Scholar]
- 6. Neal ZC, Bates MK, Albertini MR, Herweijer H. Hydrodynamic limb vein delivery of a xenogeneic DNA cancer vaccine effectively induces antitumor immunity. Mol Ther 2007; 15: 422–30. [DOI] [PubMed] [Google Scholar]
- 7. Ciesielski MJ, Apfel L, Barone TA, Castro CA, Weiss TC, Fenstermaker RA. Antitumor effects of a xenogeneic survivin bone marrow derived dendritic cell vaccine against murine GL261 gliomas. Cancer Immunol Immunother 2006; 55: 1491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gallo P, Dharmapuri S, Nuzzo M et al. Xenogeneic immunization in mice using HER2 DNA delivered by an adenoviral vector. Int J Cancer 2005; 113: 67–77. [DOI] [PubMed] [Google Scholar]
- 9. Palomba ML, Roberts WK, Dao T et al. CD8+ T‐cell‐dependent immunity following xenogeneic DNA immunization against CD20 in a tumor challenge model of B‐cell lymphoma. Clin Cancer Res 2005; 11: 370–9. [PubMed] [Google Scholar]
- 10. Wei YQ, Wang QR, Zhao X et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med 2000; 6: 1160–6. [DOI] [PubMed] [Google Scholar]
- 11. Liu JY, Wei YQ, Yang L et al. Immunotherapy of tumors with vaccine based on quail homologous vascular endothelial growth factor receptor‐2. Blood 2003; 102: 1815–23. [DOI] [PubMed] [Google Scholar]
- 12. He QM, Wei YQ, Tian L et al. Inhibition of tumor growth with a vaccine based on xenogeneic homologous fibroblast growth factor receptor‐1 in mice. J Biol Chem 2003; 278: 21831–6. [DOI] [PubMed] [Google Scholar]
- 13. Wei YQ, Huang MJ, Yang L et al. Immunogene therapy of tumors with vaccine based on xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA 2001; 98: 11545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rafii S. Vaccination against tumor neovascularization: promise and reality. Cancer Cell 2002; 2: 429–31. [DOI] [PubMed] [Google Scholar]
- 15. Sahin U, Tureci O, Schmitt H et al. Human neoplasms elicit multiple specific immune response in the autologous host. Proc Natl Acad Sci USA 1995; 92: 11810–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krackhardt AM, Witzens M, Harig S et al. Identification of tumor‐associated antigens in chronic lymphocytic leukemia by SEREX. Blood 2002; 100: 2123–31. [DOI] [PubMed] [Google Scholar]
- 17. Jäger D, Stockert E, Güre AO et al. Identification of a tissue‐specific putative transcription factor in breast tissue by serological screening of a breast cancer library. Cancer Res 2001; 61: 2055–61. [PubMed] [Google Scholar]
- 18. Yang H, Wang C, Wang R et al. Characterization of the novel gene for rat testicular antigen protein 2 by cross‐reactive serological analysis of recombinant cDNA. J Biosci Bioeng 2009; 107: 589–95. [DOI] [PubMed] [Google Scholar]
- 19. Wang CT, Zhang P, Wang YS et al. RNA interference against Biot2, a novel mouse testis‐specific gene, inhibits the growth of tumor cells. Cell Mol Biol Lett 2009; 14: 363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quenby S, Brazeau C, Drakeley A, Lewis‐Jones DI, Vince G. Oncogene and tumour suppressor gene products during trophoblast differentiation in the first trimester. Mol Hum Reprod 1998; 45: 477–81. [DOI] [PubMed] [Google Scholar]
- 21. Murray MJ, Lessey BA. Embryo implantation and tumor metastasis: common pathways of invasion and angiogensis. Semin Reprod Endocrinol 1999; 17: 275–90. [DOI] [PubMed] [Google Scholar]
- 22. Tetens F, Kliem A, Tscheudschilsuren G, Navarrete Santos A, Fischer B. Expression of proto‐oncogenes inbovine prei‐implantation blastocysts. Anat Embryol (Berl) 2000; 201: 349–55. [DOI] [PubMed] [Google Scholar]
- 23. Growe DL, Parsa B, Sinha UK. Relationships between stem cells and cancer stem cells. Histol Histopathol 2004; 19: 505–9. [DOI] [PubMed] [Google Scholar]
- 24. Marin V, Kaplanski G, Gres S, Farnarier C, Bongrand P. Endothelial cell culture: protocol to obtain and cultivate human umbilical endothelial cells. J Immunol Methods 2001; 254: 183–90. [DOI] [PubMed] [Google Scholar]
- 25. Sparmann A, Bar‐Sagi D. Ras‐induced interleukin‐8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004; 6: 447–58. [DOI] [PubMed] [Google Scholar]
- 26. Roberts WG, Whalen PM, Soderstrom E et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP‐673,451. Cancer Res 2005; 65: 957–66. [PubMed] [Google Scholar]
- 27. Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol 2000; 18: 927–74. [DOI] [PubMed] [Google Scholar]
- 28. Line A, Slucka Z, Stengrevics A, Silina K, Li G, Rees RC. Characterisation of tumour‐associated antigens in colon cancer. Cancer Immunol Immunother 2002; 51: 574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukui M, Ueno K, Suehiro Y, Hamanaka Y, Imai K, Hinoda Y. Anti‐tumor activity of dendritic cells transfected with mRNA for receptor for hyaluronan‐mediated motility is mediated by CD4+ T cells. Cancer Immunol Immunother 2006; 55: 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lokeshwar VB, Selzer MG. Differences in hyaluronic acid‐mediated functions and signaling in arterial, microvessel, and vein‐derived human endothelial cells. J Biol Chem 2000; 275: 27641–9. [DOI] [PubMed] [Google Scholar]
- 31. Hardwick C, Hoare K, Owens R et al. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol 1992; 117: 1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turley EA. Hyaluronan and cell locomotion. Cancer Metastasis Rev 1992; 11: 21–30. [DOI] [PubMed] [Google Scholar]
- 33. Hall CL, Yang B, Yang X et al. Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H‐ras transformation. Cell 1995; 82: 19–28. [DOI] [PubMed] [Google Scholar]
- 34. Rein DT, Roehrig K, Schondorf T et al. Expression of the hyaluronan receptor RHAMM in endometrial carcinomas suggests a role in tumour progression and metastasis. J Cancer Res Clin Oncol 2003; 129: 161–4. [DOI] [PubMed] [Google Scholar]
- 35. Greiner J, Ringhoffer M, Taniguchi M et al. Receptor for hyaluronan acid‐mediated motility (RHAMM) is a new immunogenic leukemia‐associated antigen in acute and chronic myeloid leukemia. Exp Hematol 2002; 30: 1029–35. [DOI] [PubMed] [Google Scholar]
- 36. Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA‐mediated motility in endothelial cell function and angiogenesis. J Biol Chem 2001; 276: 36770–8. [DOI] [PubMed] [Google Scholar]
- 37. Hofmann M, Fieber C, Assmann V et al. Identification of IHABP, a 95 kDa intracellular hyaluronate binding protein. J Cell Sci 1998; 111: 1673–84. [DOI] [PubMed] [Google Scholar]
- 38. Groen AC, Cameron LA, Coughlin M, Miyamoto DT, Mitchison TJ, Ohi R. XRHAMM functions in ran‐dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr Biol 2004; 14: 1801–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Specificity of purified immunoglobulin to RHAMM. The specificity of purified immunoglobulin to RHAMM was detected with western‐blot. pcDNA3.1‐mRHAMM plasmids were transfected into RHAMM‐negative COS7 cells. Purified immunoglobulin from control or immunized mice was used to detect the RHAMM in control and transfected COS7 cells. The results indicated that immunized immunoglobulin could specifically recognize RHAMM.
Fig. S2. Expression of RHAMM in B16 and MS1 cells. The expression of RHAMM in B16 and MS1 cells were detected with RT‐PCR. The results indicated that RHAMM was expressed both in B16 and MS1 cells.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


