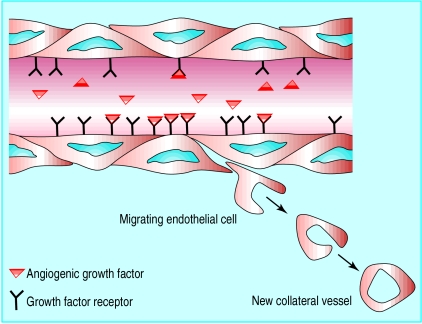
Therapeutic angiogenesis is the clinical use of growth factors to enhance or promote the development of collateral blood vessels in ischaemic tissue. Progress in understanding the process of angiogenesis, the isolation of angiogenic growth factors, successful preclinical studies, and promising early results in clinical trials have created great excitement about the potential of therapeutic angiogenesis.1 Although many questions remain, therapeutic angiogenesis may be the next major advance in the treatment of ischaemic heart disease.
The clinical problem
Ischaemic heart disease caused 6.3 million deaths worldwide in 1990, and it remains the leading cause of morbidity and mortality in the world.2 Advances in secondary prevention, reperfusion treatment for acute myocardial infarction, and revascularisation via coronary artery bypass graft surgery and percutaneous coronary interventions have improved long term survival of patients with established coronary heart disease. With these improvements in survival, and the ageing of populations, increasing numbers of patients are left with substantial myocardial ischaemia that is not amenable to revascularisation. Despite major advances in revascularisation techniques, these patients may constitute 5-15% of patients undergoing coronary angiography.
Potential of therapeutic angiogenesis
The importance of the coronary collateral circulation has become increasingly clear over the past 20 years.3–5 The coronary collateral circulation is a complex network of interconnecting vessels, most of which are <200 μm in diameter. Highly variable from patient to patient, this network develops from the recruitment of existing blood vessels as well as the creation of new vessels. The main stimulants for collateral growth are duration and severity of ischaemia, shear stress on the arterial wall, and inflammation.5 In acute myocardial infarction the presence of collateral circulation decreases infarct size, improves left ventricular function, decreases the likelihood of an aneurysm forming, and improves survival.3,4 While collateral circulation may provide adequate coronary perfusion at rest and limit the severity of myocardial ischaemia, coronary blood flow is seldom normal with maximal exercise or drug induced stress.
Future developments
Angiogenic treatment for patients with myocardial ischaemia not amenable to revascularisation, as an alternative to high risk percutaneous coronary intervention or coronary artery bypass graft surgery, or in combination with surgery to provide more complete revascularisation
Treatment of choice for patients with critical limb ischaemia and claudication
Adjunct to treatment for congestive heart failure in patients with ischaemic cardiomyopathy and hibernating myocardium
Treatment for diffuse accelerated atherosclerosis in recipients of heart transplants
Major advances in our understanding of the complex process of angiogenesis have raised the possibility of therapeutic angiogenesis.5–10 Angiogenesis is the sprouting of new vessels from pre-existing blood vessels—as opposed to vasculogenesis, the embryonic development of blood vessels from angioblasts. In therapeutic angiogenesis exogenous angiogenic growth factors (or genes encoding these growth factors) are used to stimulate the growth of collateral vessels to ischaemic tissues (figure). Many angiogenic growth factors have been identified (see box), but early trials have focused on vascular endothelial growth factor and fibroblast growth factor.
Angiogenic growth factors
| •Angiopoietin | • Placental growth factor |
| • Fibroblast growth factors (FGF) | • Platelet derived growth factor |
| • Granulocyte colony stimulating factor | • Proliferin |
| • Hepatocyte growth factor | • Thyroxine |
| • Insulin-like growth factor | • Transforming growth factor (α and β) |
| • Interleukin 8 | • Tumour necrosis factor α |
| • Leptin | • Vascular endothelial growth factor (VEGF) |
Vascular endothelial growth factor
is an angiogenic growth factor that exists in four isoforms (121, 165, 189, and 206 amino acids) that vary in permeability and heparin binding properties.9 It binds to receptors on endothelial cells, resulting in their growth, proliferation, and migration. The physiological effects of the growth factor include enhanced vascular permeability and vasodilatation induced by nitric oxide. Expression of both the growth factor and its receptors is upregulated by hypoxia and ischaemia, allowing a targeted therapeutic response and also limiting the potential for pathological angiogenesis.
Fibroblast growth factor
is a family of polypeptides that are potent stimulants of angiogenesis.10 Unlike vascular endothelial growth factor, fibroblast growth factor is not specific for endothelial cells, having receptors on many other cells including fibroblasts and vascular smooth muscle cells. It has a role in wound healing, is cardioprotective in acute myocardial infarction, and may have cytoprotective qualities elsewhere (for example, in the central nervous system).
Trials of angiogenic growth factors
Successful therapeutic angiogenesis has been demonstrated in models of coronary ischaemia in which vascular endothelial growth factor and fibroblast growth factor were administered by intracoronary, intramyocardial, intrapericardial, and intravenous routes, and also in models of peripheral ischaemia in which intra-arterial and intramuscular routes were used.9,10 Improved blood flow and functional improvement have been found not only in studies using growth factors but also in studies using genes coding for these growth factors. These results have prompted a dramatic increase in the number of human clinical trials.
Two small trials have been conducted in patients with peripheral vascular disease. Nine patients with critical limb ischaemia received two intramuscular injections of naked plasmid DNA (DNA not carried within in a vector) encoding the 165 amino acid isoform of vascular endothelial growth factor (VEGF165). These resulted in increased serum concentrations of vascular endothelial growth factor, angiographic evidence of improved collateral blood flow, and clinical improvement (healing of ischaemic ulcers, limb salvage, and resolution of rest pain).11 Preliminary results of a double blind, placebo controlled trial of fibroblast growth factor 2 in 19 patients with claudication indicated that the 13 treated patients had improved blood flow in the calf (measured by strain gauge plethysmography) and a decrease in claudication at the highest dose (30 μg/kg given on each of two days).12
Four small trials in patients with ischaemic heart disease have been published. Twenty patients undergoing coronary artery bypass graft surgery received an intramyocardial injection of fibroblast growth factor 1 near the insertion of the internal mammary artery graft. Compared with 20 patients who received placebo, the treated patients had evidence of increased collateral growth at follow up digital subtraction angiography.13 In another trial eight patients undergoing coronary artery bypass graft surgery received intramyocardial injections of fibroblast growth factor 2 in slow release beads in an area of the myocardium not amenable to revascularisation. Three patients had improved perfusion in the non-revascularised region on follow up nuclear perfusion tests.14 In a preliminary dose finding study 15 patients with viable but underperfused myocardium who were not suitable for coronary revascularisation received increasing doses of intracoronary VEGF165. Promising clinical results included improved myocardial perfusion in seven patients, angiographic evidence of increased collateral density in seven patients, and symptomatic improvement in 13 patients.15 Finally, five patients who were given intramyocardial injection of naked DNA plasmid coding for VEGF165 during minimally invasive surgery showed improvements in angina, nuclear perfusion scans, and angiography.16 Firm conclusions from these studies are limited by the small numbers and lack of placebo controls.
Phase I trials with intravenous VEGF165 and intracoronary fibroblast growth factor 2 have been completed, but the results are not yet published. Currently under way are phase I trials with intramyocardial injections of naked DNA plasmid coding for vascular endothelial growth factor during minimally invasive surgery, intramyocardial injections of adenovirus coding for VEGF121 in combination with coronary artery bypass graft surgery, and intracoronary infusions of adenovirus coding for fibroblast growth factor 4. Finally, the first randomised, placebo controlled trial of therapeutic angiogenesis has recently been completed: intracoronary and intravenous VEGF165 was given to 178 patients with myocardial ischaemia who were not candidates for revascularisation. The dramatic increase in the number of angiogenic trials reflects not only the unmet need but also the enthusiasm generated by successful preclinical models and encouraging initial clinical results.
Questions about the future of therapeutic angiogenesis
Despite these promising initial results, questions remain. Will pathological angiogenesis be a problem? What will be the optimal angiogenic growth factor, method of delivery, route of administration, and dosing schedule? Will protein or gene therapy provide the most potent angiogenic stimulus? If early results are confirmed in placebo controlled trials will the benefits be sustained? Will treatment with angiogenic growth factors alone be sufficient, or will it be adjunctive therapy to coronary artery bypass graft surgery, percutaneous coronary interventions, or the more recent techniques of transmyocardial laser revascularisation17 or enhanced external counterpulsation,18 which are also thought to stimulate angiogenesis?
Risk of pathological angiogenesis
The main concern about therapeutic angiogenesis is the potential for pathological angiogenesis. This is thought to play a part in several diseases, including tumour growth, diabetic proliferative retinopathy, macular degeneration, rheumatoid pannus formation, and progression of atherosclerosis or plaque rupture resulting from neovascularisation of atherosclerotic plaques.19
Both vascular endothelial growth factor and fibroblast growth factor may contribute to the growth of malignant tumours.6,9 Because of this, patients with a history of cancer have been excluded from the trials of these growth factors, but the potential to stimulate the growth of undetected malignancies exists. Fortunately, there are no reports of malignancy at this time. The development of diabetic retinopathy is also associated with elevated intraocular levels of vascular endothelial growth factor.9 At present, there are no reports of neovascularisation in the retina, despite formal ophthalmological examinations in most of the phase I and II trials. Initially, diabetic patients were excluded, but several trials now include diabetics without retinopathy. Expression of vascular endothelial growth factor and its receptors is increased in human coronary atherosclerotic lesions,19 and administration of the growth factor has been reported to exacerbate neointimal thickening after vascular injury in dogs. However, in the early clinical trials no increase in acute ischaemic syndromes or progression of atherosclerosis has been observed on serial angiography.
Despite these concerns, treatment with both vascular endothelial growth factor and fibroblast growth factor has been well tolerated in the early trials. Both drugs may cause hypotension at high doses or with rapid infusion. There have been reports of proteinuria and thrombocytopenia with fibroblast growth factor, and spider angiomas and peripheral oedema were reported with VEGF165 gene therapy in the trial of patients with critical limb ischaemia.
Defining the optimal agent and method of delivery
The ideal agent for therapeutic angiogenesis would be safe, effective, inexpensive, and easy to administer (see box). It would stimulate angiogenesis in the targeted ischaemic tissue without systemic effects, provide adequate exposure time to maximise angiogenesis with a limited number of treatments, and have sustained clinical benefit. It is far too early to know which growth factor or method of delivery will be optimal. The major advantage of vascular endothelial growth factor seems to be its specificity for endothelial cells, but this may also be a disadvantage if it stimulates the growth of only small, non-muscular arteries unable to provide adequate blood flow. Fibroblast growth factor may stimulate the growth of larger, muscularised arteries with increased perfusion capacity, but its lack of specificity may lead to more systemic side effects.10 The natural process of angiogenesis is complex, involving a number of growth factors, and there is evidence that fibroblast growth factor and vascular endothelial growth factor act synergistically in animal models.2
Ideal agent for therapeutic angiogenesis
Potent angiogenesis
Sustained clinical benefit
Specific to targeted ischaemic tissue
No acute side effects
Absence of unwanted angiogenesis
High local concentrations
Adequate exposure time
Readministration feasible
Non-invasive method of delivery (oral or intravenous)
Inexpensive (cost effective)
In gene therapy naked DNA or a virus vector containing the gene is injected directly into ischaemic tissue and taken up by the host cells, leading to increased expression of the protein growth factor for about a fortnight. The ideal vector would have high rates of transfection (uptake of the gene by host cells), leading to local production of the protein for a limited period of time. Concerns remain over the long term safety of incorporating naked DNA or a virus vector into the genome. Direct treatment with the growth factor has the theoretical advantage of delivering a more precise quantity in a more controlled fashion, but proponents of gene therapy think that this allows a more sustained exposure to the growth factor. However, binding of the exogenous protein to its receptor or to heparin may allow prolonged exposure with a single infusion of growth factor. In addition, new proliferating endothelial cells provide a new source of endogenous growth factor.
Invasive approaches, such as intramyocardial or intrapericardial injections during open chest procedures, have the advantage of providing high local concentrations of growth factor with low systemic exposure. Less invasive approaches, such as intracoronary or intravenous delivery, allow repeat administration without the risk inherent in more invasive methods. Initially, each growth factor and method of delivery will need to be tested individually in randomised controlled trials to determine the optimal dose and safety. In the future it seems likely that a combination of growth factors will be used, and the ability to administer them in a variety of ways will allow treatment plans to be individualised.
Additional applications
Current trials of therapeutic angiogenesis have been confined to patients with severe ischaemic heart disease or peripheral vascular disease. However, it may also be of benefit in other cardiovascular problems such as congestive heart failure in patients with ischaemic cardiomyopathies, accelerated atherosclerosis in cardiac transplant patients, restenosis, microvascular disease, hibernating myocardium, stunned myocardium, left ventricular hypertrophy, and pulmonary hypertension. Non-cardiovascular uses include treating renovascular, cerebrovascular, and peptic ulcer diseases and wound healing.
Figure.
Therapeutic angiogenesis—attachment of intravascular exogenous growth factor to receptors on endothelial cells results in migration, proliferation, and differentiation of endothelial cells into new collateral blood vessels
Editorial by Brindle et al
Footnotes
Competing interests: None declared.
References
- 1.Henry TD. Can we really grow new blood vessels? Lancet. 1998;351:1826–1827. doi: 10.1016/S0140-6736(98)22025-3. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 3.Sasayama S, Fujita M. Recent insight into the collateral circulation. Circulation. 1992;85:1197–1203. doi: 10.1161/01.cir.85.3.1197. [DOI] [PubMed] [Google Scholar]
- 4.Charney R, Cohen M. The role of the coronary collateral circulation in limiting myocardial ischemia and infarct size. Am Heart J. 1993;126:937–945. doi: 10.1016/0002-8703(93)90710-q. [DOI] [PubMed] [Google Scholar]
- 5.Schaper W, Ito WD. Molecular mechanisms of coronary collateral vessel growth. Circ Res. 1996;79:911–919. doi: 10.1161/01.res.79.5.911. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 7.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 8.Irwela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997;78:672–677. [PubMed] [Google Scholar]
- 9.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine Reviews. 1997;18:1–22. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 10.Ware JA, Simons M. Angiogenesis in ischemic heart disease. Nature Med. 1997;3:158–163. doi: 10.1038/nm0297-158. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 12.Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Quyyumi AA. Effect of basic fibroblast growth factor on lower extremity blood flow in patients with intermittent claudication: preliminary results. Circulation. 1998;98:I-456. [Google Scholar]
- 13.Schumacher MD, Pecher MD, von Sprecht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 14.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg. 1998;65:1540–1544. doi: 10.1016/s0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 15.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, et al. Results of intracoronary recombinant human vascular endothelial growth factor (rhVEFG) administration trial. J Am Coll Cardiol. 1998;31:65A. [Google Scholar]
- 16.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto N, Kohmoto T, Gu A, DeRosa C, Smith CR, Burkhoff D. Angiogenesis is enhanced in ischemic canine myocardium by transmyocardial laser revascularization. J Am Coll Cardiol. 1998;31:1426–1433. doi: 10.1016/s0735-1097(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 18.Lawson WE, Hui JC, Zheng ZS, Oster Z, Katz JP, Diggs P, et al. Three-year sustained benefit from enhanced external counterpulsation in chronic angina pectoris. Am J Cardiol. 1995;75:840–841. doi: 10.1016/s0002-9149(99)80427-5. [DOI] [PubMed] [Google Scholar]
- 19.Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions. Circulation. 1998;98:2108–2116. doi: 10.1161/01.cir.98.20.2108. [DOI] [PubMed] [Google Scholar]