Abstract
The incidence of endometrial cancer is predicted to increase in developed countries. Because of the relatively high incidence of complications and low diagnostic sensitivity associated with endometrial tissue sampling, there is an urgent need for the development of a safe and non‐invasive diagnostic method. The proteomic spectrum of albumin‐associated peptides was obtained from a total of 125 serum samples (92 from endometrial cancer patients and 33 from controls) by matrix‐assisted laser desorption/ionization hybrid quadrupole time‐of‐flight mass spectrometry, and the candidate markers were selected by the Mann–Whitney U‐test and receiver operator characteristics analysis. We selected three mass peaks at 4769, 6254 and 11 792 m/z from a total of 507 peaks as distinguishing cancer patients from controls (P < 0.00001 and area under curve of over 0.8). When the cut‐off points were defined as the averages of the values in the controls + 2 SD, the combination of the three peptides detected endometrial cancer with a sensitivity of 65.2% (60/92). Even stage I early endometrial cancers were detected with a sensitivity of 60.3% (38/63). Unfortunately, the three peptides were also detected in 44.6% (33/74) of myoma uteri patients, indicating that they are not specific to endometrial cancer. Although a large‐scale study is necessary to confirm the clinical significance of the peptide biomarkers identified in this study, direct profiling of serum‐albumin‐bound peptides by high‐resolution mass spectrometry was proven to have potential as a means of identifying biomarkers for a variety of diseases. (Cancer Sci 2007; 98: 822–829)
Abbreviations:
- AUC
area under the curve
- CC
correlation coefficient
- CHCA
cyano‐4‐hydroxycinnamic acid
- ELISA
enzyme‐linked immunosorbent assay
- ESI
electrospray ionization
- MALDI QqTOF‐MS
matrix‐assisted laser desorption/ionization hybrid quadrupole time‐of‐flight mass spectrometry
- ROC
receiver operator characteristics.
The incidence of endometrial cancer is high in developed countries, and its morbidity has increased in recent years. In 1970, endometrial cancer constituted only 3% of all uterine cancers in Japan, but the proportion had increased to 40% in 1998.( 1 ) Excessive fat consumption, overweight, physical inactivity, high energy intake, hypertension and a high serum glucose concentration have been identified as risk factors for endometrial cancer.( 2 , 3 , 4 , 5 , 6 ) A medical history of breast cancer and use of tamoxifen to treat breast cancer increase the risk of endometrial cancer,( 7 ) but pregnancy reduces it.( 8 , 9 ) Because most of the above are characteristic of the lifestyle in developed countries, the incidence of endometrial cancer is predicted to continue to increase in the future, and thus the development of an effective mass screening method is needed urgently.
Abnormal uterine bleeding is the most frequent initial symptom of endometrial cancer, but many other disorders also give rise to this symptom. Endometrial cancer is usually diagnosed by histological examination of endometrial tissue obtained with miniature endometrial biopsy devices. However, endometrial biopsy cannot be carried out in postmenopausal patients with a closed external os of the uterus, and endometrial biopsy is often associated with complications, such as infection, bleeding and perforation of the uterus. Transvaginal ultrasonography has been used as an alternative non‐invasive diagnostic method for the diagnosis of endometrial diseases, but its diagnostic accuracy is not satisfactory.( 10 )
The circulating serum proteome holds great promise as a reservoir of information that will be useful for the diagnosis of various diseases. A large variety of low molecular weight protein fragments and peptides are known to be produced as a consequence of the proteolytic processes occurring in the microenvironment of diseased tissues,( 11 ) and these protein fragments are released into the blood circulation and become bound to high‐abundance proteins, such as serum albumin.( 12 , 13 ) Serum albumin‐associated peptides are protected from renal clearance and may be concentrated over time during the course of chronic diseases, such as cancer.( 14 ) However, it has never been determined whether disease‐related peptides actually accumulate in the serum of cancer patients and whether detection of such peptides can be applied to cancer diagnosis.
Mass spectrometry‐based quantitative proteomics approaches have gained considerable attention as effective modalities for identifying new biomarkers of various diseases.( 15 , 16 , 17 ) To answer the above questions we directly quantified the serum albumin‐associated peptides of a large number of endometrial cancer patients with a high‐resolution mass spectrometer. In this paper we report that a certain set of peptides accumulate in the serum of endometrial cancer patients and that quantification of these peptides has diagnostic significance.
Materials and Methods
Subjects and serum samples. The serum samples (n = 199) used in this study were collected at the National Hospital Organization Hokkaido Cancer Center Hospital (Sapporo, Japan) between 2000 and 2004 with the informed consent of all donors. The 199 subjects consisted of patients with untreated endometrial cancer (n = 92), metroptosis patients (n = 16), myoma uteri patients (n = 74), and healthy volunteers (n = 17) (Table 1). The samples were collected in glass tubes, and after allowing them to clot, the serum was separated and cryopreserved at –80°C until analyzed. The protocol of the study was reviewed and approved by the ethics committees of the National Hospital Organization Hokkaido Cancer Center and National Cancer Center (Tokyo Japan). The characteristics of the subjects are summarized in Table 1. The endometrial cancer patients were classified as surgical stage 0, I, II, III or IV.( 18 )
Table 1.
Clinical features of the subjects
| Feature | Cancer | Control† | Metroptosis | Myoma | Healthy |
|---|---|---|---|---|---|
| Number of cases | 92 | 33 | 16 | 74 | 17 |
| Mean age ± SD (years) | 59.4 ± 10.5 | 50.8 ± 18.5 | 65.8 ± 9.8 | 48.8 ± 4.1 | 36.7 ± 12.5 |
| Surgical stage‡ | |||||
| 0 (%) | 6 (6.50%) | ||||
| I (%) | 63 (68.5%) | ||||
| II (%) | 8 (8.70%) | ||||
| III (%) | 13 (14.1%) | ||||
| IV (%) | 2 (2.20%) | ||||
The controls (n = 33) consisted of the metroptosis patients (n = 16) and healthy volunteers (n = 17).
Classified according to the 2nd edition of The General Rules for Clinical and Pathological Management of Uterine Corpus Cancer.( 18 )
Purification of serum albumin‐associated peptides. Native albumin‐associated peptides were separated and concentrated from the serum samples with a proXPRESSION kit (PerkinElmer, Boston, MA, USA) according to the instructions provided by the supplier (Suppl. Fig. S1). Briefly, a 40‐µL sample of serum was diluted 1:10 with Biomarker Enrichment Binding Buffer (PerkinElmer), and the 400‐µL diluted serum sample was loaded onto a spin column and spun at 200 g for 10 min. The column was washed with binding buffer three times. The sample was desalted with ZipPlate C‐18 (Millipore, Bedford, MA, USA) and spotted directly onto a disposable MALDI plate (PerkinElmer) with CHCA (Ciphergen Biosystems, Fremont, CA, USA). Experiments were carried out in triplicate, and only reproducible assays with a correlation coefficient over 0.75 were analyzed further.
MALDI QqTOF‐MS. Mass spectra were obtained with a high‐resolution orthogonal QqTOF‐MS instrument (prOTOF 2000; PerkinElmer). The instrument was set to measure the range between 1000 and 80 000 m/z. Laser shot, laser energy, laser rate, declustering, cooling flow and laser pattern were set at 50, 84%, 29.0 Hz, 30 V, 150.0 mL/min and 2 mm ring 96 spot, respectively. After mass calibration the mass data were converted to text files consisting of m/z and peak intensity. The text files were processed with in‐house peak detection, normalization and quantification software (called NCC‐ProteoJudge)( 19 , 20 ) and the peak data were visualized with Mass Navigator software (Mitsui Knowledge Industry, Tokyo, Japan). Mass accuracy was calibrated externally on the day of the measurements with an all‐in‐one peptide molecular mass standard (Ciphergen Biosystems).
Statistical analysis. Statistically significant differences were detected using the Mann–Whitney U‐test. ROC curves were generated and AUC values were calculated using StatFlex software (version 5.0; Artech, Osaka, Japan).( 21 )
ELISA of CA125. The serum CA125 value was measured with a commercial ELISA kit (Elecsys CA125 II reagent kit; Roche Diagnostics, Mannheim, Germany) according to the instructions provided by the supplier.
Results
Detection and quantification of serum albumin‐associated peptides. A total of 507 unique serum albumin‐bound peptide peaks were detected constantly in the range between 2000 and 30 000 m/z across 125 serum samples (92 endometrial cancer patients and 33 controls) by MALDI QqTOF‐MS. Detection and quantification of the peptides was highly reproducible as revealed by visual inspection (Fig. 1) and by calculating the CC of triplicate separation and measurements in every sample (Fig. 2). The mean CC ± SD for the 507 peaks in the 125 serum samples was 0.979 ± 0.035. Reliable quantification seems possible in the range exceeding 103 (Fig. 2).
Figure 1.

Direct profiling of serum albumin‐associated peptides by matrix‐assisted laser desorption/ionization hybrid quadrupole time‐of‐flight mass spectrometry. Mass spectra of triplicate preparations of serum albumin‐associated peptides from a representative healthy volunteer are shown in the ranges of 2500–10 000 m/z (top) and 3400–5500 m/z (bottom).
Figure 2.
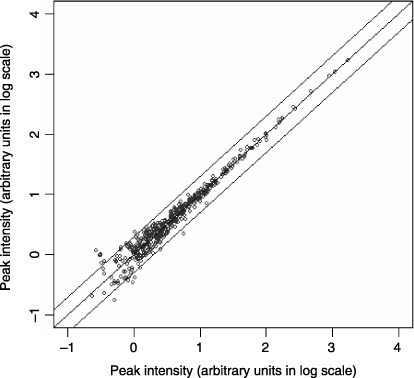
Reproducibility of profiling of albumin‐associated peptides. Two‐dimensional plots indicate the high correlation between the relative intensity (in log scale) of corresponding peaks in the duplicate (x‐axis and y‐axis) separation and measurements of albumin‐associated peptides from a representative serum sample. The correlation coefficient value between the duplicate was 0.975, and 97.83% of the peaks were plotted within a two‐fold difference (solid lines).
Selection of peptides associated with endometrial cancer. We selected mass peaks whose mean intensity in triplicate measurements differed significantly between the 92 endometrial cancer patients and 33 controls based on predefined statistical criteria (P < 0.00001, Mann–Whitney U‐test and AUC value > 0.80). Three peaks at 4769, 6254 and 11 792 m/z (Fig. 3) were found to fulfill the criteria with P‐values of 8.99E‐8, 1.25E‐9 and 7.46E‐8 (Table 2) as well as AUC values of 0.813, 0.857 and 0.815 (Fig. 4), respectively. Representative mass spectra of the three peptide peaks (one cancer patient and one control) and gel‐like images (30 cancer patients and 30 controls) are shown in Fig. 3.
Figure 3.

Marker peptides in the serum of uterine endometrial cancer patients. Representative mass spectra of the serum peptides of a healthy volunteer and an endometrial cancer patient showing the peaks at 4769, 6254 and 11 792 m/z, and gel‐like images converted from mass spectra of 30 controls and 30 cancer patients.
Table 2.
Distribution of the 4786‐, 6254‐ and 11 792‐m/z peaks
| Peaks | Cancer (n = 92) | Myoma (n = 74) | Control (n = 33) | P‐value* | P‐value** | P‐value*** |
|---|---|---|---|---|---|---|
| 4769 m/z | 2.24 ± 0.59† | 2.00 ± 0.49† | 1.62 ± 0.32† | 8.99E‐8 | 1.40E‐2 | 2.67E‐4 |
| 6254 m/z | 3.60 ± 1.16† | 3.01 ± 0.96† | 2.47 ± 0.56† | 1.25E‐9 | 4.72E‐5 | 2.34E‐4 |
| 11 792 m/z | 4.43 ± 2.27† | 3.55 ± 2.14† | 2.35 ± 0.84† | 7.46E‐8 | 4.93E‐3 | 9.80E‐5 |
Mann–Whitney U‐test between: *cancer patients and controls; **cancer patients and myoma patients; ***myoma patients and controls.†Mean intensity ± SD in arbitrary units.
Figure 4.

Receiver operator characteristics analysis of peptides of uterine endometrial cancer. Receiver operator characteristics curves and area under the curve values showing the discrimination capacities of the 4769‐, 6254‐ and 11 792‐m/z peaks.
Sensitivity and specificity of the three peptides. The distribution of the intensity of the three peaks is shown in Fig. 5. When the cut‐off values were defined as the mean + 2 SD of the values of the 33 control samples (solid lines), the sensitivity of the 4769‐, 6254‐ and 11 792‐m/z peaks was 42.4, 38.0 and 47.8%, respectively, and their specificity was 100, 97.0 and 97.0%, respectively (Fig. 5; Table 3). Endometrial cancer could be diagnosed with a sensitivity of 65.2% when at least one of the three peptides exceeded the cut‐off value, but specificity remained high (93.9%).
Figure 5.

Scatter graph of the 4769‐, 6254‐ and 11 792 m/z‐peaks. The difference in distribution of the intensity of each peak between the controls and cancer patients was statistically significant (Mann–Whitney U‐test). Solid lines represent the average intensity values of the healthy controls + 2 SD. The broken lines indicate the average intensity in the controls and cancer patients.
Table 3.
Sensitivity and specificity of 3 albumin‐associated peptides and CA125
| 4769 m/z | 6254 m/z | 11 792 m/z | Combination | CA125 | |
|---|---|---|---|---|---|
| Sensitivity | |||||
| % | 42.4† | 38.0† | 47.8† | 65.2‡ | 22.1§ |
| n | 39/92 | 35/92 | 44/92 | 60/92 | 17/77 |
| Specificity | |||||
| % | 100† | 97.0† | 97.0† | 93.9‡ | 90.0§ |
| n | 33/33 | 32/33 | 32/33 | 31/33 | 27/30 |
Cut‐off values were defined as the averages of the values in the controls (n = 33) + 2 SD.
‡Any of the three peaks exceeded the cut‐offs.
Cut‐off was defined as 35 IU/mL.
The sensitivity of each marker and of the combination of the three according to the surgical stage of the patients is shown in Table 4. There was no significant difference in the distribution of peak intensity among endometrial cancer patients of different surgical stages (Suppl. Fig. S2). The combination detected 60.3% of early endometrial cancer at stage I (Fig. 6; Table 4).
Table 4.
Sensitivity according to surgical stage
| Surgical stage† | 4769 m/z | 6254 m/z | 11 792 m/z | Combination | CA125 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | % | n | % | n | |
| 0 | 50.0 | 3/6 | 33.3 | 2/6 | 50.0 | 3/6 | 66.7 | 4/6 | 0 | 0/4 |
| I | 36.5 | 23/63 | 34.9 | 22/63 | 42.9 | 27/63 | 60.3 | 38/63 | 17.3 | 9/52 |
| II | 37.5 | 3/8 | 37.5 | 3/8 | 75.0 | 6/8 | 75.0 | 6/8 | 12.5 | 1/8 |
| III | 61.5 | 8/13 | 46.2 | 6/13 | 46.2 | 6/13 | 76.9 | 10/13 | 45.5 | 5/11 |
| IV | 100 | 2/2 | 100 | 2/2 | 100 | 2/2 | 100 | 2/2 | 100 | 2/2 |
| Total | 42.4 | 39/92 | 38.0 | 35/92 | 47.8 | 44/92 | 65.2 | 60/92 | 22.1 | 17/77 |
Classified according to the 2nd edition of The General Rules for Clinical and Pathological Management of Uterine Corpus Cancer.( 18 )
Figure 6.

Comparison of peptides and CA125. Individuals whose values for the single 4769‐, 6254‐ and 11 792‐m/z peaks, their combination, and CA125 exceeded the cut‐off values are darker. Asterisks, not examined.
The levels of the three peptides in sera of myoma uteri patients were lower than those of endometrial cancer patients and higher than those of controls, and the differences were statistically significant (Table 2). When the cut‐off values were defined as the mean + 2 SD of the controls, the intensities of the 4769‐, 6254‐, and 11 792‐m/z peaks were above the cut‐offs in 31.1 (23/74), 13.5 (10/74) and 24.3% (18/74) of myoma patients, respectively (Fig. 6), indicating that none of the three peptides is specific to endometrial cancer.
Comparison to CA125. CA125 is a known serum marker of endometrial cancer and ovarian cancer.( 22 ) We analyzed the level of CA125 in the serum of the 77 cancer patients and 30 controls whose remaining serum sample was sufficient to make the measurement. When the cut‐off value was set at 35 IU/mL, the sensitivity of CA125 was 22.1% (17/77), and its specificity was 90.0% (27/30) (Table 3), indicating that each peptide marker identified in this study as well the combination of the three peptides was superior to CA125 in detecting endometrial cancer. The sensitivity of CA125 was highly dependent on the surgical stage of the cancer, and only 17.3% (9/52) of the stage I cancers were detected with CA125 (Table 4). The reactivity of CA125 and peptide markers in each subject is shown in Fig. 6.
Discussion
Various cytokines, growth factors, ligands, enzymes and their inhibitors are secreted in the local tumor microenvironment and participate in tumor growth, metastasis and angiogenesis. Profiling of circulating serum proteins unquestionably provides useful information for cancer diagnosis. However, direct detection and quantification of biomarkers with low‐abundance by mass spectrometry is often complicated by a handful of abundant proteins, including albumin, immunoglobulin and transferrin. Various proteases, including serine proteases and matrix metalloproteinases, are activated during the process of tissue remodeling‐associated tumor expansion and invasion, and a large variety of proteolytic fragments are released into the bloodstream. These low molecular weight protein fragments and peptides are bound to large proteins, such as albumin, and are stably retained in the circulation. Lowenthal et al. recently analyzed the serum albumin‐bound peptides of ovarian cancer patients by ESI‐MS coupled with liquid chromatography and identified proteolytic fragments of a tumor suppressor protein, BRCA2 (breast cancer 2).( 14 ) However, the clinical utility of these albumin‐associated peptides as cancer tumor markers has not been established. In the present study we demonstrated that a large number of albumin‐associated peptides can be detected and quantified reproducibly by high‐resolution MALDI QqTOF‐MS (1, 2).
The serum of the endometrial cancer patients and control subjects contained a large variety of albumin‐associated peptides, but most peptide peaks did not differ significantly between the controls and endometrial cancer patients (Fig. 3). We used a very strict statistical criterion (P < 0.00001, Mann–Whitney U‐test) to search for peptides associated with endometrial cancer, because it was estimated that 5 of the 507 detectable peaks would by chance achieve a P‐value of 0.01. We found that the intensity of only three peptides differed significantly between the endometrial cancer patients and controls with P‐values at the 10−8 level (Table 2), meaning that chance identification of these peptides is unlikely.
ROC analysis (Fig. 4) and the distribution of peak intensity (Fig. 5) revealed that these three peaks had high discriminatory capacity, indicating their potential as novel serum tumor markers of endometrial cancer. CA125 has been one of the most reliable tumor markers for adenocarcinoma of the uterus and is used frequently in clinical settings( 23 ) but the sensitivity (65.2%) of the peptide marker set identified in this study was clearly higher than that of CA125 (22.1%) (Table 3). Furthermore, the marker set detected even early (stage I and II) endometrial cancer with a sensitivity of 60.3 and 75.0%, respectively (Table 4). The serum CA125 level is a prognostic indicator of endometrial cancer, but CA125 has been found to be unsatisfactory for the diagnosis of early stage endometrial cancer. Consistent with the results of this study the CA125 level has been reported to be elevated in only 13–22% of patients with early endometrial cancer.( 24 , 25 )
Thus far no attempts to obtain the amino acid sequences of the three albumin‐associated peptides identified in this study have been successful, because it was impossible to separate the proteins from these low‐abundance peaks by multidimensional liquid chromatography without contamination by neighboring peaks (Fig. 3). The size of the three peptides (4769, 6254 and 11 792 m/z) was beyond the range manageable by the direct tandem MS (MS/MS) of QqTOF‐MS. Fourier transform MS may overcome this problem, because it does not require enzymatic digestion and complete purification as preconditions for protein identification. However, the interface to the MALDI is not used for Fourier MS in practice. Furthermore, the identification of circulating blood proteins by MS/MS may not be as straightforward as previously thought, because the inclusion of novel exons and previously nonannotated gene sequences has resulted in computing errors and false identification of plasma proteins.( 26 ) The high reproducibility of QqTOF‐MS (1, 2), however, warrants direct application of its measurements to clinical use and does not necessitate actual protein identification of the peaks.
A mass screening program for endometrial cancer has been conducted in Japan since 1987 under the Health and Medical Service Law for the Aged.( 27 ) Although extensive screening by endometrial cytology is expected to increase the rate of detection of early endometrial cancer and improve the overall survival rate,( 28 ) only 5–6% of the eligible population of women enrolled in the mass screening program, probably because of shame and fear of the pain and complications associated with the cytological examination. The introduction of an effective blood test into mass screening for endometrial cancer might lower the psychological hurdle and increase enrolment dramatically. However, the current peptide marker set seems insufficient for its application to mass screening because of the high prevalence of asymptomatic myoma uteri in the general population. Its use might be limited to ambulatory gynecologic practice as a safe option to reduce the chance of missing asymptomatic endometrial cancer and myoma cases for which cytological and ultrasound examinations give negative results.
Although a confirmatory study will be necessary to verify the diagnostic accuracy of the peptides, our data clearly indicate the feasibility of direct profiling of albumin‐associated peptides for tumor marker discovery.
Supporting information
Supporting info item
Acknowledgments
We thank Mr H. Kuwabara, Mr T. Sakuma, and Ms M. Sato of the Mitsui Knowledge Industry Co. (Tokyo, Japan) for the statistical analyses, and Mr S. Sagara of PerkinElmer Japan (Tokyo, Japan) for technical assistance. S. Kikuchi is the recipient of a ‘Research Resident Fellowship’ from the Foundation for the Promotion of Cancer Research (Tokyo, Japan). This study was supported by grants from the Ministry of Health, Labor and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, the National Institute of Biomedical Innovation of Japan, and the Naito Foundation.
References
- 1. Kuwabara Y, Susumu N, Banno K et al. Clinical characteristics of prognostic factors in poorly differentiated (G3) endometrioid adenocarcinoma in Japan. Jpn J Clin Oncol 2005; 35: 23–7. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 1975; 15: 617–31. [DOI] [PubMed] [Google Scholar]
- 3. Zeleniuch‐Jacquotte A, Akhmedkhanov A, Kato I et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer 2001; 84: 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348: 1625–38. [DOI] [PubMed] [Google Scholar]
- 5. Anderson KE, Anderson E, Mink PJ et al. Diabetes and endometrial cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev 2001; 10: 611–16. [PubMed] [Google Scholar]
- 6. Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer 2003; 104: 669–76. [DOI] [PubMed] [Google Scholar]
- 7. Gehrig PA, Bae‐Jump VL, Boggess JF, Groben PA, Fowler WC Jr, Van Le L. Association between uterine serous carcinoma and breast cancer. Gynecol Oncol 2004; 94: 208–11. [DOI] [PubMed] [Google Scholar]
- 8. Hinkula M, Pukkala E, Kyyronen P, Kauppila A. Grand multiparity and incidence of endometrial cancer: a population‐based study in Finland. Int J Cancer 2002; 98: 912–15. [DOI] [PubMed] [Google Scholar]
- 9. Chubak J, Tworoger SS, Yasui Y, Ulrich CM, Stanczyk FZ, McTiernan A. Associations between reproductive and menstrual factors and postmenopausal sex hormone concentrations. Cancer Epidemiol Biomarkers Prev 2004; 13: 1296–301. [PubMed] [Google Scholar]
- 10. Langer RD, Pierce JJ, O’Hanlan KA et al. Transvaginal ultrasonography compared with endometrial biopsy for the detection of endometrial disease. Postmenopausal Estrogen/Progestin Interventions Trial. N Engl J Med 1997; 337: 1792–8. [DOI] [PubMed] [Google Scholar]
- 11. Petricoin EF, Liotta LA. SELDI‐TOF‐based serum proteomic pattern diagnostics for early detection of cancer. Curr Opin Biotechnol 2004; 15: 24–30. [DOI] [PubMed] [Google Scholar]
- 12. Mehta AI, Ross S, Lowenthal MS et al. Biomarker amplification by serum carrier protein binding. Dis Markers 2003; 19: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hortin GL. The MALDI‐TOF mass spectrometric view of the plasma proteome and peptidome. Clin Chem 2006; 52: 1223–37. [DOI] [PubMed] [Google Scholar]
- 14. Lowenthal MS, Mehta AI, Frogale K et al. Analysis of albumin‐associated peptides and proteins from ovarian cancer patients. Clin Chem 2005; 51: 1933–45. [DOI] [PubMed] [Google Scholar]
- 15. Issaq HJ, Conrads TP, Prieto DA, Tirumalai R, Veenstra TD. SELDI‐TOF MS for diagnostic proteomics. Anal Chem 2003; 75: 148A–55A. [DOI] [PubMed] [Google Scholar]
- 16. Chapman K. The ProteinChip biomarker system from ciphergen biosystems: a novel proteomics platform for rapid biomarker discovery and validation. Biochem Soc Trans 2002; 30: 82–7. [DOI] [PubMed] [Google Scholar]
- 17. Von Eggeling F, Junker K, Fiedle W et al. Mass spectrometry meets chip technology: a new proteomic tool in cancer research? Electrophoresis 2001; 22: 2898–902. [DOI] [PubMed] [Google Scholar]
- 18. Japan Society of Obstetrics and Gynecology, Japanese Society of Pathology, Japan Radiological Society . The General Rules for Clinical and Pathological Management of Uterine Corpus Cancer, 2nd edn. Tokyo: Kanahara Shuppan, 1996. [Google Scholar]
- 19. Honda K, Hayashida Y, Umaki T et al. Possible detection of pancreatic cancer by plasma protein profiling. Cancer Res 2005; 65: 10 613–22. [DOI] [PubMed] [Google Scholar]
- 20. Hayashida Y, Honda K, Osaka Y et al. Possible prediction of chemoradiosensitivity of esophageal cancer by serum protein profiling. Clin Cancer Res 2005; 11: 8042–7. [DOI] [PubMed] [Google Scholar]
- 21. Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978; 8: 283–98. [DOI] [PubMed] [Google Scholar]
- 22. Niloff JM, Klug TL, Schaetzl E, Zurawski VR Jr, Knapp RC, Bast RC Jr. Elevation of serum CA125 in carcinomas of the fallopian tube, endometrium, and endocervix. Am J Obstet Gynecol 1984; 148: 1057–8. [DOI] [PubMed] [Google Scholar]
- 23. Kukura V, Zaninovic I, Hrdina B. Concentrations of CA‐125 tumor marker in endometrial carcinoma. Gynecol Oncol 1990; 37: 388–9. [DOI] [PubMed] [Google Scholar]
- 24. Bast RC Jr, Klug TL, St John E et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309: 883–7. [DOI] [PubMed] [Google Scholar]
- 25. Duk JM, Aalders JG, Fleuren GJ, De Bruijn HW. CA 125: a useful marker in endometrial carcinoma. Am J Obstet Gynecol 1986; 155: 1097–102. [DOI] [PubMed] [Google Scholar]
- 26. States DJ, Omenn GS, Blackwell TW et al. Challenges in deriving high‐confidence protein identifications from data gathered by a HUPO plasma proteome collaborative study. Nat Biotechnol 2006; 24: 333–8. [DOI] [PubMed] [Google Scholar]
- 27. Statistics and Information Department, Minister's Secretariat, Ministry of Health and Welfare, Japan . Report on the Health Services for the Elderly. Tokyo: Health and Welfare Statistics Association, 1997. [Google Scholar]
- 28. Nakagawa‐Okamura C, Sato S, Tsuji I et al. Effectiveness of mass screening for endometrial cancer. Acta Cytol 2002; 46: 277–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


