Abstract
The development and progression of tumor cells is controlled by their interactions with neighboring host cells and a variety of microenvironmental factors including extracellular matrix (ECM) molecules, growth factors and proteinases. Cell‐adhesive ECM proteins are a prerequisite for growth and migration of many types of cells. Their interactions with integrins and other cell surface receptors induce intracellular signaling that regulates the actin cytoskeleton and gene expression. The basement membrane protein laminin‐5 is a notable cell adhesion molecule, which promotes cellular adhesion and migration much more efficiently than other ECM proteins. There is accumulating evidence that laminin‐5 is involved in tumor growth and progression. With special reference to laminin‐5, this article reviews the regulatory mechanisms of cellular adhesion and migration by ECM molecules and their significance in tumor progression. (Cancer Sci 2006; 97: 91 – 98)
Abbreviations
- BM
basement membrane
- ECM
extracellular matrix
- EHS
Engelbreth‐Holm‐Swarm
- ERK
extracellular signal‐regulated kinase
- FAK
focal adhesion kinase
- HSPG
heparan sulfate proteoglycans
- JNK
c‐Jun N‐terminal kinase
- LG
laminin‐like globular
- MAPK, mitogen‐activated protein kinase; MMP
matrix metalloproteinase
- MT1
membrane type 1; PI3K, phosphatidylinositol 3‐kinase; PKC, protein kinase C; TGF‐β, transforming growth factor‐β.
Tissue microenvironments are thought to play a critical role in the development and progression of tumor cells.( 1 , 2 ) Tumor cells interact with surrounding host cells such as fibroblasts, inflammatory cells and vascular endothelial cells, and also with a variety of soluble and insoluble microenvironmental factors, including ECM molecules, growth factors, cytokines and proteinases. These interactions determine the behavior of tumor cells in vivo. Microenvironmental factors are produced by both host and tumor cells, and their production is regulated in either a paracrine or autocrine manner by the factors themselves. Tumor cells are likely to create microenvironments suitable for their own growth. For example, tumor cells stimulate neighboring vascular endothelial cells to induce angiogenesis, thus allowing tumor cells to grow rapidly. Tumor cells stimulate surrounding stromal cells to express MMP, which are the molecules that promote invasive growth of tumor cells by degrading surrounding ECM barriers.( 3 ) It should also be noted that metastatic carcinoma cells must pass through many different environments, such as the original tumor nest and connective tissues as well as the vasculature, to reach distant metastatic sites. Different functions are required for tumor cells to survive and grow in these different environments. Therefore, to respond to and to modulate host microenvironments seem to be the principle capabilities of metastatic tumor cells. Autocrine factors and cell surface receptors of tumor cells obviously play pivotal roles in tumor–host interactions.
Approximately 20 years ago, Lance A. Liotta and his group proposed the three‐step hypothesis of tumor cell invasion, where it was suggested that tumor cells first attach to laminin on a BM, locally degrade type IV collagen in the BM with tumor‐associated proteinases, and then finally migrate into the interstitial stroma.( 4 ) His group also discovered that the stromal ECM protein fibronectin suppresses the metastatic potential of mouse melanoma cells, whereas laminin enhances it. Since their findings, numerous other studies have further revealed details of tumor cell–ECM interactions. In particular, these studies revealed the detailed mechanisms involved in the ECM degradation by tumor cells, showing new members of the ECM‐degrading MMP.( 3 , 5 ) For a long period of time, the ECM was generally regarded as a physical barrier that invading tumor cells have to penetrate.( 3 , 4 ) A recent study has demonstrated that the three‐dimensional ECM structure also blocks tumor cell growth and that expression of MT1‐MMP can rescue the suppressed cell growth.( 6 ) However, it is becoming evident that ECM molecules play a more active role in the control of tumor growth and invasion.
Tumor cell and ECM interactions
ECM molecules, such as collagens, fibronectin, laminins, vitronectin and proteoglycans, not only create tissue architecture but also regulate complex cellular functions by binding specific cell‐surface receptors, most typically integrins. Integrins are large transmembrane proteins consisting of α and β subunits. In mammals, 18 α and eight β subunits associate in different combinations to form at least 24 integrins that bind to distinct ligands. Interactions between ECM molecules and integrins activate many intracellular signal mediators, including FAK, src, PKC, small GTPases, p130CAS, PI3K and MAPKs, resulting in alterations in the actin cytoskeleton and gene expression (Fig. 1).( 7 ) The ECM/integrin‐mediated signaling pathways cooperate with signaling pathways from growth factor receptors to promote cell proliferation and to prevent cell apoptosis. Thus, integrin binding to ECM ligands is essential for cellular adhesion and migration, as well as for cellular survival and proliferation. It is believed that, compared to normal cells, tumor cells are less dependent on adhesion substrates (i.e. less dependent on integrin signaling); this is termed ‘anchorage‐independent cell growth’ or ‘lack of anchorage dependency’. However, many kinds of tumor cells require cell adhesion substrates for sufficient cell growth in vitro. Therefore, the integrin‐mediated signals from cell adhesion substrates support tumor growth and invasion in vivo.
Figure 1.
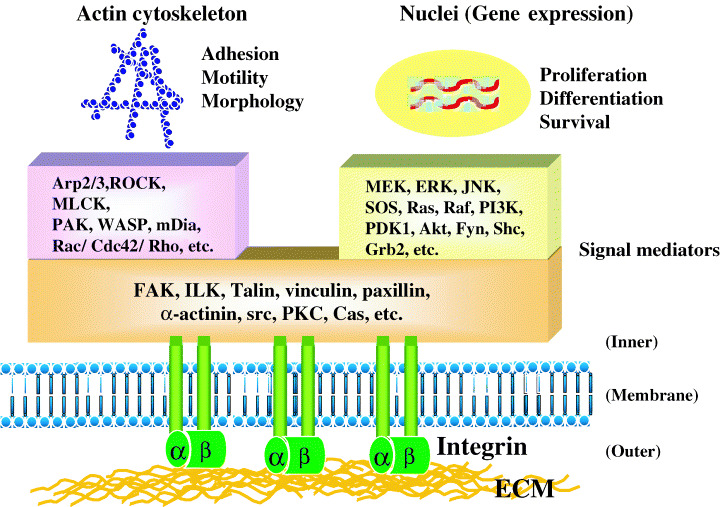
Regulation of cellular functions by integrin signaling. Integrins, when bound to extracellular matrix (ECM) molecules, induce intracellular signaling pathways through scaffold proteins, cytoskeletal proteins, protein kinases and other signal mediators. In concert with the signal transduction from receptor tyrosine kinases (RTK), the integrin‐mediated signaling regulates actin organization and gene expression, leading to changes in cellular functions such as adhesion, motility, morphology, proliferation, apoptosis and differentiation. The activation of two small GTPases, Rac and Cdc42, and phosphatidylinositol‐3‐kinase (PI3K) is particularly important for tumor cell motility and invasion.
Many ECM molecules also regulate tumor cell functions via non‐integrin receptors. For example, laminins interact with syndecans, α‐dystroglycan and a 67‐kDa receptor, besides integrins.( 8 ) Some ECM molecules contain cryptic domains. Limited proteolysis of these ECM proteins releases biologically active fragments that regulate cellular functions. For example, the anti‐angiogenic proteins endostatin and tumostatin are released by limited proteolysis from the BM proteins type XVIII collagen and type IV collagen, respectively.( 9 ) It should be emphasized that a variety of ECM molecules interact with specific cell receptors, thus inducing different intracellular signals and different biological effects.
Laminins are major cell adhesion substrates in epithelial BM, and their interaction with integrins α6β1, α3β1 or α6β4 at the cell surface regulates not only epithelial cell adhesion to the BM but also normal cellular functions such as proliferation, polarity and differentiation. Early stage carcinoma cells or differentiated carcinomas are often surrounded with BM structures and exhibit normal epithelia‐like structures (Fig. 2a).( 10 ) The normal cell‐like morphology and behavior of benign tumor cells are likely dependent on their interaction with laminins, as well as an intercellular interaction via E‐cadherin. When tumor cells invade into the stroma, they change their morphology from an epithelial type to a mesenchymal (or fibroblastic) type (Fig. 2b). This epithelial–mesenchymal transition in cell morphology seems to depend at least in part on the tumor cell interaction with stromal ECM molecules, such as fibronectin, interstitial collagens (e.g. type I, II, III and V collagens) and various kinds of glycosaminoglycans. Stromal growth factors such as TGF‐β, fibroblast growth factors and hepatocyte growth factor also play an important role in the epithelial–mesenchymal transition of tumor cells.( 2 ) A recent model experiment of in vitro cell transformation has shown that the transforming growth factor‐β‐mediated epithelial–mesenchymal transition of mammary epithelial cells is accompanied by the loss of laminin‐5 production and the upregulation of fibronectin and its α5β1 integrin receptor.( 11 ) Another study has shown that type I collagen downregulates expression of the tumor suppressor gene BRCA2 in prostate cancer cells, leading to enhanced cell proliferation.( 12 ) Thus, the migration of tumor cells from the primary tumor site into the interstitial stroma is one of the most critical steps for the malignant conversion and metastasis of tumor cells. This step requires the detachment of tumor cells from the BM laminins, as well as the matrix degradation by proteinases.
Figure 2.
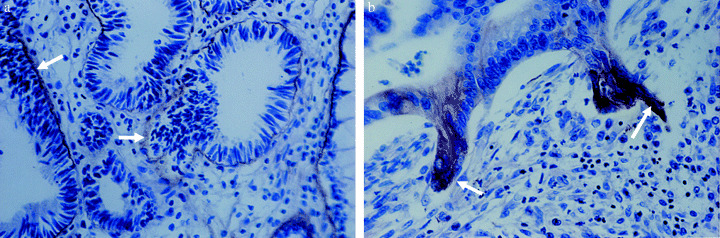
Two distinct types of morphology of well‐differentiated human gastric carcinomas.( 10 ) (a) Tumor cells forming glandular structures surrounded by continuous basement membranes (BM). The laminin‐5 in the BM was immunostained for the laminin γ2 chain (arrows). (b) Tumor cells invading the stroma. Only invading or budding tumor cells show strong intracellular staining for the γ2 chain. Note that invading tumor cells in the right panel have lost the epithelial cell polarity, which is seen in the tumor cells on the neoplastic BM in the left panel. The laminin γ2 chain was immunostained with the anti γ2‐chain monoclonal antibody D4B5.
Regulation of cell adhesion and migration by laminin‐5 (laminin‐332)
Laminins are a family of large glycoproteins present in various types of BM, which plays important roles in both tissue construction and regulation of cellular functions.( 13 ) The laminin molecules all consist of three subunits (or chains) of α, β and γ, linked by disulfide bonds to form the well‐known cross‐shape structure (Fig. 3). To date, more than 15 laminin isoforms with different combinations of the α1–α5, β1–β3 and γ1–γ3 chains have been identified. Each laminin chain contains many functional domains, allowing the laminins to interact with various molecules in the ECM. For example, laminin α chains (α1–α5) contain a large LG domain in their C‐terminus, which is divided into five homologous subdomains (LG1–LG5). The LG domain is thought to be a major site of interaction with specific receptors on the cell surface, including integrins, syndecans and α‐dystroglycan. The N‐terminal region (or short arm) of the three chains contains functional domains that are mainly involved in matrix assembly.( 13 )
Figure 3.
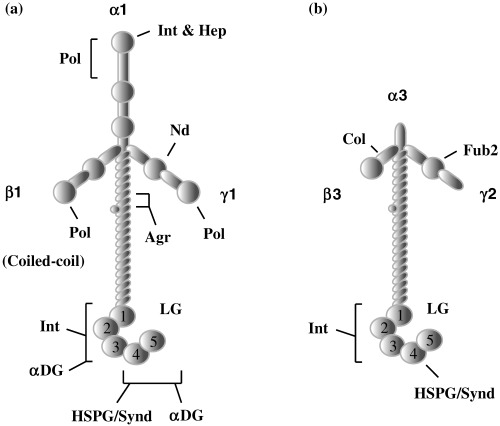
Comparison of domain structures of (a) laminin‐1 and (b) laminin‐5. (a) Laminins containing the α1, α2 and α5 chains have a typical cross‐shaped structure. (b) Several domains present in the short arms of the three laminin‐1 chains are absent in those of the α3, β3 and γ2 chains of laminin‐5. The following letters indicate the functional domains capable of binding to the respective ligands: Agr, agrin; Col, collagen; αDG, α‐dystroglycan; Fub2, fibullin‐2; Hep, heparin; HSPG/Synd, heparansulfate proteoglycans/syndecan; Int, integrin; Nd, nidogen. Pol indicates a site for self‐polymerization.
For a long time, the biological activity of laminin was studied exclusively using mouse laminin‐1, because it can be easily prepared from the mouse EHS tumor. Recently, this laminin was found to be a fetal type of laminin and rarely expressed in adult tissues. It has also become clear that each laminin has a specific biological activity. Of the many laminin isoforms, laminin‐5 (α3β3γ2) is unique in both structure and activity (Fig. 3). Laminin‐5 is a major adhesive component of the epidermal BM.( 14 , 15 ) The short arms of the three laminin‐5 chains are truncated and lack some domains present in other laminins, and the β3 and γ2 chains are found only in laminin‐5. A recent study has characterized a new laminin‐5 isoform, laminin‐5B, that contains a long α3 chain, called α3B.( 16 ) Although laminin‐5B has not been detected in cultured cells, it appears to be expressed in normal tissues at higher levels than laminin‐5 (or laminin‐5A).( 17 ) Very recently, new laminin nomenclature, based on the number of α, β and γ chains, has been proposed.( 18 ) In the new nomenclature, laminin‐1 (α1β1γ), laminin‐5 (α3β3γ2) and laminin‐5B (α3Bβ3γ2) are referred to as laminin‐111, laminin‐332 and laminin‐3B32, respectively. In this article, however, the long‐used name of laminin‐5 is used for laminin‐332.
According to its unique structure, laminin‐5 shows a characteristic biological activity. In 1993, we purified a large cell‐scattering factor with a novel laminin‐like structure from the conditioned medium of human gastric adenocarcinoma cells.( 19 ) This protein, named ladsin, was also secreted by many squamous cell carcinoma lines. Its unique activity and secretion by human cancer cells suggested its possible involvement in tumor metastasis and invasion. Earlier, three other groups found novel keratinocyte‐derived ECM proteins, designating them nicein, kalinin and epiligrin.( 14 , 15 ) Later studies revealed that all of these proteins were an identical laminin isoform and the proteins were collectively given the new name of ‘laminin‐5’. In vitro, laminin‐5 promotes attachment, spreading, scattering and migration of non‐tumorigenic epithelial cells by interacting mainly with integrin α3β1 at far lower concentrations than other cell adhesive proteins (Fig. 4a).( 20 , 21 ) Laminin‐5 also stimulates human tumor cells to form marked lamellipodia (Fig. 4b), leading to enhanced cell migration and invasion in vitro. ( 22 , 23 ) Interaction of laminin‐5 with integrin α3β1 or α6β4 induces intracellular signal transduction to support cellular survival, proliferation and migration by activating many signal mediators such as focal adhesion kinase, protein kinase C, phosphatidylinositol 3‐kinase, Rac, ERK, JNK and nuclear factor κB.( 24 , 25 , 26 ) Such activity of laminin‐5 contrasts with the activity of fibronectin, which induces marked stress fibers and supports stable cell adhesion by activating RhoA via integrin α5β1.( 25 ) Studies with recombinant laminin‐5, in its entirety or in its functional domains, have revealed its structure and function relationship. The major integrin‐binding site is located in the LG3 domain of the α3 chain,( 23 , 27 ) while the short arms of the β3 chain( 28 ) and the γ2 chain,( 29 , 30 ) and the LG4‐5 domain of the α3 chain( 31 ) contain active sites required for the matrix assembly of laminin‐5.
Figure 4.
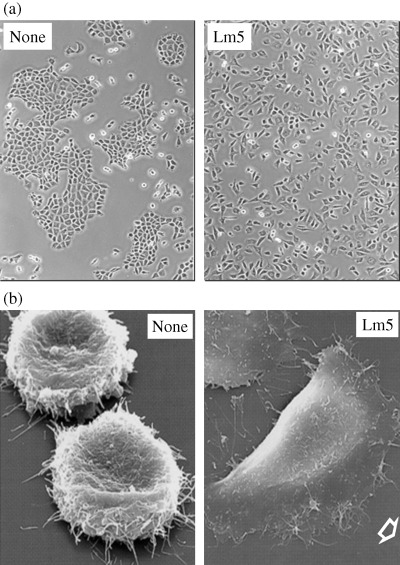
(a) Cell‐scattering activity and (b) cell‐spreading activity of laminin‐5. (a) The rat liver cell line BRL was incubated with (right) or without (left) 60 ng/mL of purified laminin‐5 for 2 days in serum‐free culture.( 19 ) Marked cell scattering is seen with laminin‐5. (b) The human bladder carcinoma cell line EJ‐1 was incubated in a serum‐free medium for 6 h on culture plates precoated with (right) or without (left) 0.3 µg/mL laminin‐5. The cells were fixed and then examined by scanning electron microscopy in collaboration with Dr H. Sawada, Yokohama City University Medical School (unpublished data). EJ‐1 cells can not spread on the non‐coated plate (left), but they rapidly spread and migrate on the laminin‐5 substrate, forming notable lamellipodia (right).
In the epidermal BM, laminin‐5 is a component of the anchoring filaments and plays an essential role in the stable anchorage of basal keratinocytes to the underlying connective tissue.( 14 , 15 ) The association of laminin‐5 with integrin α6β4 is critical to form stable hemidesmosome structures in the skin.( 32 ) Therefore, genomic defects in any of the three laminin‐5 subunits causes the lethal skin disease known as Herlitz's junctional epidermolysis bullosa.( 33 ) When the skin is injured, laminin‐5 is overexpressed by the keratinocytes at the wound edge. The potent cell migration‐promoting activity of laminin‐5 is thought to contribute to wound healing( 24 , 34 ) as well as tumor invasion.( 35 ) However, it remains unclear how laminin‐5 regulates both stable cell adhesion and cell migration.
Much attention has been focused on the proteolytic processing of laminin‐5. Human laminin‐5 is synthesized and secreted as a precursor form consisting of a 190‐kDa α3 chain, a 135‐kDa β3 chain and a 150‐kDa γ2 chain. After secretion, the α3 and γ2 chains undergo specific proteolytic processing to produce a mature form of laminin‐5 containing cleaved α3 and γ2 chains. Initially, Quaranta and his group reported that the proteolytic cleavage of the 150‐kDa γ2 chain of rat laminin‐5 to a 80‐kDa form by gelatinase A (MMP‐2) or MT1‐MMP elevates the cell migration activity of the laminin‐5.( 36 , 37 ) The γ2 chain of human laminin‐5 is cleaved mainly to a 105‐kDa form by astacin‐like metalloproteinase families, which include bone morphogenetic protein‐1 and mammalian Tolloid.( 38 ) A recent study with recombinant laminin‐5 mutants clearly shows that the cleavage of human laminin γ2 chain to the 105‐kDa isoform increases the cell motility activity of laminin‐5 but decreases its cell adhesion activity (Fig. 5).( 30 ) In contrast to the laminin γ2 chain, the 190‐kDa α3 chain is almost completely cleaved between LG3 and LG4 in the C‐terminal LG domain, producing laminin‐5 with the 160‐kDa α3 chain and releasing the LG4‐LG5 fragment with heparin‐binding activity.( 39 ) It was reported that laminin‐5 with the uncleaved α3 chain stimulates cell migration, whereas the cleaved laminin‐5 isoform supports stable cell adhesion.( 34 ) However, recent studies with laminin mutants have demonstrated that the cleavage of the α3 chains in human laminin‐6 (or laminin‐311) and laminin‐5 leads to an enhancement in both cell adhesion and motility activities, that is, activation of the latent or less active forms (Fig. 5).( 31 , 40 ) In addition, it has been shown that the proteolytic cleavage of the γ2 chain( 29 , 30 ) or the α3 chain( 31 ) impairs the ability of laminin‐5 to deposit onto, or to be assembled into, the ECM. The β3 chain is relatively resistant to proteolysis, but it has been shown to be partially cleaved at the short arm in normal keratinocyte cultures as well as some cancer cell lines.( 28 ) The β3 chain cleavage leads to a decrease in cell adhesion activity and the complete loss of the type VII collagen‐binding activity of laminin‐5.
Figure 5.
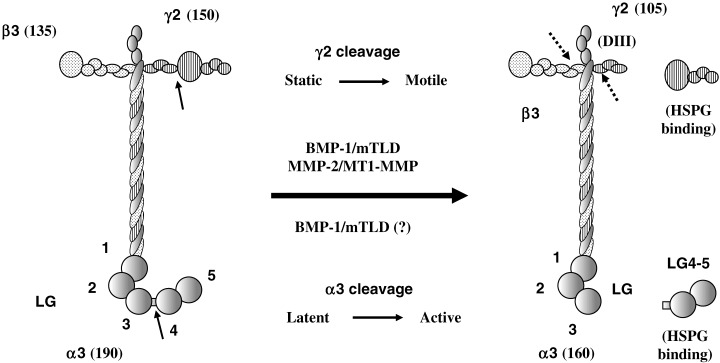
Regulation of activities of laminin‐5 by proteolytic processing of α3 and γ2 chains. In many cultures, the α3 chain is almost completely cleaved between the LG3 and LG4 domains, while the γ2 chain is partially cleaved at domain III to produce the 105‐kDa chain. Arrows in the left model indicate the cleavage sites. The processing of the α3 chain converts a less active laminin‐5 to an active form regarding both adhesion and motility activities. The processing of the γ2 chain converts laminin‐5 from a static adhesion state to a migratory state. The α3 and γ2 chain fragments released from laminin‐5 by the proteolytic cleavages, both of which contain a heparin‐binding site, modulate cellular adhesion and migration independently or in concert with processed laminin‐5.( 39 , 40 In rat laminin‐5, the 105‐kDa γ2 chain is further cleaved by MT‐MMP at the site shown by a dotted arrow to produce an 80‐kDa γ2 chain and a 30‐kDa domain III fragment.( 37 ) Another dotted arrow indicates a proteolytic cleavage of the β3 chain, which occurs far less frequently than that of the γ2 chain.( 28 )
The above‐mentioned studies demonstrate that the biological activity of laminin‐5 is regulated by the proteolytic processing of the three chains. However, it is not reasonable to explain the conversion of laminin‐5 from the stable cell adhesion state to the migratory state, or vice versa, only by its proteolytic processing, as laminin‐5 with an unprocessed γ2 chain and a processed α3 chain still exhibits high cell motility activity compared to other laminins.( 30 ) A recent study has shown that a soluble form of laminin‐5 is able to stimulate cell migration by binding to integrin α3β1 on the cell surface.( 25 ) Such activity is not seen in other laminins or in other cell adhesion proteins. In vivo, laminin‐5 is assembled into the hemidesmosome structures of BM and supports stable cell adhesion. When the BM structure is broken by injury or proteolysis, cells are stimulated to migrate. Based on these facts, it is speculated that the laminin‐5 assembled into the BM supports stable cell adhesion, whereas unassembled laminin‐5, like growth factors, stimulates active cell migration (Fig. 6). The proteolytic cleavage of laminin‐5 chains is likely to prevent laminin‐5 from its matrix assembly and keep it in an active state for cell migration.
Figure 6.
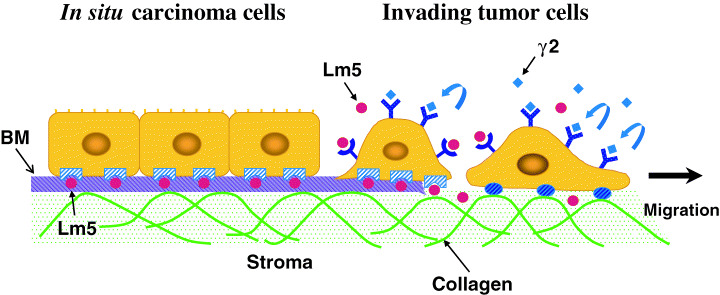
A model for regulation of tumor cell migration by laminin‐5 and its γ2 chain fragments. In situ carcinoma cells often deposit laminin‐5 onto the underlying basement membrane (BM) structures (left side) (also see the left panel of Fig. 2). The laminin‐5 (red circles) assembled into the BM matrix stably anchors these cells to the BM through interaction with integrin α6β4. When the BM structures are not synthesized or disrupted, tumor cells are able to migrate into interstitial stroma (right side). Tumor cells at the invasion front overexpress the laminin γ2 chain monomer rather than the laminin‐5 trimer (see the right panel of Fig. 2). Proteolytic fragments of the laminin γ2 monomer (blue diamonds) may promote the tumor cell invasion by binding to EGF receptor and other unidentified receptors.( 52 ) It is also possible that laminin‐5 that has not been assembled into the BM structure stimulates tumor cell invasion as a soluble ligand.
Role of laminin‐5 in tumor growth and invasion
As described above, laminin‐5 promotes cell migration as a soluble factor, as well as an insoluble substrate. Expression of laminin‐5 in tumor cells is stimulated by growth factors and a tumor promoter in vitro. ( 17 ) Forced expression of laminin‐5 promotes the growth of human tumor cells in nude mice.( 41 ) Interaction of laminin‐5 with type VII collagen plays an important role in the development of skin cancers.( 42 ) Interaction of integrin α3β1 with vascular laminin‐5 mediates pulmonary arrest and metastasis.( 43 ) Furthermore, there are a number of studies showing that two laminin‐5 receptors, integrins α3β1 and α6β4, are associated with the malignant behavior of tumor cells.( 44 ) All of these studies strongly support the hypothesis that laminin‐5 expression in cancer cells promotes their growth, invasion and metastasis.
Many immunohistochemical studies have shown that laminin‐5 or its subunits are highly expressed in various types of human cancers. In particular, the laminin γ2 chain is expressed in tumor cells at the invasion front or in budding tumor cells in many types of human cancers such as adenocarcinomas of the colon, breast, pancreas and lung, squamous cell carcinomas, and melanomas.( 35 , 45 , 46 ) However, some other studies have shown that expression of laminin‐5 is reduced during the progression of human carcinomas, and its expression is associated with lower invasive and metastatic activity.( 47 , 48 ) This discrepancy seems to have arisen from the fact that most studies have analyzed only the γ2 chain in order to detect the laminin‐5 protein. Our immunohistochemical studies, using three separate antibodies against the α3, β3 or γ2 chains, have demonstrated that in adenocarcinomas of the stomach( 10 ) and lung( 49 ) well‐differentiated carcinoma cells often deposit laminin‐5 on the neoplastic BM, but carcinoma cells invading into the underlying stroma strongly express only the γ2 chain and accumulate it intracellularly (Fig. 2). As the α3 and β3 chains are scarcely detected in budding or invading tumor cells, the γ2 chain is thought to be solely overexpressed in the tumor cells. It has also been found that some cancer cell lines secrete the γ2 chain as a monomer in vitro. ( 10 ) Many other studies have shown strong immunostaining for the γ2 chain in invading tumor cells. The laminin γ2 chain is now regarded as one of the most typical invasion markers. The β‐catenin (Wnt) signaling pathway is known to induce a coordinate expression of the laminin γ2 chain and MT1‐MMP in colorectal carcinomas.( 50 )
What is the significance of the laminin γ2 chain expression in tumor invasion? In many cases, in situ carcinomas progress to invasive carcinomas. The BM structures surrounding or supporting tumor cell clusters are correlated with a better prognosis, while the lack or discontinuity of these BM structures is one of the important prognostic factors.( 51 ) BM structures can be disrupted by the failure of tumor cells to make BM components, as well as by their proteolytic degradation. These changes are expected to allow tumor cells to invade into interstitial space (Fig. 6). Overexpression of the laminin γ2 chain by tumor cells, as well as lowered or impaired expression of the laminin α3 and/or β3 chains, may contribute to the loss of BM structures in invasive carcinomas, as laminin‐5 is an important BM component (Fig. 2b). There is another possibility, that the laminin γ2 chain monomer itself enhances tumor invasion. As the laminin γ2 chain does not contain any integrin‐binding sites, it does not support cellular adhesion. However, a recent study has shown that domain III (or the laminin epidermal growth factor‐like domain LE) of the laminin γ2 chain, which can be released from the γ2 short arm by cleavage at two separate sites by MT1‐MMP or MMP‐2, is able to stimulate cell migration by binding to the epidermal growth factor receptor.( 52 ) This suggests that the γ2 fragment may support tumor cell invasion into the stroma (Fig. 6). The activity of the γ2 fragment may also contribute to cell migration in other pathological and physiological conditions. Further studies are needed to clarify whether this mechanism occurs in vivo.
Conclusion
Studies with purified laminin‐5 have shown that it efficiently promotes cellular adhesion and migration through binding to integrin α3β1. Development of recombinant laminin‐5 expression systems have revealed its structure and function relationship. Furthermore, it has been shown that proteolytic cleavage of the three laminin‐5 chains modulates the biological activity of laminin‐5. The cleavage of laminin‐5 also prevents its matrix assembly, leaving laminin‐5 as an active cell motility factor. MMP and other matrix proteinases are often overexpressed in tumor microenvironments. Thus, coordinated action between laminin‐5 and matrix proteinases appears to be important for enhanced cell migration in tumor tissues. Immunohistochemical studies have shown that in situ carcinomas often deposit laminin‐5 on neoplastic BM structures, while tumor cells infiltrating into stromal tissues and budding tumor cells overexpress the laminin γ2 chain monomer. The failure of laminin‐5 deposition is expected to enhance the dissemination of tumor cells from the original tumor nest and their epithelial–mesenchymal transition. Proteolytic fragments of the γ2 chain may stimulate tumor cell invasion. These possibilities should be verified in future studies.
Acknowledgments
Our studies described in this article were performed in collaboration with Drs Y. Kariya, Y. Tsubota, T. Ogawa, Y. Nakashima, J. Hashimoto, T. Hirosaki, H. Mizushima, Y. Kikkawa, N. Koshikawa, H. Yasumitsu, Y. Miyagi, and many others. I am grateful to all of these collaborators.
References
- 1. Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nature 2001; 411: 375–9. [DOI] [PubMed] [Google Scholar]
- 2. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004; 432: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000; 18: 1135–49. [DOI] [PubMed] [Google Scholar]
- 4. Liotta LA. Tumor invasion and metastases − role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res 1986; 46: 1–7. [PubMed] [Google Scholar]
- 5. Sato H, Takino T, Okada Y et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994; 370: 61–5. [DOI] [PubMed] [Google Scholar]
- 6. Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three‐dimensional extracellular matrix. Cell 2003; 114: 33–45. [DOI] [PubMed] [Google Scholar]
- 7. Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004; 5: 816–26. [DOI] [PubMed] [Google Scholar]
- 8. Givant‐Horwitz V, Davidson B, Reich R. Laminin‐induced signaling in tumor cells. Cancer Lett 2005; 223: 1–10. [DOI] [PubMed] [Google Scholar]
- 9. Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol 2003; 13: 366–75. [DOI] [PubMed] [Google Scholar]
- 10. Koshikawa N, Moriyama K, Takamura H et al. Overexpression of laminin γ2 chain monomer in invading gastric carcinoma cells. Cancer Res 1999; 59: 5596–601. [PubMed] [Google Scholar]
- 11. Maschler S, Wirl G, Spring H et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 2005; 24: 2032–41. [DOI] [PubMed] [Google Scholar]
- 12. Moro LD, Rabin RA, Mara B, Greco M. Down‐regulation of BRCA2 expression by collagen type I promotes prostate cancer cell proliferation. J Biol Chem 2005; 280: 22 482–91. [DOI] [PubMed] [Google Scholar]
- 13. Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn 2000; 218: 213–34. [DOI] [PubMed] [Google Scholar]
- 14. Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium‐specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol 1991; 114: 567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3 β1 in epithelial basement membranes. Cell 1991; 65: 599–610. [DOI] [PubMed] [Google Scholar]
- 16. Kariya Y, Yasuda C, Nakashima Y, Ishida K, Tsubota Y, Miyazaki K. Characterization of laminin‐5B (α3Bβ3γ2) and NH2‐terminal proteolytic fragment of its α3B chain: Promotion of cellular adhesion, migration and proliferation. J Biol Chem 2004; 279: 24 774–84. [DOI] [PubMed] [Google Scholar]
- 17. Mizushima H, Miyagi Y, Kikkawa Y et al. Differential expression of laminin‐5/ladsin subunits in human tissues and cancer cell lines and their induction by tumor promoter and growth factors. J Biochem 1996; 120: 1196–202. [DOI] [PubMed] [Google Scholar]
- 18. Aumailley M, Bruckner‐Tuderman L, Carter WG et al. A simplified laminin nomenclature. Matrix Biol 2005; 24: 326–32. [DOI] [PubMed] [Google Scholar]
- 19. Miyazaki K, Kikkawa Y, Nakamura A, Yasumitsu H, Umeda M. A large cell‐adhesive scatter factor secreted by human gastric carcinoma cells. Proc Natl Acad Sci USA 1993; 90: 11 767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kikkawa Y, Umeda M, Miyazaki K. Marked stimulation of cell adhesion and motility by ladsin, a laminin‐like scatter factor. J Biochem 1994; 116: 862–9. [DOI] [PubMed] [Google Scholar]
- 21. Rousselle P, Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J Cell Biol 1994; 125: 205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukushima Y, Ohnishi T, Arita N, Hayakawa T, Sekiguchi K. Integrin α3β1‐mediated interaction with laminin‐5 stimulates adhesion, migration and invasion of malignant glioma cells. Int J Cancer 1998; 76: 63–72. [DOI] [PubMed] [Google Scholar]
- 23. Kariya Y, Tsubota Y, Hirosaki T et al. Differential regulation of cellular adhesion and migration by recombinant laminin‐5 forms with partial deletion or mutation within the G3 domain of α3 chain. J Cell Biochem 2003; 88: 506–20. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen BP, Gil SG, Carter WG. Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J Biol Chem 2000; 275: 31 896–907. [DOI] [PubMed] [Google Scholar]
- 25. Kariya Y, Miyazaki K. The basement membrane protein laminin‐5 acts as a soluble cell motility factor. Exp Cell Res 2004; 297: 508–20. [DOI] [PubMed] [Google Scholar]
- 26. Nikolopoulos SN, Blaikie P, Yoshioka T et al. Targeted deletion of the integrin β4 signaling domain suppresses laminin‐5‐dependent nuclear entry of mitogen‐activated protein kinases and NF‐κB, causing defects in epidermal growth and migration. Mol Cell Biol 2005; 25: 6090–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirosaki T, Mizushima H, Tsubota Y, Moriyama K, Miyazaki K. Structural requirement of carboxyl‐terminal globular domains of laminin α3 chain for promotion of rapid cell adhesion and migration by laminin‐5. J Biol Chem 2000; 275: 22 495–502. [DOI] [PubMed] [Google Scholar]
- 28. Nakashima Y, Kariya Y, Yasuda C, Miyazaki K. Regulation of cell adhesion and type VII collagen binding by β3 chain short arm of laminin‐5: effect of its proteolytic cleavage. J Biochem 2005; 138: 539–52. [DOI] [PubMed] [Google Scholar]
- 29. Gagnoux‐Palacios L, Allegra M, Spirito F et al. The short arm of the laminin γ2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J Cell Biol 2001; 153: 835–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogawa T, Tsubota Y, Maeda M, Kariya Y, Miyazaki K. Regulation of biological activity of laminin‐5 by proteolytic processing of γ2 chain. J Cell Biochem 2004; 92: 701–14. [DOI] [PubMed] [Google Scholar]
- 31. Tsubota Y, Yasuda C, Kariya Y et al. Regulation of biological activity and matrix assembly of laminin‐5 by COOH‐terminal, LG4‐5 domain of α3 chain. J Biol Chem 2005; 280: 14 370–7. [DOI] [PubMed] [Google Scholar]
- 32. Baker SE, Hopkinson SB, Fitchmun M et al. Laminin‐5 and hemidesmosomes: role of the α3 chain subunit in hemidesmosome stability and assembly. J Cell Sci 1996; 109: 2509–20. [DOI] [PubMed] [Google Scholar]
- 33. Kivirikko S, McGrath JA, Baudoin C et al. A homozygous nonsense mutation in the α3 chain gene of laminin 5 (LAMA3) in lethal (Herlitz) junctional epidermolysis bullosa. Hum Mol Genet 1995; 4: 959–62. [DOI] [PubMed] [Google Scholar]
- 34. Goldfinger LE, Stack MS, Jones JC. Processing of laminin‐5 and its functional consequences: role of plasmin and tissue‐type plasminogen activator. J Cell Biol 1998; 141: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin‐5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res 1995; 55: 4132–9. [PubMed] [Google Scholar]
- 36. Giannelli G, Falk‐Marzillier J, Schiraldi O, Stetler‐Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease‐2 cleavage of laminin‐5. Science 1997; 277: 225–8. [DOI] [PubMed] [Google Scholar]
- 37. Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1‐MMP in epithelial cell migration over laminin‐5. J Cell Biol 2000; 148: 615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veitch DP, Nokelainen P, McGowan KA et al. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin‐5 in keratinocytes and skin. J Biol Chem 2003; 278: 15 661–8. [DOI] [PubMed] [Google Scholar]
- 39. Tsubota Y, Mizushima H, Hirosaki T, Higashi S, Yasumitsu H, Miyazaki K. Isolation and activity of proteolytic fragment of laminin‐5 α3 chain. Biochem Biophys Res Commun 2000; 278: 614–20. [DOI] [PubMed] [Google Scholar]
- 40. Hirosaki T, Tsubota Y, Kariya Y, Moriyama K, Mizushima H, Miyazaki K. Laminin‐6 is activated by proteolytic processing and regulates cellular adhesion and migration differently from laminin‐5. J Biol Chem 2002; 277: 49 287–95. [DOI] [PubMed] [Google Scholar]
- 41. Mizushima H, Hirosaki T, Miyata S, Takamura H, Miyagi Y, Miyazaki K. Expression of laminin‐5 enhances tumorigenicity of human fibrosarcoma cells in nude mice. Jpn J Cancer Res 2002; 93: 652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ortiz‐Urda S, Garcia J, Green CL et al. Type VII collagen is required for Ras‐driven human epidermal tumorigenesis. Science 2005; 307: 1773–6. [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Fu W, Im JH et al. Tumor cell α3β1 integrin and vascular laminin‐5 mediate pulmonary arrest and metastasis. J Cell Biol 2004; 164: 935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giannelli G, Astigiano S, Antonaci S et al. Role of the α3β1 and α6β4 integrins in tumor invasion. Clin Exp Metastasis 2002; 19: 217–23. [DOI] [PubMed] [Google Scholar]
- 45. Sordat I, Bosman FT, Dorta G et al. Differential expression of laminin‐5 subunits and integrin receptors in human colorectal neoplasia. J Pathol 1998; 185: 44–52. [DOI] [PubMed] [Google Scholar]
- 46. Ono Y, Nakanishi Y, Ino Y et al. Clinicopathologic significance of laminin‐5 γ2 chain expression in squamous cell carcinoma of the tongue. Cancer 1999; 85: 2315–21. [PubMed] [Google Scholar]
- 47. Martin KJ, Kwan CP, Nagasaki K et al. Down‐regulation of laminin‐5 in breast carcinoma cells. Mol Med 1998; 4: 602–13. [PMC free article] [PubMed] [Google Scholar]
- 48. Soini Y, Määttä M, Salo S, Tryggvason K, Autio‐Harmainen H. Expression of the laminin γ2 chain in pancreatic adenocarcinoma. J Pathol 1996; 180: 290–4. [DOI] [PubMed] [Google Scholar]
- 49. Kagesato Y, Mizushima H, Koshikawa N et al. Sole expression of laminin γ2 chain in invading tumor cells and its association with stromal fibrosis in lung adenocarcinomas. Jpn J Cancer Res 2001; 92: 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T. Beta‐catenin activates a coordinated expression of the proinvasive factors laminin‐5 gamma2 chain and MT1‐MMP in colorectal carcinomas. Int J Cancer 2004; 108: 321–6. [DOI] [PubMed] [Google Scholar]
- 51. Nishino T, Ishida T, Oka T, Yasumoto K, Sugimachi K. Prognostic significance of laminin in adenocarcinoma of the lung. J Surg Oncol 1990; 43: 214–18. [DOI] [PubMed] [Google Scholar]
- 52. Schenk S, Hintermann E, Bilban M et al. Binding to EGF receptor of a laminin‐5 EGF‐like fragment liberated during MMP‐dependent mammary gland involution. J Cell Biol 2003; 161: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]


