Abstract
Extranodal NK/T‐cell lymphoma (NTCL) is characterized by clinical heterogeneity based on clinical prognostic factors and survival outcome. NTCL subsets are classified as upper aerodigestive tract (UAT) NTCL or non‐UAT NTCL; non‐UAT has pathologic similarity to UAT‐NTCL but is a clinically distinct subtype. Due to the clinical heterogeneity of NTCL, optimal treatment modalities and prognostic factors have been difficult to determine. Ann Arbor staging for lymphomas and the International Prognostic Index (IPI) have been used to predict prognosis for UAT‐NTCL; however, local tumor invasiveness (bony invasion or perforation or invasion of the overlying skin) is the most significant factor for poor outcomes in localized UAT‐NTCL. Thus, a new staging system is proposed: limited disease (stage I/II UAT‐NTCL without local tumor invasiveness) and extensive disease (stage I/II with local invasiveness or stage III/IV disease of UAT NTCL, and non‐UAT NTCL) based on treatment outcomes. NTCL is resistant to anthracycline‐based chemotherapy, whereas non‐anthracycline combination chemotherapy (such as ifosfamide, methotrexate, etoposide, and prednisolone) has an activity against NTCL as either a front‐line or as a second‐line treatment. The effectiveness of radiotherapy is evident in limited disease, but questionable in extensive disease. (Cancer Sci 2009; 100: 2242–2248)
Extranodal natural killer (NK)/T‐cell lymphoma, nasal type (NTCL) is characterized by angioinvasion, prominent necrosis, a cytotoxic phenotype and a strong association with Epstein–Barr virus (EBV).( 1 , 2 ) The Hong Kong workshop participants initially defined the clinical, phenotypic, and genotypic features of NTCL as having a distinct clinicopathologic entity,( 3 ) which was clarified by World Health Organization (WHO) classification.( 1 , 2 ) NTCL frequently destroys the facial midline of the upper aerodigestive tract (UAT) and spreads to or relapses at extranodal sites including skin, gastrointestinal tract, bone marrow, lung, extremities, orbit, adrenal gland, testis, or the central nervous system.( 1 , 2 , 3 ) NTCL is very rare in Western populations but relatively common among Asians, Mexicans, and South Americans of American Indian descent.( 3 , 4 ) It is also common in Korea, with a marked preponderance of the NK cell phenotype, comprising up to 8.7% to 10.5% of all non‐Hodgkin’s lymphomas (NHLs) and 74.1% of lymphomas arising in the nasal cavity and paranasal sinuses.( 5 , 6 ) Due to the clinical heterogeneity and various prognostic factors of NTCL,( 7 , 8 , 9 , 10 ) subtypes have been variously described as: nasal versus nasal‐type,( 1 , 3 ) nasal versus non‐nasal,( 11 ) UAT versus non‐UAT,( 7 ) and nasal (UAT) versus extranasal.( 2 , 9 ) One group attempted to further divide UAT disease into nasal and Waldeyer ring NTCL based on a clinical heterogeneity of this subtype.( 10 ) In this review, we focus on the subsets based on treatment outcomes.
Subtypes of NK/T‐Cell Lymphoma
Nasal NTCLs were initially defined as those involving the nasal region, while nasal‐type (or extranasal) NTCLs were defined as those involving the entire UAT as well as non‐UAT regions, including the skin, gastrointestinal tract, and testis.( 1 , 3 ) Differences between clinical prognostic factors were not observed between nasal and nasal‐type UAT lymphoma, whereas treatment outcomes and clinical parameters differed significantly between UAT and non‐UAT lymphomas (Fig. 1 and Table 1).( 12 , 13 ) Although NTCL subtypes share identical morphology, phenotype, and genotype,( 1 , 2 , 3 ) clinical differences were observed during the International Peripheral T‐cell Lymphoma Project( 9 ) and the Korean Cancer Study Group study.( 7 )
Figure 1.
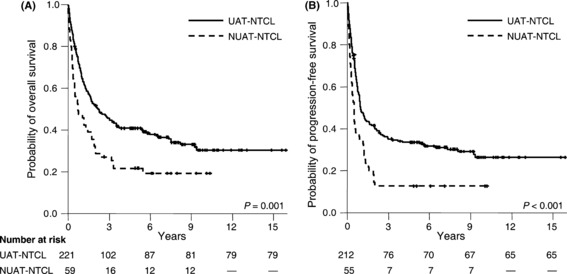
Kaplan–Meier plots of (A) overall survival and (B) progression‐free survival according to the disease subtypes.
Table 1.
Comparison of clinical parameters between the NK/T‐cell lymphoma subtypes
| Korean Cancer Study Group | International Peripheral T‐cell Lymphoma Project | |||||
|---|---|---|---|---|---|---|
| UAT | Non‐UAT | P | Nasal | Extranasal | P | |
| Patients, number (%) | 221 (79) | 59 (21) | 92 (72) | 35 (28) | ||
| Male, % | 67 | 59 | 0.244 | 64 | 66 | 1.000 |
| Median age, years | 47 | 42 | 0.199 | 52 | 45 | 0.670 |
| Ann Arbor stage III/IV, % | 15 | 59 | <0.001 | 27 | 68 | <0.001 |
| Number of extranodal sites ≥ 2 | 17 | 44 | <0.001 | 16 | 55 | <0.001 |
| Elevated LDH, % | 38 | 66 | <0.001 | 45 | 60 | 0.009 |
| Hemophagocytosis, % | 7 | 12 | 0.188 | 3 | 3 | 1.000 |
| B symptoms, % | 38 | 58 | 0.007 | 39 | 54 | 0.161 |
| ECOG performance status ≥2, % | 18 | 42 | <0.001 | 9 | 37 | <0.001 |
| Bone marrow involvement, % | 3 | 22 | <0.001 | 10 | 14 | 0.530 |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; UAT, upper aerodigestive tract.
Upper aerodigestive tract NK/T‐cell lymphoma. UAT‐NTCL was defined as a primary tumor involving nasal UAT (nasal cavity or nasopharynx) or the extra‐nasal UAT (oral cavity, oropharynx, or hypopharynx), regardless of dissemination to other sites. Comparable survival outcomes were observed between nasal and extra‐nasal UAT‐NTCLs (5‐year overall survival [OS] 41%vs 41%, respectively; and 5‐year progression‐free survival [PFS] 33%vs 38%, respectively), whereas regional lymphadenopathy and advanced stage were significantly higher in patients with extra‐nasal UAT‐NTCL than in those with nasal UAT‐NTCL. Hemophagocytosis, a common complication in NTCL (2–8% of patients),( 7 , 14 , 15 ) reduced survival time in patients with UAT‐ and non‐UAT‐NTCL.
Recently, Li et al. ( 8 ) revealed distinct clinical features of Waldeyer ring‐NTCL and compared clinical presentations of nasal and Waldeyer ring‐NTCLs.( 10 ) Patients with Waldeyer ring‐NTCL demonstrated a higher propensity for nodal involvement, advanced stage disease, intermediate chemosensitivity, and favorable prognosis. In particular, nodal involvement was found to be significantly higher in Waldeyer ring lymphoma (80–82%) compared to nasal NTCL (17%) in Chinese studies.( 8 , 10 ) These findings differ somewhat from results of other studies, which found that 17% to 46% of NTCLs exhibited regional lymph node involvement.( 7 , 16 , 17 , 18 , 19 ) Nevertheless, nasal and Waldeyer ring lymphomas still represent a clinical heterogeneity within the UAT‐NTCL category,( 10 ) thus, for a risk‐stratified treatment approach, it is crucial to classify subtypes based on the prognostic factors.
Non‐upper aerodigestive tract NK/T‐cell lymphoma. Non‐UAT (NUAT)‐NTCL corresponds to a primary tumor outside the UAT, in places such as the skin, gastrointestinal tract, bone marrow, lung, extremities, orbit, adrenal gland, testis, or central nervous system.( 7 ) NUAT‐NTCL is associated with significantly higher proportions of advanced stage disease, two or more extranodal sites, elevated lactate dehydrogenase (LDH) levels, B symptoms (e.g., fever, night sweats, weight loss), and poor performance status (Table 1).( 7 ) Similar findings were obtained from a nationwide survey in Japan( 20 ) and from the International Peripheral T‐cell Lymphoma Project.( 9 ) Korean patients with NUAT‐NTCL exhibited more B symptoms and bone marrow involvement than those with UAT‐NTCL; however, the International Peripheral T‐cell Lymphoma Project did not find these differences. Although advanced stage was associated with survival time in a few studies,( 19 , 21 ) other studies failed to demonstrate the predictive value of the Ann Arbor staging system,( 7 , 9 , 22 ) and the Ann Arbor stages III/IV were not significantly associated with shortened survival time in NUAT disease in our study.( 7 ) The IPI score also failed to distinguish between low‐intermediate and high‐intermediate risk groups in NUAT disease (2‐year OS 25%vs 36%, respectively; P = 0.520).( 7 ) Furthermore, the IPI score was not found to affect survival of extranasal NTCL in the International Peripheral T‐cell Lymphoma Project (IPI score 0–1 vs IPI score 4–5 for 5‐year OS, 17%vs 20%).( 23 )
Although still used in the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues,( 2 ) the term ‘nasal‐type or extranasal’ was originally defined by the Hong Kong workshop( 3 ) and included in the 2001 WHO classification( 1 ) is ambiguous (Fig. 2). That is, extranasal NTCL refers to either a tumor outside the nasal region according to the 2001 WHO classification or a tumor outside the UAT according to the 2008 WHO classification. Consequently, other designations (e.g., NUAT) are appropriate than ‘extranasal or nasal‐type’ NTCL. Taken together, these findings demonstrate that NUAT NTCL is a distinct subtype compared to UAT‐NTCL.
Figure 2.
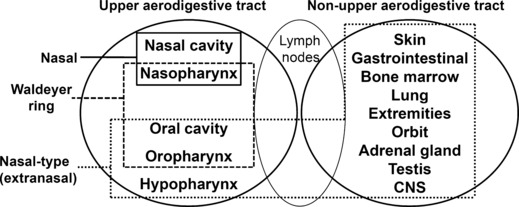
Anatomic boundary of NK/T‐cell lymphoma.
Prognostic Factors of Upper Aerodigestive Tract NK/T‐Cell Lymphoma
Ann Arbor staging. The utility of Ann Arbor staging as a predictor of prognosis in nasal lymphoma is unclear. In early reports regarding nasal lymphoma, multivariate analysis revealed that only Ann Arbor stage I was positively associated with survival (5‐year OS, 66%); however, immunophenotyping was performed only in 45% of patients (T‐cell, 78%).( 16 ) A study of NHL of the nose and nasopharynx also showed that Ann Arbor stage was an independent prognostic factor for survival (NK/T‐cell, 45%).( 17 ) Similarly, Ann Arbor stage was a significant factor for survival in patients with early‐stage nasal NTCL (5‐year OS, 42–78% for stage I and 19–46% for stage II).( 14 , 24 ) Furthermore, univariate analysis revealed that advanced stage had marginal significance as a predictor of survival.( 9 ) However, an Italian survey found no significant differences between early‐stage and advanced diseases in the complete remission (CR) rate or in survival rates.( 25 ) In addition, the Ann Arbor staging system did not predict survival in Korean patients with early‐stage disease (5‐year OS 56% for stage I vs 44% for stage II).( 26 , 27 ) This finding was consistent with results from patients with early‐stage UAT‐NTCL by Korean multicenter study.( 7 ) Because infiltrative nature is not considered in Ann Arbor staging system, the predictive role of the staging system is limited in early‐stage NTCL with extensively destructive lesions.
International prognostic index (IPI). Although the IPI failed to predict survival in NTCL patients in a few studies,( 14 , 27 , 28 ) most studies showed that low IPI score was associated with good prognosis for survival.( 7 , 9 , 24 , 26 , 29 , 30 ) Low IPI scores (≤1) were significantly associated with improved CR rate (77%vs 36%, P = 0.017) and 10‐year OS (64%vs 27%, P = 0.003) compared to high IPI score (≥2) in nasal NTCL patients who received anthracycline‐based chemotherapy.( 29 ) Additionally, the IPI score or stage‐modified IPI score independently predicted survival in early‐stage UAT‐NTCL.( 24 , 26 ) Although the IPI score was able to identify four distinct risk groups with different median OS rates, it could not discriminate between high‐intermediate and high‐risk groups with UAT‐NTCL (2‐year OS 9%vs 17%, respectively; P = 0.361).( 7 ) Furthermore, the distribution of patients was unbalanced, with most having low IPI scores, suggesting that including the IPI score in a prognostic model should be done cautiously.( 19 , 20 , 26 )
Local tumor invasiveness. Paranasal extension based on T stage was initially identified as a prognostic factor in stage I/II lymphomas of nasal cavity and paranasal sinuses.( 31 ) Similarly, Logsdon et al. ( 32 ) reclassified stage I lymphomas of nasal cavity and paranasal sinuses into T stages based on the extent of disease, and demonstrated that T stage is the strongest predictor of outcome. Paranasal extension in NTCL was found to be a significant predictive factor for early‐stage disease;( 24 , 30 ) although one study showed no prognostic significance of paranasal extension in stage I NTCL.( 14 ) In another study, 21% of patients presented with local tumor invasiveness and all of these patients died earlier (median OS, 10.6 months; range, 1.4–64.8 months); local tumor invasiveness was defined as bony invasion or perforation or invasion of the overlying skin (Fig. 3A–B).( 26 ) Presence of local invasiveness reduced survival (5‐year OS 4%vs 68%, P < 0.001 [Fig. 3C]; and 1‐year disease‐free survival 13%vs 68%, P < 0.001 [Fig. 3D]) in both the combined modality group and in the chemotherapy only group. Local tumor invasiveness has been shown to be the most important prognostic factor in predicting low probability of CR and reduced survival in stage I/II UAT‐NTCL.( 26 ) Therefore, local invasiveness should be taken into consideration to the selection of treatment modalities in early‐stage UAT‐NK/T‐cell lymphoma.
Figure 3.
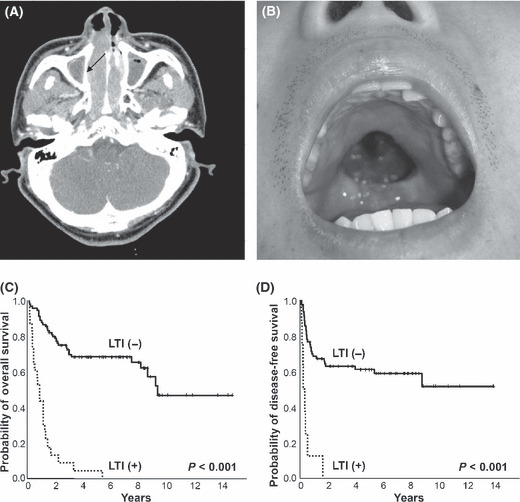
Local tumor invasiveness (LTI) included (A) thinning of right medial wall of maxillary bone (arrow) in CT of paranasal sinuses and (B) palatal perforation. Kaplan–Meier plots of (C) overall survival and (D) disease‐free survival according to the local tumor invasiveness.
NTCL prognostic index. A Korean retrospective study developed a prognostic model for NTCL based on the following adverse factors: B symptoms, Ann Arbor stage, LDH level, and regional lymphadenopathy.( 19 ) Four distinct risk groups were identified: group 1, no adverse factors (5‐year OS, 81%); group 2, one factor (5‐year OS, 64%); group 3, two factors (5‐year OS, 34%); and group 4, three or more factors (5‐year OS, 7%). This model was validated only in UAT‐NTCL patients, not in non‐UAT disease in the Korean Cancer Study Group survey( 7 ) and International Peripheral T‐cell Lymphoma Project.( 9 ) Because multivariate analysis determined independent predictors for survival differed between each subtype,( 7 ) a prognostic model should be developed for each subtype.
Tumor biologic and microenvironmental factors. High Ki‐67 nuclear antigen (≥65%) is a marker for actively proliferating tumor cells and is correlated with tumor bulk and adverse survival outcomes in stage I/II UAT‐NTCL.( 27 ) Patients with nasal UAT‐NTCL and high Ki‐67 expression demonstrated worse survival outcomes. Histologically, high Ki‐67 proliferation (>50%) or transformed tumor cells (>40%) had prognostic significance for survival in nasal NTCL.( 9 )
Because cyclooxygenase‐2 (COX‐2) promotes tumor proliferation, growth, and metastasis, its expression was also evaluated in early‐stage NTCL.( 33 ) COX‐2 expression by tumor cells was associated with significantly lower 2‐year disease‐free survival rates and 2‐year systemic relapse‐free survival rates. However, COX‐2 positivity was not a significant predictor of 5‐year OS (28%vs 47%, P = 0.060).( 33 )
NK cell receptors, including CD94, NKG2, and killer immunoglobulin‐like receptors, have also been investigated as potential prognostic factors. Lin et al. ( 34 ) found that patients positive for CD94 expression more often survived beyond 1 year compared with those negative for CD94 expression (median OS 60 months vs 10 months, respectively; P = 0.026). Expression of anti‐apoptotic proteins, such as activated caspase‐3, granzyme B protease inhibitor 9 (PI9), and Bcl‐2 proteins were analyzed in NTCL by the Groupe d’Etude des Lymphomes de l’ Adulte group.( 35 ) Interestingly, high expression of PI9 by lymphoma cells was associated with a favorable outcome (5‐year OS 64 ± 9%vs 23 ± 11%, P = 0.009). Additionally, PI9 expression independently predicted overall and event‐free survivals in NTCL patients.
Regarding EBV status, high copies of EBV DNA at presentation was the most significant prognostic factor for reduced disease‐free survival (median, 0.5 months vs >27.0 months, P = 0.025) and lower OS in NTCL.( 36 ) Patients with bone marrow that is positive for EBV by in situ hybridization demonstrated significantly poorer survival in early‐stage NTCL.( 37 , 38 )
Recently, our colleague hypothesized that tumor‐infiltrating regulatory T cells may suppress NTCL cells that have an activated NK cell phenotype, thereby contributing to a favorable outcome.( 39 ) Low numbers of tumor‐infiltrating regulatory T cells, which is associated with poor performance status and non‐UAT tumor, independently predicted worse survival. Furthermore, the number of tumor‐infiltrating regulatory T cells predicted survival independent of Ann Arbor stage, IPI score, or NTCL prognostic index.
The positive rate of 18fluoro‐2‐deoxyglucose positron emission tomography to detect a lymphoma lesion was 100% in NTCL.( 40 ) Furthermore, pre‐treatment maximal standardized uptake value was associated with local tumor invasiveness in NTCL of the head and neck, and predicted disease‐specific survival.( 41 )
New Staging System and Treatment Strategies
Patients with UAT‐NTCL have demonstrated superior outcomes compared to those with non‐UAT NTCL (5‐year OS 41–42%vs 9–22%, respectively; Fig. 1).( 7 , 23 ) Although patients with stage I/II UAT‐NTCL experienced favorable outcomes (5‐year OS, 37–77%),( 10 , 18 , 24 , 26 , 30 ) local tumor invasiveness significantly reduced survival and response regardless of treatment modality.( 26 ) Based on these treatment outcomes, a new staging system for NTCL is proposed: limited disease (stage I/II UAT‐NTCL without invasiveness) and extensive disease (stage I/II with local invasiveness or stage III/IV disease of UAT NTCL, and non‐UAT NTCL) based on treatment outcomes (2, 3).( 7 , 26 )
Table 2.
New staging system for extranodal NK/T‐cell lymphoma, nasal type
| Limited disease | Extensive disease | |
|---|---|---|
| Category | UAT presentation: Stage I/II without local tumor invasiveness | Non‐UAT presentation UAT presentation: Stage I/II with local tumor invasiveness Stage III/IV |
| Long‐term survival | Yes | No |
| Prognosis | Favorable | Unfavorable |
| Usefulness of radiotherapy | Yes | Questionable |
UAT, upper aerodigestive tract.
Table 3.
| Treatment outcomes | Limited disease | Extensive disease | |
|---|---|---|---|
| Stage I/II UAT‐NTCL without LTI | Stage I/II UAT‐NTCL with LTI | Stage III/IV UAT‐NTCL | |
| CR/CRu rate | 90% | 36% | 17% |
| Relapse/failure | |||
| Locoregional | 34% | 65% | 40% |
| Systemic | 14% | 35% | 60% |
| Survival outcome | |||
| 2‐year OS | 79% | 0% | 16% |
| 2‐year DFS | 63% | 13% | 52% |
| Role of radiotherapy | Yes | Unknown | No |
CR/CRu, complete remission/CR unconfirmed; DFS, disease‐free survival; LTI, local tumor invasiveness; NTCL, NK/T‐cell lymphoma; OS, overall survival; UAT, upper aerodigestive tract.
Limited disease. Due to high systemic failure rate after treatment with radiotherapy alone,( 18 ) chemoradiation has been widely used in patients with localized NTCL.( 24 , 26 , 30 , 42 , 43 ) However, anthracycline‐based chemotherapy followed by radiation produced a CR rate of 58% and planned radiation was delivered in only 35% of patients due to early progression.( 42 ) The role of early radiotherapy compared with initial chemotherapy was highlighted in a few studies.( 24 , 30 , 43 ) Front‐line use of radiotherapy produced superior survival compared to initial chemotherapy in localized nasal NTCL (5‐year OS 83%vs 29%, respectively).( 30 ) Li et al. ( 24 ) recommended a front‐line radiotherapy for early‐stage nasal NTCL and suggested that radiotherapy followed by chemotherapy should be considered for patients with adverse factors or stage II NTCL. In particular, high‐dose radiotherapy (≥50 Gy) significantly improved the 5‐year local control rate patients with stage I nasal lymphoma compared with low‐dose therapy (100%vs 67%, respectively; P = 0.013). Additional chemotherapy did not improve treatment outcomes in stage I nasal lymphoma.( 44 ) Likewise, the addition of chemotherapy to radiotherapy in stage I nasal NTCL did not improve survival or response rate.( 24 ) The benefit of additional chemotherapy on survival was observed in early‐stage Waldeyer ring NTCL, but not in early‐stage nasal NTCL.( 10 )
Due to the frequent expression of multidrug‐resistance protein 1, anthracycline‐based chemotherapy has limited activity against NTCL. Our study revealed that initial anthracycline‐based combination chemotherapy resulted in a CR rate of 43% and a 2‐year OS of 53% in stage I/II UAT‐NTCL.( 45 ) Patients who achieved CR after initial chemotherapy did not benefit from additional radiotherapy. Subsequent study of a nonanthracycline‐based regimen (IMEP: combination chemotherapy of ifosfamide, methotrexate, etoposide, and prednisolone) demonstrated CR rates of 79% and 93% after initial IMEP alone and IMEP ± radiotherapy, respectively.( 46 ) However, 64% of CR patients relapsed at local sites; these local failures were successfully treated with additional radiotherapy. Based on high local failure rate after IMEP chemotherapy alone, a phase II multicenter trial of IMEP followed by radiotherapy was initiated by the Korean Cancer Study Group (KCSG‐LY04‐03) in stage I/II UAT‐NTCL. This study is now closed with the planned numbers of patients registered and its results will be presented.
Extensive disease. Although combined modality treatment (i.e., chemotherapy and radiotherapy) provided a superior outcome in stage I/II UAT‐NTCL with local tumor invasiveness compared to chemotherapy alone,( 26 ) combined modality treatment has not been compared with radiotherapy alone. Furthermore, neither the role of radiotherapy nor the optimal sequence of chemotherapy and radiation have been verified in this disease subset with local invasiveness. Thus, various therapeutic interventions should be investigated in these patients to improve treatment outcomes.
For patients with stage III/IV UAT‐NTCL, combination chemotherapy is considered to be the standard treatment; however, its prognosis is dismal.( 47 , 48 ) An anthracycline‐based regimen( 45 ) and IMEP chemotherapy( 46 ) were both disappointing in this group (CR rate of 13%; and median OS 2.7 months for patients treated with IMEP). Successful treatment outcomes were observed in refractory or relapsed NTCL using l‐asparaginase alone( 49 ) or in combination with other chemotherapy.( 50 , 51 ) In addition, l‐asparaginase demonstrated anti‐tumor activity against NK cell lymphoma/leukemia cells in vitro.( 52 ) Thus, a phase I study of dexamethasone, methotrexate, ifosfamide, l‐asparaginase, and etoposide was conducted for patients with advanced, relapse, or refractory NTCL.( 53 ) Of three patients with newly diagnosed advanced‐stage NTCL, one patient achieved CR and one achieved partial remission. Because nuclear factor‐kappa B (NF‐κB) is constitutively activated in NK leukemia/lymphoma cells, NF‐κB inhibitors were tested in vitro.( 54 ) NF‐κB inhibitors suppressed the growth of NK leukemia/lymphoma cells by reducing constitutive NF‐κB activation. Another study demonstrated that bortezomib induces apoptosis in NK cell leukemia/ lymphoma in vitro and in a preclinical animal model.( 55 ) Based on these results, a phase I study of bortezomib plus cyclophosphamide, doxorubicin, vincristine, and prednisolone was undertaken with patients with advanced‐stage T‐ or NK/T‐cell lymphoma.( 56 ) Only one of three patients treated with this regimen achieved CR.
Salvage treatment. l‐asparaginase‐based chemotherapy was delivered as a salvage treatment for refractory or relapsed NTCL.( 53 , 57 ) Two of three patients with refractory or relapsed NTCL attained CR after l‐asparaginase regimen and were alive at 15 months and 7 months, respectively.( 53 ) One large retrospective study of l‐asparaginase‐based regimen demonstrated an overall response rate of 82% (CR, 56%; 5‐year OS 67%).( 57 ) In addition, salvage treatment significantly prolonged the 5‐year OS compared with the best supportive care alone (38%vs 0%, respectively; P < 0.001).( 58 ) Salvage radiotherapy improved survival in relapsed stage III/IV NTCL patients, whereas relapsed patients with stage I/II NTCL did not benefit from salvage chemotherapy.
Hematopoietic stem cell transplantation (HSCT) does not seem to improve treatment outcomes for disseminated or early refractory NTCL. Allogeneic HSCT when used with reduced‐intensity regimen, is a favorable treatment option for advanced‐stage NTCL patients.( 59 ) Autologous HSCT at the first CR showed a trend toward better OS compared with historic controls.( 60 ) However, retrospective matched controlled study demonstrated no significant difference in overall survival between the HSCT and control groups (median, not reached vs 43.5 months), although disease‐specific survival rates at 5 years were significantly higher in the HSCT group than in the control group in patients who achieved CR (87%vs 68%, P = 0.027).( 61 ) The overall survival data from HSCT was not superior to recent chemotherapy without HSCT (3‐year OSs, 80.4% for limited disease patients and 60% for all patients treated with first‐line IMEP chemotherapy ± involved‐field radiotherapy).( 46 ) Furthermore, treatment with IMEP and l‐asparaginase showed 5‐year OS in 80% of extensive disease NTCL patients in our series (TM Kim and DS Heo, unpublished data). Taken together, the role of HSCT or any other new treatment in NTCL should be investigated in a prospective randomized trial.
Second‐line IMEP chemotherapy was evaluated in our institution after the failure of anthracycline‐based chemotherapy.( 62 ) The overall response rate was 44% (CR rate, 38%) and median OS was 8.2 months (5‐year OS, 25%). Locoregional (n = 1) or systemic relapse (n = 4) was observed in 42% (n = 5) of patients who achieved CR. This salvage regimen was well‐tolerated and moderately effective for refractory or relapsed NTCL.
Because NTCL cells that specifically express interleukin‐9 and its receptor were inhibited by neutralizing antibody, its signaling pathway can be a new target for NTCL.( 63 ) Heat‐shock protein 90 inhibitor induced NTCL cells having activated phsophatidylinositol‐3 kinase/Akt pathway to an apoptosis.( 64 ) Thus, this pathway can be exploited as a new therapeutic target. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) was observed in NTCL cells and STAT3 inhibition resulted in cell death induction.( 65 ) Accordingly, STAT3 protein can be another therapeutic target in NK/T‐cell lymphoma.
Concluding Remarks
A new staging system has been proposed based on treatment outcomes: limited disease (stage I/II UAT‐NTCL without local tumor invasiveness) and extensive disease (stage I/II with local invasiveness or stage III/IV disease of UAT NTCL, and non‐UAT NTCL). Due to the limited numbers of well conducted trials, there is no consensus on treatment strategy. In our cohorts, non‐anthracycline combination chemotherapy followed by radiotherapy is effective for limited stage disease, but new treatment strategy is needed for extensive stage disease.
Acknowledgments
We apologize to all of the authors whose work could not be included in this review due to limited space. This study was supported by grants from the Korea Research Foundation (KRF‐2004‐015‐E00155) and from the Innovative Research Institute for Cell Therapy (A062260), Republic of Korea.
References
- 1. Chan JK, Jaffe ES, Ralfkiaer E. Extranodal NK/T‐cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC, 2001; 204–7. [Google Scholar]
- 2. Chan JK, Quintanilla‐Martinez L, Ferry JA, Peh S‐C. Extranodal NK/T‐cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC, 2008; 285–8. [Google Scholar]
- 3. Jaffe ES, Chan JK, Su IJ et al. Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol 1996; 20: 103–11. [DOI] [PubMed] [Google Scholar]
- 4. Gaal K, Sun NC, Hernandez AM, Arber DA. Sinonasal NK/T‐cell lymphomas in the United States. Am J Surg Pathol 2000; 24: 1511–7. [DOI] [PubMed] [Google Scholar]
- 5. Ko YH, Kim CW, Park CS et al. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European‐American lymphoma. Cancer 1998; 83: 806–12. [PubMed] [Google Scholar]
- 6. Kang YK, Kim BS, Kim TW et al. Clinicopathologic characteristics of Korean non‐Hodgkin’s lymphomas based on REAL classification. Cancer Res Treat 1999; 31: 641–52. [Google Scholar]
- 7. Kim TM, Lee SY, Jeon YK et al. Clinical heterogeneity of extranodal NK/T‐cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol 2008; 19: 1477–84. [DOI] [PubMed] [Google Scholar]
- 8. Li YX, Fang H, Liu QF et al. Clinical features and treatment outcome of nasal‐type NK/T‐cell lymphoma of Waldeyer ring. Blood 2008; 112: 3057–64. [DOI] [PubMed] [Google Scholar]
- 9. Au WY, Weisenburger DD, Intragumtornchai T et al. Clinical differences between nasal and extranasal natural killer/T‐cell lymphoma: a study of 136 cases from the International Peripheral T‐Cell Lymphoma Project. Blood 2009; 113: 3931–7. [DOI] [PubMed] [Google Scholar]
- 10. Li YX, Liu QF, Fang H et al. Variable clinical presentations of nasal and Waldeyer ring natural killer/T‐cell lymphoma. Clin Cancer Res 2009; 15: 2905–12. [DOI] [PubMed] [Google Scholar]
- 11. Kwong YL. Natural killer‐cell malignancies: diagnosis and treatment. Leukemia 2005; 19: 2186–94. [DOI] [PubMed] [Google Scholar]
- 12. Lee SY, Park K, Ryoo BY et al. Korean multicenter study of extranodal NK/T‐cell lymphoma: failure of Ann Arbor staging in predicting prognosis. Seoul, Korea: The 29th World Congress of the International Society of Hematology, 2002. [Google Scholar]
- 13. Lee J, Park YH, Kim WS et al. Extranodal nasal type NK/T‐cell lymphoma: elucidating clinical prognostic factors for risk‐based stratification of therapy. Eur J Cancer 2005; 41: 1402–8. [DOI] [PubMed] [Google Scholar]
- 14. Cheung MM, Chan JK, Lau WH, Ngan RK, Foo WW. Early stage nasal NK/T‐cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys 2002; 54: 182–90. [DOI] [PubMed] [Google Scholar]
- 15. Cuadra‐Garcia I, Proulx GM, Wu CL et al. Sinonasal lymphoma: a clinicopathologic analysis of 58 cases from the Massachusetts General Hospital. Am J Surg Pathol 1999; 23: 1356–69. [DOI] [PubMed] [Google Scholar]
- 16. Liang R, Todd D, Chan TK et al. Treatment outcome and prognostic factors for primary nasal lymphoma. J Clin Oncol 1995; 13: 666–70. [DOI] [PubMed] [Google Scholar]
- 17. Cheung MM, Chan JK, Lau WH et al. Primary non‐Hodgkin’s lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 1998; 16: 70–7. [DOI] [PubMed] [Google Scholar]
- 18. Kim GE, Cho JH, Yang WI et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol 2000; 18: 54–63. [DOI] [PubMed] [Google Scholar]
- 19. Lee J, Suh C, Park YH et al. Extranodal natural killer T‐cell lymphoma, nasal‐type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006; 24: 612–8. [DOI] [PubMed] [Google Scholar]
- 20. Oshimi K, Kawa K, Nakamura S et al. NK‐cell neoplasms in Japan. Hematology 2005; 10: 237–45. [DOI] [PubMed] [Google Scholar]
- 21. Lee J, Kim WS, Park YH et al. Nasal‐type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer 2005; 92: 1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ko YH, Cho EY, Kim JE et al. NK and NK‐like T‐cell lymphoma in extranasal sites: a comparative clinicopathological study according to site and EBV status. Histopathology 2004; 44: 480–9. [DOI] [PubMed] [Google Scholar]
- 23. Armitage J, Vose J, Weisenburger D. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008; 26: 4124–30. [DOI] [PubMed] [Google Scholar]
- 24. Li YX, Yao B, Jin J et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T‐cell lymphoma. J Clin Oncol 2006; 24: 181–9. [DOI] [PubMed] [Google Scholar]
- 25. Pagano L, Gallamini A, Trape G et al. NK/T‐cell lymphomas ‘nasal type’: an Italian multicentric retrospective survey. Ann Oncol 2006; 17: 794–800. [DOI] [PubMed] [Google Scholar]
- 26. Kim TM, Park YH, Lee SY et al. Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T‐cell lymphoma, nasal type. Blood 2005; 106: 3785–90. [DOI] [PubMed] [Google Scholar]
- 27. Kim SJ, Kim BS, Choi CW et al. Ki‐67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T‐cell lymphoma, nasal type. Ann Oncol 2007; 18: 1382–7. [DOI] [PubMed] [Google Scholar]
- 28. Aviles A, Diaz NR, Neri N, Cleto S, Talavera A. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol 2000; 22: 215–20. [DOI] [PubMed] [Google Scholar]
- 29. Chim CS, Ma SY, Au WY et al. Primary nasal natural killer cell lymphoma: long‐term treatment outcome and relationship with the International Prognostic Index. Blood 2004; 103: 216–21. [DOI] [PubMed] [Google Scholar]
- 30. You JY, Chi KH, Yang MH et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T‐cell lymphoma: a single institute survey in Taiwan. Ann Oncol 2004; 15: 618–25. [DOI] [PubMed] [Google Scholar]
- 31. Robbins KT, Fuller LM, Vlasak M et al. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer 1985; 56: 814–9. [DOI] [PubMed] [Google Scholar]
- 32. Logsdon MD, Ha CS, Kavadi VS, Cabanillas F, Hess MA, Cox JD. Lymphoma of the nasal cavity and paranasal sinuses: improved outcome and altered prognostic factors with combined modality therapy. Cancer 1997; 80: 477–88. [DOI] [PubMed] [Google Scholar]
- 33. Shim SJ, Yang WI, Shin E et al. Clinical significance of cyclooxygenase‐2 expression in extranodal natural killer (NK)/T‐cell lymphoma, nasal type. Int J Radiat Oncol Biol Phys 2007; 67: 31–8. [DOI] [PubMed] [Google Scholar]
- 34. Lin CW, Chen YH, Chuang YC, Liu TY, Hsu SM. CD94 transcripts imply a better prognosis in nasal‐type extranodal NK/T‐cell lymphoma. Blood 2003; 102: 2623–31. [DOI] [PubMed] [Google Scholar]
- 35. Bossard C, Belhadj K, Reyes F et al. Expression of the granzyme B inhibitor PI9 predicts outcome in nasal NK/T‐cell lymphoma: results of a Western series of 48 patients treated with first‐line polychemotherapy within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood 2007; 109: 2183–9. [DOI] [PubMed] [Google Scholar]
- 36. Au WY, Pang A, Choy C, Chim CS, Kwong YL. Quantification of circulating Epstein–Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV‐positive lymphomas in immunocompetent patients. Blood 2004; 104: 243–9. [DOI] [PubMed] [Google Scholar]
- 37. Huang WT, Chang KC, Huang GC et al. Bone marrow that is positive for Epstein–Barr virus encoded RNA‐1 by in situ hybridization is related with a poor prognosis in patients with extranodal natural killer/T‐cell lymphoma, nasal type. Haematologica 2005; 90: 1063–9. [PubMed] [Google Scholar]
- 38. Lee J, Suh C, Huh J et al. Effect of positive bone marrow EBV in situ hybridization in staging and survival of localized extranodal natural killer/T‐cell lymphoma, nasal‐type. Clin Cancer Res 2007; 13: 3250–4. [DOI] [PubMed] [Google Scholar]
- 39. Kim WY, Jeon YK, Kim TM et al. Increased quantity of tumor‐infiltrating FOXP3‐positive regulatory T cells is an independent predictor for improved clinical outcome in extranodal NK/T‐cell lymphoma. Ann Oncol 2009; doi: DOI: 10.1093/annonc/mdp056. [DOI] [PubMed] [Google Scholar]
- 40. Kako S, Izutsu K, Ota Y et al. FDG‐PET in T‐cell and NK‐cell neoplasms. Ann Oncol 2007; 18: 1685–90. [DOI] [PubMed] [Google Scholar]
- 41. Suh C, Kang YK, Roh JL et al. Prognostic value of tumor 18F‐FDG uptake in patients with untreated extranodal natural killer/T‐cell lymphomas of the head and neck. J Nucl Med 2008; 49: 1783–9. [DOI] [PubMed] [Google Scholar]
- 42. Kim WS, Song SY, Ahn YC et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T‐cell lymphoma? Ann Oncol 2001; 12: 349–52. [DOI] [PubMed] [Google Scholar]
- 43. Ribrag V, Ell Hajj M, Janot F et al. Early locoregional high‐dose radiotherapy is associated with long‐term disease control in localized primary angiocentric lymphoma of the nose and nasopharynx. Leukemia 2001; 15: 1123–6. [DOI] [PubMed] [Google Scholar]
- 44. Shikama N, Izuno I, Oguchi M et al. Clinical stage IE primary lymphoma of the nasal cavity: radiation therapy and chemotherapy. Radiology 1997; 204: 467–70. [DOI] [PubMed] [Google Scholar]
- 45. Kim BS, Kim TY, Kim CW et al. Therapeutic outcome of extranodal NK/T‐cell lymphoma initially treated with chemotherapy – result of chemotherapy in NK/T‐cell lymphoma. Acta Oncol 2003; 42: 779–83. [DOI] [PubMed] [Google Scholar]
- 46. Lee KW, Yun T, Kim DW et al. First‐line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/− radiotherapy is active in stage I/II extranodal NK/T‐cell lymphoma. Leuk Lymphoma 2006; 47: 1274–82. [DOI] [PubMed] [Google Scholar]
- 47. Chan JK, Sin VC, Wong KF et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood 1997; 89: 4501–13. [PubMed] [Google Scholar]
- 48. Kwong YL, Chan AC, Liang R et al. CD56+ NK lymphomas: clinicopathological features and prognosis. Br J Haematol 1997; 97: 821–9. [DOI] [PubMed] [Google Scholar]
- 49. Nagafuji K, Fujisaki T, Arima F, Ohshima K. l‐asparaginase induced durable remission of relapsed nasal NK/T‐cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol 2001; 74: 447–50. [DOI] [PubMed] [Google Scholar]
- 50. Yong W, Zheng W, Zhang Y et al. l‐asparaginase‐based regimen in the treatment of refractory midline nasal/nasal‐type T/NK‐cell lymphoma. Int J Hematol 2003; 78: 163–7. [DOI] [PubMed] [Google Scholar]
- 51. Yong W, Zheng W, Zhu J et al. Midline NK/T‐cell lymphoma nasal‐type: treatment outcome, the effect of l‐asparaginase based regimen, and prognostic factors. Hematol Oncol 2006; 24: 28–32. [DOI] [PubMed] [Google Scholar]
- 52. Ando M, Sugimoto K, Kitoh T et al. Selective apoptosis of natural killer‐cell tumours by l‐asparaginase. Br J Haematol 2005; 130: 860–8. [DOI] [PubMed] [Google Scholar]
- 53. Yamaguchi M, Suzuki R, Kwong YL et al. Phase I study of dexamethasone, methotrexate, ifosfamide, l‐asparaginase, and etoposide (SMILE) chemotherapy for advanced‐stage, relapsed or refractory extranodal natural killer (NK)/T‐cell lymphoma and leukemia. Cancer Sci 2008; 99: 1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim K, Ryu K, Ko Y, Park C. Effects of nuclear factor‐kappaB inhibitors and its implication on natural killer T‐cell lymphoma cells. Br J Haematol 2005; 131: 59–66. [DOI] [PubMed] [Google Scholar]
- 55. Shen L, Au WY, Guo T et al. Proteasome inhibitor bortezomib‐induced apoptosis in natural killer (NK)‐cell leukemia and lymphoma: an in vitro and in vivo preclinical evaluation. Blood 2007; 110: 469–70. [DOI] [PubMed] [Google Scholar]
- 56. Lee J, Suh C, Kang HJ et al. Phase I study of proteasome inhibitor bortezomib plus CHOP in patients with advanced, aggressive T‐cell or NK/T‐cell lymphoma. Ann Oncol 2008; 19: 2079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yong W, Zheng W, Zhu J et al. L‐asparaginase in the treatment of refractory and relapsed extranodal NK/T‐cell lymphoma, nasal type. Ann Hematol 2009; 88: 647–52. [DOI] [PubMed] [Google Scholar]
- 58. Zhang XX, Xie CH, Xu Y et al. Salvage treatment improved survival of patients with relapsed extranodal natural killer/t‐cell lymphoma, nasal type. Int J Radiat Oncol Biol Phys 2009; 74: 747–52. [DOI] [PubMed] [Google Scholar]
- 59. Murashige N, Kami M, Kishi Y et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer‐cell neoplasms. Br J Haematol 2005; 130: 561–7. [DOI] [PubMed] [Google Scholar]
- 60. Au WY, Lie AK, Liang R et al. Autologous stem cell transplantation for nasal NK/T‐cell lymphoma: a progress report on its value. Ann Oncol 2003; 14: 1673–6. [DOI] [PubMed] [Google Scholar]
- 61. Lee J, Au WY, Park MJ et al. Autologous hematopoietic stem cell transplantation in extranodal natural killer/T cell lymphoma: a multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant 2008; 14: 1356–64. [DOI] [PubMed] [Google Scholar]
- 62. Kim BS, Kim DW, Im SA et al. Effective second‐line chemotherapy for extranodal NK/T‐cell lymphoma consisting of etoposide, ifosfamide, methotrexate, and prednisolone. Ann Oncol 2009; 20: 121–8. [DOI] [PubMed] [Google Scholar]
- 63. Nagato T, Kobayashi H, Kishibe K et al. Expression of interleukin‐9 in nasal natural killer/T‐cell lymphoma cell lines and patients. Clin Cancer Res 2005; 11: 8250–7. [DOI] [PubMed] [Google Scholar]
- 64. Jeon YK, Park CH, Kim KY et al. The heat‐shock protein 90 inhibitor, geldanamycin, induces apoptotic cell death in Epstein–Barr virus‐positive NK/T‐cell lymphoma by Akt down‐regulation. J Pathol 2007; 213: 170–9. [DOI] [PubMed] [Google Scholar]
- 65. Coppo P, Gouilleux‐Gruart V, Huang Y et al. STAT3 transcription factor is constitutively activated and is oncogenic in nasal‐type NK/T‐cell lymphoma. Leukemia 2009; doi: DOI: 10.1038/leu.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


