Abstract
The cell wall skeleton of Mycobacterium bovis BCG has been investigated as an immunopotentiating adjuvant for immuno‐therapy of malignant tumors via Toll‐like receptor (TLR) 2 and TLR4. However, due to its high molecular weight, highly complicated lipoglycan structure, and complicated purification and isolation procedure, its exact structure–activity relationship has not been well established. We have newly isolated the cell wall skeleton from M. bovis BCG Tokyo (SMP‐105) and examined the binding of SMP‐105 with TLR. It was revealed that highly purified SMP‐105 activates the nuclear factor‐kB promoter in a TLR2‐dependent manner, not a TLR4‐dependent manner, using a reporter gene assay system. Peritoneal exudated cells of TLR2 and MyD88 knockout mice severely reduced the induction of tumor necrosis factor‐a and interleukin‐6 in the presence of SMP‐105, whereas cells from TLR4 knockout mice produced similar levels of cytokines to wild‐type mice. Dendritic cells and macrophages accumulated in the draining lymph nodes of treated mice. When mice were administered both SMP‐105 and mitomycin C‐inactivated Lewis lung carcinoma cells simultaneously, interferon‐g‐producing cells reacting to the tumor were increased distinctly in draining lymph nodes. When C57BL/6 mice, into which splenocytes from OT‐I transgenic mice had been transferred, were administered with both SMP‐105 and E.G7‐OVA, OVA‐specific cytotoxic T lymphocytes (CTL) increased markedly. Mice treated with SMP‐105 and inactivated Lewis lung carcinoma cells suppressed the growth of implanted tumors. These results suggest that the activation of TLR2 by SMP‐105 sufficiently enhanced immune responses, such as the number of interferon‐g‐producing cells and CTL, and prevented the growth of tumors without the contribution of TLR4. (Cancer Sci 2008; 99: 1435–1440)
Anticancer immunotherapy is an attractive alternative for the treatment of patients with inoperable cancer, without the application of radiotherapy or chemotherapy, and it is expected that the side effects experienced will be lower than with classical antitumor chemotherapeutics. Although several immunotherapeutic agents have been approved for some types of cancer patients, they are not generally accepted as reliable tools that show sufficient efficacy.( 1 ) One reason for the insufficient results of immunotherapy is the poor immunogenecity of most tumor antigens,( 2 ) and the lack of evidence and theoretical basis of immunotherapeutic agents for regulating the antitumor immune response. Recent progress in innate immunity, in particular the function and role of dendritic cells and Toll‐like receptors (TLR), offers a great opportunity to overcome previous problems. Appropriate activation of antigen presentation enhances the immunogenecity of tumor antigens to effectively increase cellular immune responses against tumors.
Toll‐like receptors are pathogen‐associated molecular pattern recognition receptors, and play an important role in induction of the immune response via activation of innate immunity.( 3 , 4 ) TLR agonists have been shown to enhance immune responses against tumors in both animal models and clinical studies. Poly I‐C is a ligand for TLR3( 5 ) and RIG‐I.( 6 ) Imiquimod is a ligand for TLR7 and TLR8,( 7 ) and is approved as an immunotherapeutic drug for basal cell carcinoma. CpG DNA is a ligand for TLR9,( 8 ) and its antitumor activity against various types of tumors has been investigated in several clinical trials. Although agonists for TLR3, TLR4, TLR7, TLR8, and TLR9 have been well studied for antitumor activity, there is little information solely on TLR2 agonists, except for MALP‐2,( 9 , 10 , 11 ) in cancer immunotherapy.
The cell wall skeleton (CWS) of Mycobacterium bovis (BCG‐CWS) is a unique and potent adjuvant for the innate immune system. It is a useful tool for understanding the mechanism of cancer immunotherapy, as BCG immunotherapy for superficial bladder carcinoma is used widely in the urological field. BCG‐CWS is prepared from M. bovis BCG as an insoluble fraction of the cell wall, and consists of mycolic acids, neutral sugars such as arabinose and galactose, and peptidoglycans.( 12 ) BCG‐CWS not only shows strong adjuvant activity to elicit immune responses to antigens,( 13 , 14 , 15 ) but also has potent activity to regress tumor growth in animal models and cancer patients.( 12 , 16 , 17 ) BCG‐CWS has been proposed to activate antigen‐presenting cells through both TLR2 and TLR4.( 18 , 19 )
Although BCG‐CWS has long been investigated, its clinical use is very limited because of solubility and stability difficulties related to drug efficacy. We have recently established a new optimized purification procedure, producing highly purified and non‐contaminated BCG‐CWS from M. bovis BCG Tokyo 172.( 20 ) Chemical analysis of newly manufactured SMP‐105 indicated that it is composed of mycolic acids, arabinogalactan (consisting of arabinose, galactose, and rhamnose), and peptidoglycan (consisting of alanine, glutamic acid, diaminopimeric acid, muramic acid, glucosamine, galactosamine, and a phosphate group), and the levels of potential impurities are quite low.( 20 ) In this paper, we have carefully examined the effects of this SMP‐105 on TLR and the induction of immune responses against tumors. Using reporter gene assay systems and TLR knockout mice, it was demonstrated that saline‐suspended SMP‐105 selectively activates TLR2, but does not activate TLR4 entirely. Despite a lack of activity for TLR4, SMP‐105 elicits immune responses against tumors, such as interferon (IFN)‐γ‐producing cells and cytotoxic T lymphocytes, and prevents the growth of implanted tumors.
Materials and Methods
Animals. C57BL/6J female mice were purchased from Charles River Japan (Kanazawa, Japan). TLR2‐,( 21 ) TLR4‐,( 22 ) and MyD88( 23 )‐deficient mice were obtained from Oriental Bio Service (Kyoto, Japan). C57BL/6‐Tg(OT‐I)‐RAG1tm1Mom (OT‐I transgenic mice)( 24 , 25 ) were purchased from Taconic (Hudson, NY, USA). Mice were maintained under specific pathogen‐free conditions. All animal experiments were conducted according to the guidelines of the Animal Care and Use Committee at Dainippon Sumitomo Pharma.
Cell lines. Lewis lung carcinoma 3LL was obtained from the Cancer Institute for the Japanese Foundation for Cancer Research (Tokyo, Japan). 3LL cells were maintained in RPMI‐1640 medium supplemented with 10% fetal calf serum (FCS), 50 µg/mL streptomycin, and 50 U/mL penicillin. E.G7‐OVA( 26 ) and HEK293 were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI‐1640 supplemented with 10% FCS, 50 µg/mL streptomycin, 50 U/mL penicillin, and 400 µg/mL G418, or Dulbecco's Modified Eagle Medium supplemented with 10% FCS, 50 µg/mL streptomycin, and 50 U/mL penicillin. HEK‐Blue‐4 was purchased from Invivogen (San Diego, CA, USA). 293 kB was established as a stable transfectant of HEK293 expressing the pGL3 basic vector (Promega, Madison, WI, USA), in which the nuclear factor (NF)‐κB region derived from the mouse Igκ chain was cloned. 293 kB/hTLR2 was established as a stable transfectant of 293 kB expressing human TLR2 (Origene, Rockville, MD, USA). To prepare inactivated tumor cells as antigens, cells were incubated at 37°C for 20 min at 1 × 107 cells/mL in culture medium containing 200 µg/mL mitomycin C (Kyowa Hakko Kogyo, Tokyo, Japan), following repeated washing with sufficient culture medium.
Preparation of SMP‐105. SMP‐105 was prepared as described previously.( 20 ) Contaminated endotoxin was less than 0.005 endotoxin units/mg. For in vitro experiments, SMP‐105 was suspended in saline containing 0.01% Tween80. For animal experiments, an oil‐in‐water emulsion of SMP‐105 was prepared with the following formulation: 0.6 mg/mL SMP‐105, 1.6% squalane, 1.0% polysorbate 80, and 5.0% mannitol. Vehicle preparation used the same formulation, except for SMP‐105.
Reagents. Pam3CSK4 was purchased from Calbiochem (Merck, Tokyo, Japan). Escherichia coli J5 lipopoly saccharide (LPS) was purchased from LIST Biological Laboratories (Campbell, CA, USA). LPS was further purified using phenol extraction methods.( 27 , 28 , 29 ) Heat‐killed BCG Tokyo was prepared by inactivation at 80°C for 30 min.
Reporter gene assay. 293 kB/hTLR2 or HEK‐Blue 4 was cultured with SMP‐105 for 18 h. The luciferase assay was measured using Luclite (PerkinElmer Japan, Kanagawa, Japan). An alkaline phosphatase assay was carried out in HEK‐Blue Detection Medium (Invivogen) as recommended by the manufacturer. Relative induction was calculated as the ratio of experimental groups to the control.
Macrophage stimulation. Thioglycollate‐elicited peritoneal exudate cells (TG‐PEC) were prepared from mice 5 days after intraperitoneal injection of 3% thioglycollate medium (Difco; Becton Dickinson Japan, Tokyo, Japan), and seeded at 5 × 105 cells/well in a 96‐well plate. After the removal of non‐adherent cells by washing, adherent macrophages were treated with 1 ng/mL recombinant mouse IFN‐γ (R & D Systems, Minneapolis, MN, USA) for 2 h. TG‐PEC were incubated with SMP‐105 and cultured for 18 h. The concentrations of tumor necrosis factor (TNF)‐α (R & D Systems) and interleukin (IL)‐6 (BioSource, Invitrogen Japan, Tokyo, Japan) in the supernatants were determined by enzyme‐linked immunosorbent assay.
Flow cytometry. C57BL/6J mice were injected intradermally at the base of the tail with SMP‐105 (60 µg), or Pam3CSK4 (1 µg). Inguinal lymph node cells were collected the next day and a single cell suspension was prepared. Cells were blocked with FcBlock (BD Pharmingen, Beckton Dickinson Japan, Tokyo, Japan), stained with fluorescein isothiocyanate‐labeled F4/80 (Serotec, Oxford, UK) and R‐phycoerythrin (PE)‐labeled anti‐CD11c (BD Pharmingen), and analyzed with FACScan (Becton Dickinson Japan).
In vivo vaccination and tumor challenge. C57BL/6J mice were administered a mixture of inactivated 3LL cells (3 × 104 cells) and SMP‐105 (12.5 µg) or vehicle intradermally, four times at 7‐day intervals. Mice were inoculated with 3LL (105 cells) in the left flank 7 days after the final administration. The growth of tumors was monitored by measuring the longest (l) and shortest (s) diameter of tumor pulp, and the volume of tumor was estimated according to the following equation:
| Tumor volume (mm3) = 0.5 × l (mm) × s (mm) × s (mm). |
In vitro restimulation by inactivated tumors. C57BL/6J mice were administered a mixture of inactivated 3LL (3 × 104 cells) and SMP‐105 (12.5 µg) on days 0 and 7, intradermally in the right‐flank region. Spleen and inguinal lymph nodes were collected on day 14. Splenocytes or lymphocytes were cultured in the presence of inactivated 3LL cells for 48 h. The levels of IFN‐γ in the culture supernatant were measured by enzyme‐linked immunosorbent assay (BioSource).
In vivo CTL proliferation assay. Splenocytes (1.5 × 105 cells) from OT‐I transgenic mice were transferred into C57BL/6J mice. The next day, mice were administered inactivated E.G7‐OVA cells (1 × 106 cells), a mixture of cells and SMP‐105 (80 µg) or CpG1826 ODN which is a 21‐mer containing two CpGmotifs (TTCCATGACGTTCCTGACGTT; 80 µg) into their footpads.( 30 ) Popliteal lymph nodes were collected 4 or 7 days after immunization. Lymphocytes were stained with the PE‐labeled H‐2Kb OVA (SIINFEKL) tetramer (MBL, Aichi, Japan) and PerCP5.5‐labeled rat antimouse CD8a (BD Pharmingen), and were analyzed with FACScan. OVA‐specific CTL were detected as CD8+ H‐2Kb OVA‐tetramer+.
Statistical analysis. The absolute numbers of dendritic cells, macrophages, and OVA‐specific CTL were compared with the control group using Dunnett's multiple comparison. Significant differences in tumor volumes were evaluated using Student's t‐test. All statistical analyses were carried out using SAS software (SAS Institute, Cary, NC, USA).
Results
Toll‐like receptor 2‐selective activation of mouse peritoneal macrophages (TG‐PEC) by SMP‐105. Because BCG‐CWS has already been proposed to activate both TLR2 and TLR4 signaling,( 18 , 19 ) we first investigated whether SMP‐105, a new preparation of M. bovis BCG Tokyo 172 CWS, activates TLR2 and TLR4 signaling. To measure the activity of SMP‐105 for both receptors in vitro, TLR2‐ and TLR4‐specific reporter gene assay systems were used. After overnight culture of TLR2‐ or TLR4‐expressing cells with SMP‐105, the expression of the reporter gene in TLR2‐introduced cells increased in a dose‐dependent manner (Fig. 1a). In contrast, induction of the reporter gene of HEK‐Blue 4 was not seen when cells were treated with SMP‐105 and heat‐killed BCG Tokyo at either 10 or 100 µg/mL, whereas the previous preparation of BCG‐CWS induced the reporter gene at 100 µg/mL as reported previously (Fig. 1b). The specific response of 293 kB/hTLR2 and HEK‐Blue4 to the ligands Pam3CSK4 and LPS, respectively, was confirmed (Fig. 1c,d).
Figure 1.
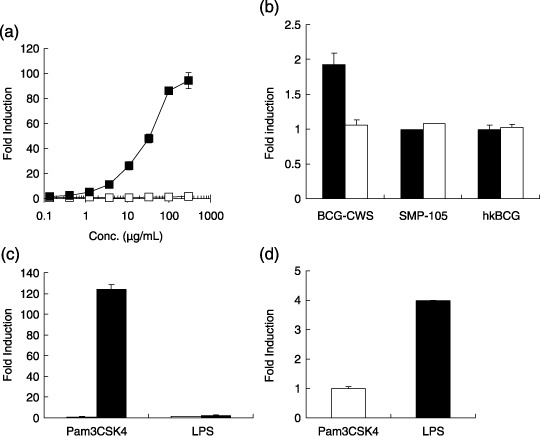
Nuclear factor (NF)‐κB activation after SMP‐105 stimulation. (a) 293 kB/hTLR2 ( ), which expressed human Toll‐like receptor (TLR) 2 and the NF‐κB‐luciferase gene, and parental 293 kB (
), which expressed human Toll‐like receptor (TLR) 2 and the NF‐κB‐luciferase gene, and parental 293 kB ( ), which carried only the NF‐κB‐luciferase gene, were stimulated with SMP‐105 for 18 h. Luciferase activity was measured with luminescence. The mean ± SD of two separate measurements is shown. (b) HEK‐Blue4 was stimulated with 100 µg/mL (black bar) or 10 µg/mL (white bar) of the previous preparation of Mycobacterium bovis cell wall skeleton (BCG‐CWS), SMP‐105, or heat‐killed BCG Tokyo (hkBCG) for 18 h. Alkaline phosphatase activity was measured. Representative data from four experiments are shown. (c) 293 kB (white bar) or 293 kB/hTLR2 (black bar) was stimulated with Pam3CSK4 (0.3 µg/mL) or lipopolysaccharide (LPS) (0.5 µg/mL) for 18 h. Reporter gene induction was measured as luciferase activity. The mean ± SD of two separate measurements is shown. (d) HEK‐Blue4 was stimulated with Pam3CSK4 (10 µg/mL) or LPS (0.1 µg/mL) for 18 h. The induction of alkaline phosphatase activity was measured.
), which carried only the NF‐κB‐luciferase gene, were stimulated with SMP‐105 for 18 h. Luciferase activity was measured with luminescence. The mean ± SD of two separate measurements is shown. (b) HEK‐Blue4 was stimulated with 100 µg/mL (black bar) or 10 µg/mL (white bar) of the previous preparation of Mycobacterium bovis cell wall skeleton (BCG‐CWS), SMP‐105, or heat‐killed BCG Tokyo (hkBCG) for 18 h. Alkaline phosphatase activity was measured. Representative data from four experiments are shown. (c) 293 kB (white bar) or 293 kB/hTLR2 (black bar) was stimulated with Pam3CSK4 (0.3 µg/mL) or lipopolysaccharide (LPS) (0.5 µg/mL) for 18 h. Reporter gene induction was measured as luciferase activity. The mean ± SD of two separate measurements is shown. (d) HEK‐Blue4 was stimulated with Pam3CSK4 (10 µg/mL) or LPS (0.1 µg/mL) for 18 h. The induction of alkaline phosphatase activity was measured.
To confirm that SMP‐105 activates the innate immune response in a TLR2‐dependent manner, the effects of SMP‐105 on TG‐PEC isolated from TLR2, TLR4, and MyD88 knock out mice were analyzed. TG‐PEC prepared from wild‐type mice responded to SMP‐105 and produced large amounts of IL‐6 (Fig. 2a) and TNF‐α (Fig. 2b) in the culture supernatant. TG‐PEC derived from TLR2 KO mice showed a marked decline in the production of these cytokines, although trace amount of cytokines was detected (IL‐6, 200.6 pg/mL; TNF‐α, 1196.4 pg/mL). However, TG‐PEC prepared from TLR4 KO mice produced as much cytokine as TG‐PEC from wild‐type mice. TG‐PEC from MyD88 KO mice also showed poor responses to SMP‐105. The responsiveness of TG‐PEC was well consitent with the results from the reporter gene assay. These results indicate that purified SMP‐105 activated cells in the manner that was largely dependent on TLR2 and MyD88, but not TLR4. Thus, using SMP‐105 it is possible to investigate whether antitumor activity needs either receptor or both TLR2 and TLR4 agonistic activity.
Figure 2.
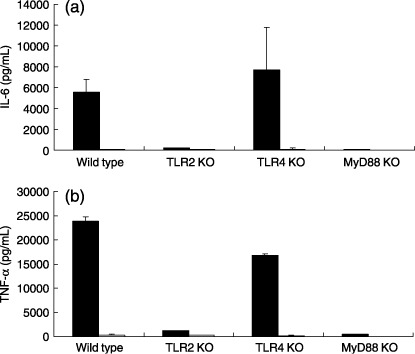
Interleukin (IL)‐6 and tumor necrosis factor (TNF)‐α production by thioglycollate‐elicited peritoneal exudate cells (TG‐PEC) from wild‐type, Toll‐like receptor (TLR) 2, TLR4, and MyD88 knockout (KO) mice. TG‐PEC were cultured in the presence of 1 ng/mL recombinant mouse interferon‐γ with medium control (white bar) or 10 µg/mL of SMP‐105 (black bar) for 18 h. Culture supernatants were recovered and assayed for the production of (a) IL‐6 and (b) TNF‐α by enzyme‐linked immunosorbent assay. The mean ± SD of two mice is shown.
Accumulation of dendritic cells and macrophages in the draining lymph nodes by administration of SMP‐105. To analyze the in vivo effect of SMP‐105 on dendritic cells and macrophages, the absolute numbers of dendritic cells and macrophages in inguinal lymph nodes were monitored after SMP‐105 was administered to mice intradermally into the base of the tail. The number of CD11c+ F4/80− dendritic cells increased significantly in SMP‐105‐treated mice compared to control mice (Table 1). Although the vehicle of oil‐in‐water emulsion slightly but significantly increased the number of CD11c− F4/80+ macrophages, CD11c− F4/80+ macrophages also increased significantly in SMP‐105‐treated mice compared with control mice (Table 1). The synthetic TLR2 ligand Pam3CSK4 increased the numbers of both dendritic cells and macrophages (Table 1). These results suggest that the activation of TLR2 in vivo induced the recruitment and accumulation of dendritic cells and macrophages in the draining lymph nodes.
Table 1.
Absolute numbers of whole lymph node cells, dendritic cells, and macrophages in the draining lymph node of SMP‐105, Pam3CSK4, or vehicle‐injected or naïve C57BL/6 mice
| Experimental group | Absolute number of cells | |||||
|---|---|---|---|---|---|---|
| Lymph node cells (×106) | CD11c+ F4/80− (×103) | CD11c− F4/80+ (×103) | ||||
| Naïve | 1.20 ± 0.18 | 34.33 ± 6.68 | 3.34 ± 0.70 | |||
| Vehicle | 1.60 ± 0.38 | 0.0417* | 45.74 ± 17.58 | 0.2372* | 6.24 ± 1.77 | 0.0093* |
| SMP‐105 | 2.45 ± 0.23 | 0.0006** | 64.89 ± 9.54 | 0.0255** | 15.00 ± 3.99 | 0.0032** |
| Pam3CSK4 | 2.57 ± 0.32 | 0.0002** | 76.06 ± 5.04 | 0.0010** | 23.62 ± 5.32 | <0.0001** |
C57BL/6 mice were injected intradermally at the base of the tail with vehicle emulsion, SMP‐105 (60 µg), or Pam3CSK4 (1 µg). Inguinal lymph nodes were collected the following day and analyzed for their composition in dendritic cells (CD11c+ F4/80−) and macrophages (CD11c− F4/80+). *P‐value compared to naïve mice (Student's t‐test) was indicated. **P‐value compared to vehicle‐injected mice (Dunnett's multiple comparison) was indicated.
Induction of Thelper (Th) 1 type immune response to tumor cells with CWS via stimulation of TLR2. To determine whether TLR2 stimulation induces Thelper (Th) 1 type immune responses for tumor cells in treated animals, IFN‐γ production against tumor cells was evaluated. Mice were administered SMP‐105 and inactivated tumor cells. Splenocytes and inguinal lymph node cells were cultured in the presence of the administered tumor cells. Splenocytes from treated mice produced a significant level of IFN‐γ (Fig. 3). Lymph node cells at the administered side also produced IFN‐γ, whereas lymph node cells on the opposite side to the administration site did not produce any IFN‐γ (Fig. 3a). The induction of immune responses in draining lymph nodes (Fig. 3b) or spleens (Fig. 3c) was not seen in mice administered vehicle emulsion and inactivated tumor cells. These data suggest that treatment with SMP‐105 and inactivated tumor cells induced a systemic Th1‐type immune response, which was detected in the spleen cell culture and, in addition, induced the local accumulation of reactive cells to lymph nodes.
Figure 3.
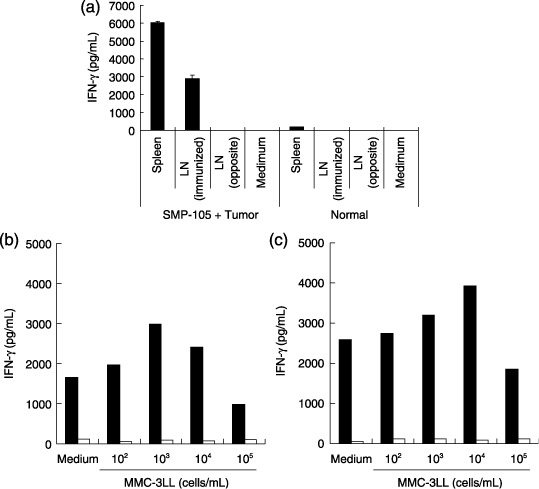
Induction of interferon (IFN)‐γ‐producing cells in vaccinated mice. (a) C57BL/6 mice were administered twice at 7‐day intervals with mitomycin C‐inactivated 3LL cells and SMP‐105. Spleen cells (spleen) and lymph node cells (LN) were collected 7 days after final administration, and were cultured with inactivated 3LL cells for 48 h. The amount of IFN‐γ in the culture supernatant was measured using enzyme‐linked immunosorbent assay. (b,c) C57BL/6 mice were administered into both hind footpads with mitomycin C‐inactivated 3LL cells and SMP‐105 (black bar) or vehicle (white bar). Single cell suspension was prepared from (b) popliteal lymph nodes or (c) spleens and was cultured in the absence or presence of mitomycin C‐inactivated 3LL cells for 24 h. The amount of IFN‐γ in the culture supernatant was measured using enzyme‐linked immunosorbent assay.
In vivo proliferation of CTL by CWS via stimulation of TLR2 in mice. Because the Th1‐type immune response was induced in SMP‐105‐treated mice, we tried to investigate whether SMP‐105 can expand CTL in vivo. To evaluate the number of CTL, an OVA‐specific CTL transfer model was used because we could not develop an in vivo quantitative method for CTL against tumor‐associated antigens expressed in 3LL carcinoma. After spleen cells from OT‐I transgenic mice were adoptively transferred to C57BL/6 mice, C57BL/6 mice were administered with SMP‐105 and an inactivated OVA‐expressing thymoma cell line, E.G7‐OVA. Popliteal lymph node cells were prepared 4 or 7 days after administration, and the absolute number of OVA‐specific CTL was monitored by flow cytometry using H‐2Kb OVA tetramer and anti‐CD8 staining. Administration of E.G7‐OVA and SMP‐105 significantly increased the number of CTL on day 7 (P = 0.0288; Fig. 4). The number of CTL increased on day 4 although there was no significant difference (P = 0.3098). The number of CTL increased in CpG1826 ODN‐treated mice on day 7 (P = 0.0307; Fig. 4). The proliferation of CTL was not clearly observed in CpG1826 ODN‐treated mice on day 4 (P = 0.9391). These results suggest that SMP‐105 had potent activity to proliferate and maintain CTL, similar to other TLR ligands, such as CpG ODN, and the activity of SMP‐105 for induction of the Th1‐type immune response and CTL via stimulation of TLR2 possibly led to the induction of antitumor immune responses.
Figure 4.
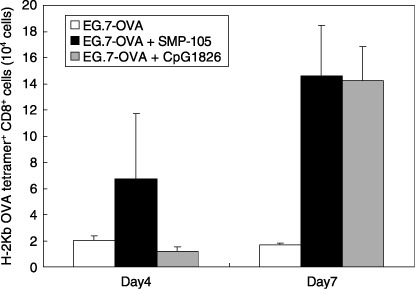
In vivo proliferation of OVA‐specific cytotoxic T lymphocytes (CTL). C57BL/6 mice, into which were adoptively transferred spleen cells of OT‐I transgenic mice, were administered with mitomycin C‐inactivated E.G7‐OVA cells alone (white bar), a mixture of cells and SMP‐105 (black bar), or CpG1826 ODN (gray bar). Popliteal lymph node cells were collected 4 or 7 days after administration and the number of OVA‐specific CTL was determined as CD8a+ OVA tetramer+ cells using a flow cytometer. The mean ± SD of two mice in each group on each day is shown.
Induction of antitumor activity by CWS via stimulation of TLR2 in wild‐type, TLR2 KO, TLR4 KO, and MyD88 KO mice. To confirm whether induction of the immune response via TLR2 stimulation by SMP‐105 leads to the suppression of tumor growth, wild‐type, TLR2, TLR4, and MyD88 KO mice were treated with SMP‐105 admixed with mitomycin C‐inactivated Lewis lung carcinoma 3LL and were inoculated with live Lewis lung carcinoma 3LL to monitor the growth of inoculated tumors. Because some of the tumor‐bearing mice developed necrotic collapses in the center of the tumor burden 15 days after inoculation, the antitumor activity of SMP‐105 was evaluated on day 15. 3LL grew progressively in vehicle‐treated wild‐type mice (Fig. 5). In contrast, 3LL was completely rejected in SMP‐105‐treated wild‐type mice (P = 0.0012; Fig. 5). Although two of five SMP‐105‐treated TLR2 KO mice allowed tumor growth on day 4, one mouse rejected the tumor growth, and the other one developed tumor growth (Fig. 5). In TLR4 KO mice, 3LL was completely rejected when mice were treated with SMP‐105 (Fig. 5). In MyD88 KO mice, the rejection of 3LL was not observed at all (Fig. 5). Thus, it was confirmed that induction of the immune response through the activation of MyD88 and TLR2 by SMP‐105 led to antitumor activity.
Figure 5.
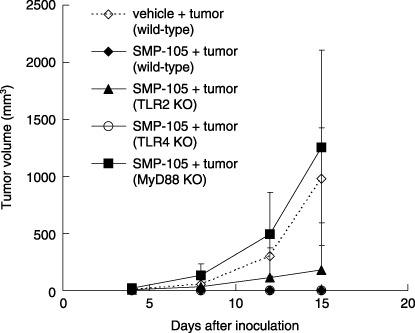
Induction of antitumor activity by vaccination of inactivated tumor with SMP‐105. Wild‐type C57BL/6, Toll‐like receptor (TLR) 2 knockout (KO), TLR4 KO, and MyD88 KO mice were administered mitomycin C‐inactivated 3LL cells and SMP‐105 or vehicle four times at 7‐day intervals. Mice were challenged with live 3LL cells in the left flank 7 days after final administration and the growth of tumors was monitered using calipers. The mean ± SD of five mice is shown.
Discussion
The induction of antitumor innate immune responses essentially requires initial TLR signaling selection. As previous papers has been reported,( 18 , 19 ) it was confirmed that a previous preparation of BCG‐CWS could induce TLR4 signaling. The present study demonstrated that SMP‐105, a new preparation of CWS from BCG Tokyo 172, selectively activated TLR2 signaling. The selectivity of TLR2 is controversial because previous studies with BCG‐CWS from different preparations has shown activity to both TLR2 and TLR4,( 18 , 19 ) which might be explained by the following possibilities: (i) the previous preparations of CWS and SMP‐105 in the present study were derived from different BCG substrains; or (ii) the decomposition of CWS or contamination with another TLR4 ligand was involved. Because there are various differences among BCG vaccine substrains, including genetic mutation, immunogenicity, and metabolites,( 31 , 32 , 33 ) it is possible that a minor component interacts with TLR4; however, as shown in Figure 1b, heat‐killed BCG, which is the raw material of SMP‐105, did not activate TLR4 signaling. Although there remains the possibility that mechanical disruption of BCG cells exposes TLR4 ligands that are masked in the whole body, the activity against TLR4 likely reflects the difference of BCG substrains. In addition, muramyl dipeptide (MDP) derivatives, such as aclyated MDP, are known to potentially activate TLR4.( 34 ) Because SMP‐105 has supramolecular architecture, it is of interest to study which part of the molecular structure contributes to TLR2 activation; however, peptidoglycans are not likely candidates because chemically synthesized partial fragments of peptidoglycan did not activate TLR2.( 35 ) Chemically synthesized mycolyl esters of arabinan showed potent activity in TNF‐α production.( 36 ) Thus, it would be interesting to find out whether these terminal branched arabinofuranosyl mycolyl esters of CWS activate TLR2.
Using SMP‐105, which selectively activated TLR2, the role of TLR2 in the induction of tumor immunity was investigated. When mice were administered SMP‐105, macrophages and dendritic cells were recruited and accumulated in draining lymph nodes (Table 1). When mice were administered SMP‐105 and inactivated tumor cells, immune responses against tumors, such as the induction of IFN‐γ‐producing cells against tumors and antigen‐specific CTL, were mounted as shown in 3, 4. These mice developed a Th1‐like immune response, which was detected as IFN‐γ‐producing activity in the spleen (Fig. 3), induced local accumulation of these cells in treated lymph nodes (Fig. 3), and maintained the increased number of CTL (Fig. 4). Interestingly, CTL in SMP‐105‐treated mice tended to grow more rapidly than in CpG1826 ODN‐treated mice. The reason for the discrepant result from CpG1826 ODN is not clearly understood at present but might be explained by several differences, such as the distribution of cells that express TLR2 or TLR9, incorporation into cells, kinetics inside cells, and pharmacokinetics. Further detailed kinetic examination is required.
The above immune responses are suggested to play an important role in antitumor activity. Indeed, as shown in Figure 5, SMP‐105 has immunoprophylaxis activity like BCG‐CWS,( 37 ) when mice were treated with inactivated tumor cells and SMP‐105. In addition, it was suggested that the induction of antitumor activity in vivo was dependent on TLR2 and MyD88 but not TLR4 because the antitumor activity of SMP‐105 was partially canceled by the lack of TLR2, completely diminished in MyD88 KO mice, and remained in TLR4 KO mice. Thus, it was suggested that TLR4 signaling is not necessary for the antitumor activity of BCG‐CWS. The reason why neutralization of immunoprophylaxis activity by the lack of TLR2 was weaker than MyD88 might be explained by the following possibilities: (i) SMP‐105 activated receptors other than TLR2 and TLR4 in vivo; and (ii) IL‐1R signaling, which shares the common adaptor MyD88 with TLR, was involved. As a trace amount of cytokines remained in the culture supernatant of TG‐PEC prepared from TLR2 KO mice as shown in Figure 2, it is possible that this effect was amplified in vivo in the induction of immune responses. Su et al. reported that adaptive immune responses, such as the delayed type hypersensitivity response, occurrs in TLR2‐, TLR4‐, and TLR9‐deficient mice, but not in MyD88‐deficient mice, and IL‐1R signaling plays a role in the adjuvant effect to induce experimental autoimmune uveitis.( 38 ) SMP‐105 consists of a peptidoglycan structure, which contains potent ligands for Nod1 and Nod2. It is possible that IL‐1R signaling compensates for the induction of antitumor activity in TLR2 KO mice. Thus, it is suggested that TLR2 activation induces innate immune responses against tumors. Although many ligands are reported to interact with TLR2, the effect of TLR2 agonists on cancer immunotherapy has been poorly reported, except for CWS and MALP‐2.( 9 , 10 , 11 ) Although both CWS and MALP‐2 were originally isolated from microorganisms, they have many different features, such as molecular structure and physicochemical properties. Because these two different TLR2 ligands have similar antitumor activity, modulating TLR2 signaling becomes more attractive for the development of immunotherapeutic agents. In addition, because the expression and distribution of TLR2 are different from those of TLR3, TLR7, TLR8, and TLR9,( 4 ) the combination of modulators acting on these receptors is expected to show more compensative benefits and tremendous potential for cancer immunotherapy.
Acknowledgments
C57BL/6‐Tg(OT‐I)‐RAG1tm1Mom line number #004175 mice were obtained through the National Institute of Allergy and Infections Diseases Exchange Program, National Institutes of Health. The author gratefully thanks Dr I. Azuma at Hokkaido Pharmaceutical University School of Pharmacy for kindly providing BCG‐CWS, Y. Ishitsubo for excellent technical assistance, and Drs N. Chiba and Y. Kashiwazaki at Dainppon Sumitomo Pharma and Dr I. Yano at Japan BCG Central Laboratory for helpful discussion and reviewing the manuscript.
References
- 1. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science 2004; 305: 200–5. [DOI] [PubMed] [Google Scholar]
- 3. Takeda K, Akira S. Toll‐like receptors in innate immunity. Int Immunol 2005; 17: 1–14. [DOI] [PubMed] [Google Scholar]
- 4. Takeda K, Kaisho T, Akira S. Toll‐like receptors. Annu Rev Immunol 2003; 21: 335–76. [DOI] [PubMed] [Google Scholar]
- 5. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double‐stranded RNA and activation of NF‐κB by Toll‐like receptor 3. Nature 2001; 413: 732–8. [DOI] [PubMed] [Google Scholar]
- 6. Yoneyama M, Kikuchi M, Natsukawa T et al . The RNA helicase RIG‐I has an essential function in double‐stranded RNA‐induced innate antiviral responses. Nat Immunol 2004; 5: 730–7. [DOI] [PubMed] [Google Scholar]
- 7. Hemmi H, Kaisho T, Takeuchi O et al . Small anti‐viral compounds activate immune cells via the TLR7 MyD88‐dependent signaling pathway. Nat Immunol 2002; 3: 196–200. [DOI] [PubMed] [Google Scholar]
- 8. Hemmi H, Takeuchi O, Kawai T et al . A Toll‐like receptor recognizes bacterial DNA. Nature 2000; 408: 740–5. [DOI] [PubMed] [Google Scholar]
- 9. Garay RP, Viens P, Bauer J et al . Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. Eur J Pharmacol 2007; 563: 1–17. [DOI] [PubMed] [Google Scholar]
- 10. Schneider C, Schmidt T, Ziske C et al . Tumour suppression induced by the macrophage activating lipopeptide MALP‐2 in an ultrasound guided pancreatic carcinoma mouse model. Gut 2004; 53: 355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shingu K, Kruschinski C, Luhrmann A et al . Intratracheal macrophage‐activating lipopeptide‐2 reduces metastasis in the rat lung. Am J Respir Cell Mol Biol 2003; 28: 316–21. [DOI] [PubMed] [Google Scholar]
- 12. Azuma I, Ribi EE, Meyer TJ, Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst 1974; 52: 95–101. [DOI] [PubMed] [Google Scholar]
- 13. Koike Y, Yoo YC, Mitobe M et al . Enhancing activity of mycobacterial cell‐derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine. Vaccine 1998; 16: 1982–9. [DOI] [PubMed] [Google Scholar]
- 14. Granatek CH, Ezaki K, Hersh EM, Keating MJ, Rasmussen S. Antibody responses of remission leukemia patients receiving active specific and nonspecific immunotherapy. Cancer 1981; 47: 272–9. [DOI] [PubMed] [Google Scholar]
- 15. Azuma I, Kanetsuna F, Taniyama T, Yamamura Y, Hori M. Adjuvant activity of mycobacterial fractions. I. Purification and in vivo adjuvant activity of cell wall skeletons of Mycobacterium bovis BCG, Nocardia asteroides 131 and Corynebacterium diphtheriae PW8. Biken J 1975; 18: 1–13. [PubMed] [Google Scholar]
- 16. Meyer TJ, Ribi EE, Azuma I, Zbar B. Biologically active components from mycobacterial cell walls. II. Suppression and regression of strain‐2 guinea pig hepatoma. J Natl Cancer Inst 1974; 52: 103–11. [DOI] [PubMed] [Google Scholar]
- 17. Taniyama T, Azuma I, Aladin AA, Yamamura Y. Effect of cell‐wall skeleton of Mycobacterium bovis BCG on cell‐mediated cytotoxicity in tumor‐bearing mice. Gann 1975; 66: 705–9. [PubMed] [Google Scholar]
- 18. Tsuji S, Matsumoto M, Takeuchi O et al . Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette–Guerin: involvement of toll‐like receptors. Infect Immun 2000; 68: 6883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uehori J, Matsumoto M, Tsuji S et al . Simultaneous blocking of human Toll‐like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette–Guerin peptidoglycan. Infect Immun 2003; 71: 4238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uenishi Y, Okada T, Okabe S, Sunagawa M. Study on the cell wall skeleton derived from Mycobacterium bovis BCG Tokyo 172 (SMP‐105): establishment of preparation and analytical methods. Chem Pharm Bull (Tokyo) 2007; 55: 843–52. [DOI] [PubMed] [Google Scholar]
- 21. Takeuchi O, Hoshino K, Kawai T et al . Differential roles of TLR2 and TLR4 in recognition of gram‐negative and gram‐positive bacterial cell wall components. Immunity 1999; 11: 443–51. [DOI] [PubMed] [Google Scholar]
- 22. Hoshino K, Takeuchi O, Kawai T et al . Cutting edge: toll‐like receptor 4 (TLR4)‐deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999; 162: 3749–52. [PubMed] [Google Scholar]
- 23. Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88‐deficient mice to endotoxin. Immunity 1999; 11: 115–22. [DOI] [PubMed] [Google Scholar]
- 24. Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell 1994; 76: 17–27. [DOI] [PubMed] [Google Scholar]
- 25. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG‐1‐deficient mice have no mature B and T lymphocytes. Cell 1992; 68: 869–77. [DOI] [PubMed] [Google Scholar]
- 26. Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 1988; 54: 777–85. [DOI] [PubMed] [Google Scholar]
- 27. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll‐like receptor 2. J Immunol 2000; 165: 618–22. [DOI] [PubMed] [Google Scholar]
- 28. Manthey CL, Perera PY, Henricson BE, Hamilton TA, Qureshi N, Vogel SN. Endotoxin‐induced early gene expression in C3H/HeJ (Lpsd) macrophages. J Immunol 1994; 153: 2653–63. [PubMed] [Google Scholar]
- 29. Manthey CL, Vogel SN. Elimination of trace endotoxin protein from rough chemotype LPS. J Endotoxin Res 1994; 1: 84–91. [Google Scholar]
- 30. Ballas ZK, Krieg AM, Warren T et al . Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol 2001; 167: 4878–86. [DOI] [PubMed] [Google Scholar]
- 31. Bedwell J, Kairo SK, Behr MA, Bygraves JA. Identification of substrains of BCG vaccine using multiplex PCR. Vaccine 2001; 19: 2146–51. [DOI] [PubMed] [Google Scholar]
- 32. Ikeda N, Honda I, Yano I, Koyama A, Toida I. Bacillus Calmette–Guerin Tokyo172 substrain for superficial bladder cancer: characterization and antitumor effect. J Urol 2005; 173: 1507–12. [DOI] [PubMed] [Google Scholar]
- 33. Matsuo T, Matsuo H, Ohara N et al . Cloning and sequencing of an MPB70 homologue corresponding to MPB83 from Mycobacterium bovis BCG. Scand J Immunol 1996; 43: 483–9. [DOI] [PubMed] [Google Scholar]
- 34. Uehori J, Fukase K, Akazawa T et al . Dendritic cell maturation induced by muramyl dipeptide (MDP) derivatives: monoacylated MDP confers TLR2/TLR4 activation. J Immunol 2005; 174: 7096–103. [DOI] [PubMed] [Google Scholar]
- 35. Fujimoto Y, Inamura S, Kawasaki A et al . Chemical synthesis of peptidoglycan fragments for elucidation of the immunostimulating mechanism. J Endotoxin Res 2007; 13: 189–96. [DOI] [PubMed] [Google Scholar]
- 36. Ishiwata A, Akao H, Ito Y, Sunagawa M, Kusunose N, Kashiwazaki Y. Synthesis and TNF‐α‐inducing activities of mycoloyl‐arabinan motif of mycobacterial cell wall components. Bioorg Med Chem 2006; 14: 3049–61. [DOI] [PubMed] [Google Scholar]
- 37. Akazawa T, Masuda H, Saeki Y et al . Adjuvant‐mediated tumor regression and tumor‐specific cytotoxic response are impaired in MyD88‐deficient mice. Cancer Res 2004; 64: 757–64. [DOI] [PubMed] [Google Scholar]
- 38. Su SB, Silver PB, Grajewski RS et al . Essential role of the MyD88 pathway, but nonessential roles of TLR 2, 4, and 9, in the adjuvant effect promoting Th1‐mediated autoimmunity. J Immunol 2005; 175: 6303–10. [DOI] [PubMed] [Google Scholar]


