Abstract
Although overexpression of human epidermal growth factor receptor 2 (HER2) protein, amplification of the gene or both are associated with poor prognosis in breast cancer, trastuzumab has clearly provided clinical benefits in metastatic breast cancer, adjuvant treatment settings and primary systemic therapy. However, even in those HER2 overexpressors, the majority of patients who achieve an initial response generally acquire resistance within 1 year. Therefore, it is critical to elucidate the mechanism of resistance and to search for better combination treatments with chemotherapeutic agents or other novel modalities. Here, we discuss both clinical and preclinical data regarding these issues. (Cancer Sci 2007; 98: 767–771)
Overexpression of human epidermal growth factor receptor 2 (HER2) protein, amplification of the gene or both occurs in approximately 20–30% of primary breast cancers, and the beneficial effects of trastuzumab treatment are seen only in patients with HER2 overexpression. However, less than 35% of patients respond to trastuzumab as a single agent. Furthermore, the majority of the patients who achieve an initial response generally acquire resistance within 1 year.( 1 , 2 ) To improve the efficacy of trastuzumab in breast cancer patients, it is critical to elucidate the mechanism of resistance of these tumors and develop better combination treatments with chemotherapeutic agents or other novel modalities.
Efficacy of trastuzumab in breast cancer patients
Metastatic breast cancer. Although 68% of the patients in a previous study were treated with anthracycline agents as adjuvant treatment after surgery, trastuzumab alone produced a response rate of only 26% in first‐line treatment. To improve this, the investigators tested a combination of chemotherapeutic agents with trastuzumab and showed additive‐to‐synergistic effects with cisplatin, carboplatin, cyclophosphamide, docetaxel, paclitaxel, vinorelbine, doxorubicin and epirubicin, among others.( 3 , 4 , 5 ) An attenuation of DNA repair activity was reported as the mechanism for synergy between trastuzumab and platinum salts (cisplatin and carboplatin).( 6 ) Pegram et al. reported that the combination of docetaxel plus trastuzumab increased antitumor efficacy against MCF7/HER2‐overexpressing xenografts compared with the combination of paclitaxel plus trastuzumab.( 7 ) The mechanism behind the unique interaction between trastuzumab and docetaxel has yet to be defined, but at least five differences between paclitaxel and docetaxel might explain the observed interaction. First, docetaxel has more potent cytotoxic antitumor effects than paclitaxel on an equimolar basis.( 8 ) Second, docetaxel achieves higher intracellular concentrations with less cellular efflux of the drug.( 9 ) Third, docetaxel has a higher affinity for microtubules than paclitaxel does.( 10 , 11 ) Fourth, coincubation of docetaxel with trastuzumab results in increased apoptosis in SK‐BR‐3 cells compared with that caused by equimolar concentrations of paclitaxel.( 12 ) Fifth, docetaxel is associated with increased phosphorylation of Bcl‐2, leading to increased apoptosis at lower concentrations of docetaxel than paclitaxel.( 13 ) Given that the combination of trastuzumab plus the chemotherapeutic agents described above showed synergistic antitumor effects, many clinical trials have been conducted and have revealed an increase in response rate, up to 50–90%.( 14 , 15 , 16 )
Adjuvant treatment
HERA trial. In the third phase III trial (HERA) (Table 1), patients were randomized after adjuvant (or neoadjuvant) chemotherapy, with or without radiation, to receive trastuzumab every 3 weeks for 1 year or for 2 years, or to receive no trastuzumab therapy (control group).( 17 ) An interim analysis was conducted after 475 events at a median follow‐up period of 1 year. The analysis included 3387 patients in the 1‐year trastuzumab arm plus the control group in whom a total of 347 events were reported (127 events in the trastuzumab group and 220 in the control group). Data from the 2‐year trastuzumab arm were not included in the interim analysis. Disease‐free survival rates 2 years after randomization were 86 and 77% for patients in the 1‐year trastuzumab group and those in the control group, respectively (hazard ratio [HR] 0.54, P < 0.0001). The study included patients of any nodal status, and patients were required to be HER2‐positive by immunohistochemistry (IHC) at the 3+ level and/or by fluorescence in situ hybridization (FISH).
Table 1.
Summary of adjuvant trastuzumab trials
| Study | Investigational treatment | Control treatment | n | HR for DFS (95% CI) | P‐value |
|---|---|---|---|---|---|
| Romond et al.( 18 ) | AC→PH | AC→P | 3351 | 0.48 (0.39–0.59) | 2 × 10−12 |
| Piccart‐Gebhart et al.( 17 ) | Chemotherapy→H | Chemotherapy | 3387 | 0.54 (0.43–0.67) | <0.0001 |
| Slamon et al.( 19 ) | AC→TH | AC→T | 3222 | 0.49 (0.37–0.65) | 4.8 × 10−7 |
| TCH | 0.61 (0.47–0.79) | 0.00015 | |||
| Joensuu et al.( 20 ) | TH→CEF | T→CEF | 232 | 0.46 (0.21–0.83) | 0.0078 |
| VH→CEF | V→CEF |
AC, doxorubicin and cyclophosphamide; CEF, cyclophosphamide, epirubicin and 5‐fluorouracil; CI, confidence interval; DFS, disease‐free survival; H, trastuzumab; HR, hazard ratio; P, paclitaxel; T, docetaxel; TCH, docetaxel, carboplatin, and trasuzumab; V, vinorelbine.
NSABP‐B31 (N9831). After a median follow‐up period of 2 years, a joint interim analysis of data from 3351 patients in two cooperative group studies from the USA (NSABP‐B31 and NCCTG N9831) showed significant improvements in the primary endpoint of disease‐free survival and secondary endpoint of overall survival with paclitaxel plus trastuzumab compared with paclitazel alone (both following anthracycline plus cyclophosphamide).( 18 ) Three years after randomization, disease‐free survival was 87% among patients in the paclitaxel plus trastuzumab group compared with 75% in the paclitaxel group (HR 0.48, P < 0.0001). After 3 years, there was also a 33% relative reduction in the number of deaths with the addition of trastuzumab (62 vs 92 deaths; HR 0.67, P = 0.015).
BCIRG 006. Interim results of the fourth phase III trial (BCIRG 006) in 3222 patients with HER2‐positive early stage breast cancer showed that, compared with a control adjuvant regimen of doxorubicin plus cyclophosphamide followed by docetaxel, there was a 51% reduction in the risk of disease recurrence with doxorubicin plus cyclophosphamide followed by docetaxel plus trastuzumab, and a 39% reduction when adjuvant therapy comprised docetaxel, carboplatin and trastuzumab.( 19 ) Results for both trastuzumab‐containing treatment arms were statistically siginificant versus the control arm (HR 0.49, P = 0.00000048; HR 0.61, P = 0.00015). The second interim analysis, BCIRG 006, presented at the San Antonio Breast Cancer Symposium 2006 showed that compared with a control adjuvant regimen of doxorubicin plus cyclophosphamide followed by docetaxel, there was a 39% reduction in the risk of disease recurrence with doxorubicin plus cyclophosphamide followed by docetaxel plus trastuzumab, and a 33% reduction when adjuvant therapy comprised docetaxel, carboplatin and trastuzumab. 19 Results for both trastuzumab‐containing treatment arms were again statistically significant versus the control arm (HR 0.61, P = 0.000011; HR 0.67, P = 0.00028). In the subset analysis, it was shown that coamplification of topoisomerase IIα may confer a therapeutic advantage to an anthracycline‐based regimen.
FinHer Study. A smaller adjuvant therapy trial from Finland, FinHer, showed a significant advantage in the use of trastuzumab for only 9 weeks in the adjuvant therapy setting (in combination with docetaxel or vinorelbine).( 20 ) The study involved 1010 patients randomized to docetaxel every 3 weeks for three doses versus 9 weeks of vinorelbine followed, in both groups, by three 3‐week cycles of cyclophosphamide, epirubicin and 5‐fluorouracil (CEF). The 232 patients found to have HER‐2/neu‐positive breast cancer by chromogenic in situ hybridization (CISH) were randomized to receive weekly trastuzumab for 9 weeks along with docetaxel and vinorelbine. At a median follow‐up of 3 years, adjuvant trastuzumab was effective in preventing breast cancer recurrences (HR 0.46; P = 0.0078).
Primary systemic therapy. Several phase II trials have evaluated the use of trastuzumab in the neoadjuvant setting.( 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ) Although not always explicitly stated, pathological complete response was the primary endpoint in most of these studies. Various preoperative regimens that included trastuzumab patients with early stage HER2‐positive breast cancer have shown promising results, as outlined in Table 2. The rates of pathological complete response ranged from 13 to 65% and those for clinical overall response ranged from 72 to 96%, with the majority being clinical complete responses.
Table 2.
Summary of neoadjuvant trastuzumab trials
| Study | Regimen of primary systemic therapy | pCR rate | Clinical OR |
|---|---|---|---|
| Bines et al.( 21 ) | Doc 36 mg/m2 q1 week × 12 (over 14 week) + Tra q1 week × 14 | 13 | 72 |
| Burstein et al.( 22 ) | Pac 175 mg/m2 q3 week × 4 + Tra q1 week × 12 | 18 | 75 |
| Buzdar et al.( 23 ) | Pac 225 mg/m2 q3 week × 4 + Tra q1 week × 12 then FEC × 4 + Tra q1 week × 12 | 65 | 96 |
| Pac 225 mg/m2 q3 week × 4 then FEC × 4 | 26 | 95 | |
| Coudert et al.( 24 ) | Doc 100 mg/m2 q3 week × 6 + Tra q1 week × 18 | 36 | 96 |
| Harris et al.( 25 ) | Vin 25 bg/m2 q1 week + Tra q1 week × 12 | 21 | 92 |
| Hurley et al.( 26 ) | Doc 70 mg/m2 + Cis 70 mg/m2 q3 week × 4 + Tra q1 week × 12 | 21 | |
| Van Pelt et al.( 27 ) | Doc 100 mg/m2 q3 week × 4 + Tra q1 week × 12 | 77 | |
| Kelly et al.( 28 ) | AC q3 week × 4 then Tra + Pac q1 week × 12 | 19 | 86 |
AC, doxorubicine + cyclophosphamide; Cis, cisplatin; Doc, docetaxel; FEC, 5‐fluorouracil + epirubicin + cyclophosphamide; OR, overall response; Pac, paclitaxel; pCR, pathological complete response; Vin, vinorelbine; qxwk, every × weeks.
One phase III trial with a planned sample size of 164 patients was halted early because an interim analysis showed a statistical advantage for trastuzumab plus chemotherapy versus chemotherapy alone in terms of the pathological complete response rate, which was the primary endpoint of this study.( 23 ) Pathological complete response rates were 65 versus 26% (P = 0.016) among 42 randomized patients. Although clinical overall response rates were similar between the groups (96 vs 95%), clinical complete response rates were numerically higher with the trastuzumab‐containing regimen (87 vs 47%). In addition, trastuzumab was generally well tolerated when used concurrently with the anthracycline‐containing regimen in this study; however, as mentioned in a comment by Ahluwalia and Daw,( 29 ) the addition of trastuzumab to anthracycline‐based chemotherapy should not be used on a routine basis for the treatment of operable breast cancer. Further research is required, particularly to further establish the long‐term cardiac safety of this regimen.
Mechanism of action of trastuzumab
Trastuzumab has been shown to have multiple mechanisms of action based on in vitro studies (Fig. 1). (1) The antibody binds to the extracellular domain of Her‐2/neu and inhibits the downstream signaling cascade, resulting in growth inhibition of Her‐2/neu‐overexpressing tumor cells. This inhibitory capacity was found to be associated with internalization of the receptor–antibody complex and movement into endocytic vesicles.( 30 ) (2) Treatment of HER2‐overexpressing breast cancer cell lines with trastuzumab results in induction of p27KIP1 and the Rb‐related protein p130, which in turn significantly reduces the number of cells undergoing the transition to S‐phase (G1 arrest).( 31 ) (3) HER2 undergoes proteolytic cleavage that results in release of the extracellular domain and production of the truncated membrane‐bound fragment p95. This HER2 shedding is activated by 4‐aminophenylmercuric acetate, a well‐known matrix metallopratease activator, in HER2‐overexpressing breast cancer cells. The HER2 p95 fragment is phosphorylated and has kinase activity. Trastuzumab inhibits basal and induced HER2 cleavage and, as a consequence, the generation of phosphorylated p95.( 32 ) (4) Antigen‐dependent cellular cytotoxicity (ADCC), a lytic attack on antibody‐targeted cells, is triggered following binding of the Fc region of an antibody to the Fcγ receptor IIIa (FcγRIIIa) expressed on natural killer (NK) cells. The clinical importance of ADCC was first demonstrated with rituximab (Rituxan), an anti‐CD20 chimeric antibody approved for non‐Hodgkin's lymphoma treatment in 1998.( 33 , 34 , 35 ) These studies have focused on the relationships between the clinical response and FcγRIIIa gene (FCGR3A) functional polymorphism that generates either phenylalanine (F) or valine (V) at amino acid position 158, with significantly better clinical responses for patients having FCGR3A‐158 V allele associated with strong IgG binding to the receptor and ADCC activation.( 36 , 37 ) More recently, ADCC involvement in the clinical response was also suggested for trastuzumab therapy with methods seemingly more direct than FCGR3A genotyping. Gennari et al. showed a significant correlation between clinical responses and ADCC‐mediated killing by patients’ peripheral blood mononuclear cells (PBMC).( 38 ) Furthermore, Arnould et al. showed an increased infiltration of NK cells into tumor tissue of trastuzumab‐responding patients.( 39 ) These reports support an in vivo role for ADCC in trastuzumab therapy. (5) Inhibition of angiogenesis has also been reported.( 40 )
Figure 1.
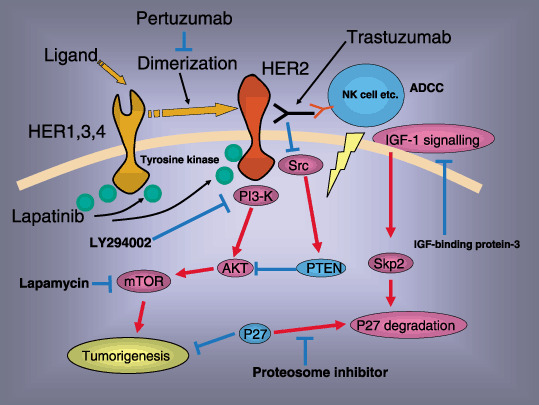
The mechanisms of action of trastuzumab. Direct activity (induction of apoptosis: phosphatidylinositol‐3‐kinase [PI3K]–Akt pathway) and antigen‐dependent cellular cytotoxicity (ADCC). The mechanisms of resistance: Downregulation of p27, loss of PTEN activity and activation of insulin‐like growth factor (IGF)‐I signaling. Lapamycin, IGF‐binding protein‐3, proteosome inhibitor or LY294002 might restore the resistance of trastuzumab. ADCC, antigen‐dependent cellular cytotoxicity; HER, human epidermal growth factor receptor; IGF, insulin‐like growth factor; NK, natural killer.
Mechanism of resistance
Although trastuzumab provides important clinical benefits for a substantial proportion of HER2‐positive breast cancer patients with well‐defined HER2 overexpression or gene amplification, many patients do not respond to trastuzumab, thus underscoring the importance of determining the mechanisms of clinical sensitivity versus resistance. Currently, there is no clinically verified factor that can be used to predict trastuzumab resistance. However, possible mechanisms of resistance have been reported. (1) Nahta et al. created two trastuzumab‐resistant (TR) pools from the SKBR3 HER2‐overexpressing breast cancer cell line and demonstrated that the cyclin‐dependent kinase inhibitor p27kip1 was decreased in the TR cells and cyclin‐dependent kinase activity was increased.( 41 ) Exogenous addition of p27kip1 increased trastuzumab sensitivity and the resistant cells displayed heightened sensitivity to proteasome inhibitor MG132, which induced p27kip1 expression. Thus, it is suggested that trastuzumab resistance may be associated with decreased p27kip1 levels and may be susceptible to treatments that induce p27kip1 expression. 41 (2) PTEN (MMAC1/TEP) is a dual phosphatase that mainly dephosphorylates position D3 of membrane phosphatidylinositol‐3,4,5 triphosphate (PI3,4,5P3), which is the site for recruiting the plecstrin‐homology domain of Akt to the cell membrane. As phosphatidylinositol‐3‐kinase (PI3K) catalyzes the production of PI3,4,5P3, PTEN antagonizes this PI3K function and negatively regulates Akt activities. Trastuzumab treatment quickly increases PTEN membrane localization and phosphatase activity by recruiting PTEN tyrosine phosphorylation via Src inhibition. Reducing PTEN in breast cancer cells by antisense oligonucleotides confers trastuzumab resistance in vitro and in vivo. Patients with PTEN‐deficient breast cancers had significantly poorer responses to trastuzumab‐based therapy than those with normal PTEN. Interestingly, LY294002, PI3K inhibitors rescued PTEN loss‐induced trastuzumab resistance, suggesting that PI3K‐targeting therapies could overcome this resistance.( 42 ) (3) Trastuzumab inhibited the growth of MCF‐7/HER2‐18 cells, which overexpress HER2/neu receptors and express insulin‐like growth factor (IGF)‐I receptors (IGFIR). In 1% fetal bovine serum (FBS), trastuzumab reduced cell proliferation by 42%; however, in 10% FBS or IGF‐I, trastuzumab had no effect on proliferation. In SKBR3 cells, which overexpress HER2/neu receptor but express few IGFIR, trastuzumab reduced proliferation by 42% regardless of IGF‐I concentration. When SKBR3 cells were genetically altered to overexpress IGFIR and were cultured with IGF‐I, trastuzumab had no effect on proliferation. However, the addition of IGF‐binding protein‐3, which decreased IGFIR signaling, restored trastuzumab‐induced growth inhibition. Thus, it is suggested that strategies that target IGFIR signaling may prevent or delay development of resistance to trastuzumab.( 43 )
Possibilities of improving the efficacy of trastuzumab therapy
Lapatinib. Lapatinib is an oral receptor tyrosine kinase inhibitor, targeting both epidermal growth factor receptor (EGFR) and HER2. Pre‐clinical in vitro and in vivo models indicate that lapatinib is active as a monotherapy, synergistically in combination with trastuzumab, and in trastuzumab‐resistant cell lines. Konecny et al. tested the therapeutic potential of lapatinib in a panel of 31 characterized human breast cancer cell lines, including trastuzumab‐conditioned HER‐2‐positive cell lines, and reported that for the combination of lapatinib plus trastuzumab, synergistic drug interactions were observed in four different HER‐2‐positive cell lines. Moreover, lapatinib retained in vitro activity against cell lines selected for long‐term outgrowth in trastuzumab‐containing culture medium. Thus, these findings might provide a biological rationale to test lapatinib in combination with trastuzumab in HER‐2‐overexpressing breast cancer and in patients with clinical resistance to trastuzumab.( 44 ) There has been one phase I trial of lapatinib plus trastuzumab in metastatic breast cancer( 45 ) and two phase II trials of single‐agent lapatinib in patients with refractory metastatic breast cancer.( 46 , 47 )
Pertuzumab. Pertuzumab, the recombinant humanized monoclonal antibody 2C4, binds to a different epitope on erbB2 than trastuzumab, and inhibits both homodimerization and heterodimerization with other erbB receptors and blocks ligand‐activated signaling from HER‐2/EGFR and HER‐2/HER‐3 heterodimers.( 48 ) The combination of trastuzumab and pertuzumab synergistically inhibits the survival of BT474 breast cancer cell lines, in part because of increased apoptosis. Trastuzumab increases 2C4‐mediated disruption of erbB2 dimerization with EGFR and erbB3. Combination drug treatment reduced levels of total and phosphorylated erbB2 protein and blocked receptor signaling through Akt, but did not affect MAPK. These results suggest that combining erbB2‐targeting agents may be a more effective therapeutic strategy in breast cancer than treatment with a single erbB2 monoclonal antibody.( 49 ) A phase II trial with trastuzumab and pertuzumab in patients with HER2‐overexpressed locally advanced and metastatic breast cancer has been conducted.( 50 )
Mammalian target of rapamycin antagonist. Mammalian target of rapamycin antagonist (mTOR) is a serine‐threonine kinase member of the cellular PI3K pathway that is involved in multiple functions such as transcriptional and translational control. Activation of mTOR as a consequence of nutrients and growth factors results in the phosphorylation and activation of the 40S ribosomal protein S6 kinase and the eukaryotic initiation factor 4E‐binding protein‐1. These proteins play a key role in ribosomal biogenesis and cap‐dependent translation, which result in increased translation of mRNA that is important to the control and progression of the cell cycle. mTOR is a downstream mediator in the PI3K–Akt signaling pathway and plays a critical role in cell survival. In breast cancer the PI3K–Akt pathway can be activated by membrane receptors, including the HER family, the IGF receptor, and the estrogen receptor.( 51 ) There is evidence suggesting that Akt promotes breast cancer cell survival and resistance to chemotherapy, trastuzumab and tamoxifen. This suggests that targeting the Akt–PI3K pathway with mTOR antagonists may increase the therapeutic efficacy of trastuzumab‐resistant breast cancer.( 52 )
Fucose‐negative trastuzumab. It was reported that removal of fucose from antibody oligosaccharides attached to Asn297 of the heavy chain (defucosylation) significantly enhanced ADCC compared to the conventional antibody.( 53 , 54 , 55 , 56 ) Thus, this modulation of antibody could be one of the most powerful approaches to improve efficacy in cancer antibody therapy, and we evaluated the ADCC of commercial trastuzumab (fucosylated) and its fucose‐negative version using PBMC drawn from the volunteers as effector cells and two breast cancer cell lines with different HER2 expression levels as target cells. ADCC was significantly enhanced with the fucose‐negative antibody compared to the fucose‐positive antibody. This preliminary study suggests that the use of fucose‐negative antibodies may improve the therapeutic effects of anti‐HER2 therapy in breast cancer.( 57 )
Future perspectives
Despite significant improvements in the analysis of mechanisms of action and resistance and clinical outcome with trastuzumab, it is still necessary to resolve the following questions. (1) Optimal timing for the induction of trastuzumab: The results of trastuzumab‐based treatment in an adjuvant setting are more impressive than those in metastatic breast cancer. It might be better to start trastuzumab treatment earlier, such as in a primary systemic therapy setting, although neoadjuvant chemotherapy did not show clinical benefit when compared with an adjuvant setting. (2) Optimal duration: Final results from the HERA trial could reveal the optimal duration of trastuzumab treatment (1 vs 2 years). (3) Optimal combination treatment: In addition to chemotherapeutic agents or hormonal treatment, novel molecular targeting therapies, such as lapatinib or bevacizumab, could show clinical benefits. (4) Search for the prediction marker for responder: PTEN could be a promising marker for selecting responders to trastuzumab. Having a clinically useful prediction marker to select responders to trastuzumab is very important for improving health economics because of the high cost of trastuzumab treatment.
References
- 1. Cobleigh MA, Vogel CL, Tripathy D et al. Multinational study of the efficacy and safety of humanized anti‐HER2 monoclonal antibody in women who have HER2‐overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17: 2639–48. [DOI] [PubMed] [Google Scholar]
- 2. Vogel CL, Cobleigh MA, Tripathy D et al. Efficacy and safety of trastuzumab as a single agent in first‐line treatment of HER2‐overexpressing metastatic breast cancer. J Clin Oncol 2002; 20: 719–26. [DOI] [PubMed] [Google Scholar]
- 3. Baselga J, Norton L, Masui L et al. Antitumor effects of doxorubicin in combination with anti‐epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst 1993; 85: 1327–33. [DOI] [PubMed] [Google Scholar]
- 4. Baselga J, Norton L, Albanell J et al. Recombinant humanized anti‐HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58: 2825–31. [PubMed] [Google Scholar]
- 5. Pegram M, Hsu S, Lewis G et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004; 96: 739–49. [DOI] [PubMed] [Google Scholar]
- 6. Pegram M, Hsu S, Lewis G et al. Inhibitory effects of combinations of HER‐2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999; 18: 2241–51. [DOI] [PubMed] [Google Scholar]
- 7. Pegram M, Konecny GE, O’Callaghan C et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004; 19: 739–49. [DOI] [PubMed] [Google Scholar]
- 8. Braakhuis BJ, Hill BT, Dietel M et al. In vitro antiproliferative activity of docetaxel (Taxotere), paclitaxel (Taxol) and cisplatin against human tumour and normal bone marrow cells. Anticancer Res 1994; 14: 205–8. [PubMed] [Google Scholar]
- 9. Riou JF, Petigenet O, Combeau C et al. Cellular uptake and efflux of docetaxel and paclitaxel in P388 cell line. Proc Am Assoc Cancer Res 1994; 35: 385. [Google Scholar]
- 10. Diaz JF, Andreu JM. Assembly of purified GDP‐tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry 1993; 32: 2747–55. [DOI] [PubMed] [Google Scholar]
- 11. Fromes Y, Gounon P, Veitia R et al. Influence of microtubule‐associated proteins on the differential effects of paclitaxel and docetaxel. J Protein Chem 1996; 15: 377–88. [DOI] [PubMed] [Google Scholar]
- 12. O'Donovan N, Beryt M, Duffy MJ et al. HER‐2/neu and apoptosis in breast cancer. Proc Am Assoc Cancer Res 2002; 43: 603. [Google Scholar]
- 13. Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res 1997; 57: 229–33. [PubMed] [Google Scholar]
- 14. Tedesco KL, Thor AD, Johnson DH et al. Docetaxel combined with trastuzumab is an active regimen in HER‐2 3+ overexpressing and fluorescent in situ hybridization‐positive metastatic breast cancer: a multi‐institutional phase II trial. J Clin Oncol 2004; 22: 1071–7. [DOI] [PubMed] [Google Scholar]
- 15. Pegram MD, Pienkowski T, Northfelt DW et al. Results of two open‐label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2‐positive advanced breast cancer. J Natl Cancer Inst 2004; 96: 759–69. [DOI] [PubMed] [Google Scholar]
- 16. Burstein HJ, Harris LN, Marcom PK et al. Trastuzumab and vinorelbine as first‐line therapy for HER2‐overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol 2003; 21: 2889–95. [DOI] [PubMed] [Google Scholar]
- 17. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B et al. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med 2005; 353: 1659–72. [DOI] [PubMed] [Google Scholar]
- 18. Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005; 353: 1673–84. [DOI] [PubMed] [Google Scholar]
- 19. Slamon D, Eiermann W, Robert N et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG study. Breast Cancer Res Treat 2005: 94 (Suppl 1): S5a. [Google Scholar]
- 20. Joensuu H, Kellokumpu‐Lehtinen PL, Bono P et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006; 354: 809–20. [DOI] [PubMed] [Google Scholar]
- 21. Bines J, Murad A, Lago S et al. Primary treatment with weekly docetaxel (Taxotere) and trastuzumab (Herceptin) for HER‐2 overexpressing locally advanced breast cancer. Eur J Cancer 2003; 1 (Suppl 5): S114. [Google Scholar]
- 22. Burstein HJ, Harris LN, Gelman R et al. Use of the peroxisome proliferator‐activated receptor (PPAR) gamma ligand troglitazone as treatment for refractory breast cancer: a phase II study. J Clin Oncol 2003; 21: 46–53. [DOI] [PubMed] [Google Scholar]
- 23. Buzdar AU, Ibrahim NK, Francis D et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2‐positive operable breast cancer. J Clin Oncol 2005; 23: 3676–85. [DOI] [PubMed] [Google Scholar]
- 24. Coudert BP, Arnould L, Moreau L, et al. Pathological complete response rate with neoadjuvant trastuzumab and decetaxel chemotherapy in HER‐2 positive (3+) locally advanced breast cancer (Abstract no. 128P). Ann Oncol 2004; 15 (Suppl 3): 34. [Google Scholar]
- 25. Harris LN, Burstein H, Gelman R et al. Preoperative trastuzumab and vinorelbine is a well‐tolerated, active regimen for Her2, 3+/FISH+ stage II/III breast cancer (Abstract no. 397). Eur J Cancer 2003; 1 (Suppl 5): S121. [Google Scholar]
- 26. Hurley J, Dolny P, Silva O et al. Neoadjuvant herceptin/taxotere/cisplatin in the treatment of locally advanced and inflammatory breast cancer (Abstract no. 196). 38th Annual Meeting of the American Society of Clinical Oncology, 18–21 May 2002, Orlando, FL, USA.
- 27. Van Pelt AE, Mohsin S, Elledge RM et al. Neoadjuvant trastuzumab and docetaxel in breast cancer: preliminary results. Clin Breast Cancer 2003; 4: 348–53. [DOI] [PubMed] [Google Scholar]
- 28. Kelly H, Kimmick G, Dees EC et al. Response and cardiac toxicity of trastuzumab given in conjunction with weekly paclitaxel after doxorubicin/cyclophosphamide. Clin Breast Cancer 2006; 7: 237–43. [DOI] [PubMed] [Google Scholar]
- 29. Ahluwalia MS, Daw HA. Neoadjuvant therapy with trastuzumab, paclitaxel and epirubicin for HER‐2‐positive operable breast cancer. J Clin Oncol 2005; 20: 7759–60. [DOI] [PubMed] [Google Scholar]
- 30. Hurwitz E, Stancovski I, Sela M et al. Suppression and promotion of tumor growth by monoclonal antibodies to ErbB‐2 differentially correlate with cellular uptake. Proc Natl Acad Sci USA 1995; 92: 3353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sliwkowski MX, Lofgren JA, Lewis GD et al. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol 1999; 26: 60–70. [PubMed] [Google Scholar]
- 32. Molina MA, Codony‐Servat J, Albanell J et al. Trastuzumab (herceptin), a humanized anti‐Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res, 2001; 84: 4744–9. [PubMed] [Google Scholar]
- 33. Leget GA, Czuczman MS. Use of rituximab, the new FDA‐approved antibody. Curr Opin Oncol 1998; 10: 548–51. [DOI] [PubMed] [Google Scholar]
- 34. Gopal AK, Press OW. Clinical applications of anti‐CD20 antibodies. J Lab Clin Med 1999; 134: 445–50. [DOI] [PubMed] [Google Scholar]
- 35. Smith MR. Rituximab (monoclonal anti‐CD20 antibody): mechanisms of action and resistance. Oncogene 2003; 22: 7359–68. [DOI] [PubMed] [Google Scholar]
- 36. Koene HR, Kleijer M, Algra J, Von Roos D, Dem Born AE, De Haas M. FcgRIIIa‐158V/F polymorphism influences the binding of IgG by natural killer cell FcgRIIIa, independently of the FcgRIIIa‐48L/R/H phenotype. Blood 1997; 90: 1109–14. [PubMed] [Google Scholar]
- 37. Wu J, Edberg JC, Redecha PB et al. A novel polymorphism of FcgRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest 1997; 100: 1059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gennari R, Menard S, Fagnoni F et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumours overexpressing HER2. Clin Cancer Res 2004; 10: 5650–5. [DOI] [PubMed] [Google Scholar]
- 39. Arnould L, Gelly M, Penault‐Liorca F et al. Trastuzumab‐based treatment of HER2‐positive breast cancer: an antibody‐dependent cellular cytotoxicity mechanism? Br J Cancer 2006; 94: 259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Izumi Y, Xu L, Di Tomaso E et al. Tumour biology: herceptin acts as an anti‐angiogenic cocktail. Nature 2002; 416: 279–80. [DOI] [PubMed] [Google Scholar]
- 41. Nahta R, Takahashi T, Ueno NT et al. P27 (kip1) down‐regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res 2004; 64: 2343–6. [DOI] [PubMed] [Google Scholar]
- 42. Nagata Y, Lan KH, Zhou X et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004; 6: 117–27. [DOI] [PubMed] [Google Scholar]
- 43. Lu Y, Zi X, Zhao Y et al. Insulin‐like growth factor‐I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 2001; 93: 1852–7. [DOI] [PubMed] [Google Scholar]
- 44. Konecny GE, Pegram MD, Venkatesan N et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER‐2‐overexpressing and trastuzumab‐treated breast cancer cells. Cancer Res 2006; 66: 1630–9. [DOI] [PubMed] [Google Scholar]
- 45. Storniolo A, Burris H, Pegram M et al. A phase I, open label study of lapatinib (GW572016) plus trastuzumab; a clinically active regimen (Abstract no. 559). J Clin Oncol 2005; 23 (Suppl 16): S18. [Google Scholar]
- 46. Blackwell KL, Kaplan EH, Franco SX et al. A phase II, open label, multicenter study of GW572016 in patients with trastuzumab‐refractory metastatic breast cancer. J Clin Oncol 2004; 22 (Suppl 14): 3006. [Google Scholar]
- 47. Burstein H, Storniolo AM, Franco S et al. A phase II, open label, multicenter study of GW572016 in two cohorts of patients with advanced or metastatic breast cancer who have progressed while receiving trastuzumab‐containing regimens. Ann Oncol 2004; 15 (Suppl 13): A103. [Google Scholar]
- 48. Agus DB, Akita RW, Fox WD et al. Targeting ligand‐activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2: 127–37. [DOI] [PubMed] [Google Scholar]
- 49. Nahta R, Hung MC, Esteva FJ. The HER‐2‐targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 2004; 64: 2343–6. [DOI] [PubMed] [Google Scholar]
- 50. Walshe JM, Denduluri N, Berman AW et al. A phase II trial with trastuzumab and pertuzumab in patients with HER2‐overexpressed locally advanced and metastatic breast cancer. Clin Breast Cancer 2006; 6: 535–9. [DOI] [PubMed] [Google Scholar]
- 51. Dancey J, Sausville EA. Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov 2003; 2: 296–313. [DOI] [PubMed] [Google Scholar]
- 52. Neshat MS, Mellinghoff IK, Tran C et al. Enhanced sensitivity of PTEN‐deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA 2001; 98: 10 314–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shinkawa T, Nakamura K, Yamane N et al. The absence of fucose but not the presence of galactose or bisecting N‐acetylglucosamine of human IgG1 complex‐type oligosaccharides shows the critical role of enhancing antibody‐dependent cellular cytotoxicity. J Biol Chem 2003; 278: 3466–73. [DOI] [PubMed] [Google Scholar]
- 54. Niwa R, Shoji‐Hosaka E, Sakurada M et al. Defucosylated anti‐CC chemokine receptor 4 IgG1 with enhanced antibody‐dependent cellular cytotoxicity shows potent therapeutic activity to T cell leukemia and lymphoma. Cancer Res 2004; 64: 2127–33. [DOI] [PubMed] [Google Scholar]
- 55. Okazaki A, Shoji‐Hosaka E, Nakamura K et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgRIIIa. J Mol Biol 2004; 336: 1239–49. [DOI] [PubMed] [Google Scholar]
- 56. Niwa R, Hatanaka S, Shoji‐Hosaka E et al. Enhancement of the antibody‐dependent cellular cytotoxicity of low‐fucose IgG1 is independent of FcgRIIIa functional polymorphism. Clin Cancer Res 2004; 10: 6248–55. [DOI] [PubMed] [Google Scholar]
- 57. Suzuki E, Niwa R, Sagi S et al. A non‐fucosylated anti‐HER2 antibody augments antibody‐dependent cellular cytotoxicity of breast cancer patients. Clin Cancer Res 2007; 13: 1875–82. [DOI] [PubMed] [Google Scholar]


