Abstract
Aberrant activation of Wnt signaling is a critical event in the development of human colorectal tumors. The aim of this study was to elucidate the role of B9L and its association with β‐catenin in each stage of the adenoma‐carcinoma sequence of human colorectal tumorigenesis. We investigated the expression levels of B9L in sporadic colorectal adenomas and carcinomas, categorized according to the Vienna classification, using real‐time quantitative polymerase chain reaction (RTQ‐PCR) and immunohistochemical analysis. B9L was expressed in the nuclei of non‐neoplastic colonic mucosa cells and was overexpressed in the nuclei of neoplasias and invasive carcinoma cells. Immunoreactivity to B9L protein was correlated with the progressive grades of colorectal neoplasias, as was the expression of B9L mRNA. A high level of immunoreactivity to nuclear B9L was present in 27% of low‐grade neoplasias and in more than 50% of high‐grade neoplasias and invasive carcinomas, whereas a high level of nuclear B9L immunoreactivity was not observed in any of the non‐neoplastic mucosa samples. The expression of B9L was dramatically increased in low‐grade neoplasias compared with that in non‐neoplastic mucosa. B9L may play an important role in tumorigenesis induced by aberrant activation of Wnt signaling and may act as a key protein in the progressive dysplasia of adenoma. (Cancer Sci 2007; 98: 83–87)
Sporadic and hereditary forms of colorectal cancer develop along a well‐defined sequence of histopathologic changes.( 1 ) The earliest lesions, aberrant crypt foci (ACF), occur in the colonic epithelium and are characterized by neoplastic or hyperplastic crypts. Subsequent expansion of the ACF generates a larger adenoma, which in turn may progress to carcinomas in situ and to invasive adenocarcinomas. The earliest mutations identified in the adenoma‐to‐carcinoma sequence alter the function of components of the Wnt pathway.( 1 , 2 ) The overwhelming majority (80%) of early adenomas from sporadic cases of colorectal cancer bear truncating mutations in the adenomatous polyposis coli gene (APC).( 3 ) Some of the remaining cases of colorectal cancer result from mutations in β‐catenin or in Axin2.( 4 , 5 , 6 )β‐catenin/TCF‐regulated transcription is constitutively activated by the inactivation of APC or by the activation of β‐catenin, thereby activating the transcription of Wnt target genes.( 7 , 8 , 9 )
It has recently been shown that the transactivation potential of the β‐catenin/TCF complex is activated by its interaction with B9L/BCL9‐2, a protein related to the product of BCL9, which was identified previously as a gene that is over‐expressed in a B‐cell precursor acute lymphoblastic leukemia line carrying the translocation t(1;14)(q21;q32), t(1;22)(q21;q11).( 10 , 11 ) Also, it has been shown that Legless (Lgs), the Drosophila homolog of BCL9, activates Wnt signaling by physically linking Pygopus (Pygo) to β‐catenin.( 12 , 13 , 14 ) B9L has 35% overall amino acid identity with BCL9, with six highly homologous domains, which include the Pygo binding domain HD1 and the β‐catenin binding domain HD2. B9L is overexpressed in human colorectal tumors, relative to expression in the corresponding non‐neoplastic tissues.( 15 )
To elucidate the role of B9L and its association with β‐catenin in each stage of the adenoma‐carcinoma sequence of human colorectal tumorigenesis, we investigated the expression of B9L mRNA in sporadic colorectal adenomas and carcinomas using real‐time quantitative polymerase chain reaction (RTQ‐PCR), and we examined B9L and β‐catenin protein expression by immunohistochemistry.
Materials and Methods
Patients, sampling, and tumor morphology. A total of 127 sporadic colorectal neoplasms diagnosed histologically as adenomas and/or adenocarcinomas, not invasive beyond the muscle layer, were selected from 127 patients, based on the pathologic records of Gunma University and Maebashi Red Cross Hospitals, Japan, from January 2001 to August 2004. The neoplasms had been endoscopically resected or resected during surgery for colorectal carcinoma. Samples of non‐neoplastic tissues were also obtained endoscopically or surgically. Samples were randomly selected based on tumor size and initial pathologic diagnosis in order to minimize their differences. Samples from patients with familial adenomatous polyposis, hereditary non‐polyposis colorectal cancer, or inflammatory bowel disease were excluded. In the morphological classification, ‘protruded neoplasms’ included sessile, semipedunculated, and pedunculated lesions; ‘superficial neoplasms’ included flat and depressed lesions. The clinicopathologic characteristics are shown in Table 1.
Table 1.
Paients and clinicopathologic characteristics
| RTQ‐PCR analysis | Immunohistochemical analysis | |||
|---|---|---|---|---|
| No. of patients 27 | % | No. of patients 100 | % | |
| Samples | ||||
| Endoscopically resected | 15 | 56 | 76 | 76 |
| Surgically resected | 12 | 44 | 24 | 24 |
| Characteristic | ||||
| Age | ||||
| Mean (year) | 69 | 67 | ||
| Range | 42–85 | 42–87 | ||
| ≤65 years | 8 | 30 | 42 | 42 |
| >65 years | 19 | 70 | 58 | 58 |
| Sex | ||||
| Male | 19 | 70 | 70 | 70 |
| Female | 8 | 30 | 30 | 30 |
| Location | ||||
| Right colon | 10 | 37 | 33 | 33 |
| Left colon | 8 | 40 | 42 | 42 |
| Rectum | 9 | 44 | 25 | 25 |
| Tumor size | ||||
| Mean (mm) | 13 | 12 | ||
| Range | 5–45 | 3–45 | ||
| ≤10 mm | 10 | 37 | 33 | 33 |
| >10 mm | 17 | 63 | 67 | 67 |
| Morphological type | ||||
| Protruded | 24 | 89 | 81 | 81 |
| Superficial | 3 | 11 | 19 | 19 |
The ethics committee of Gunma University Graduate School of Medicine approved the use of the tissue samples for DNA and RNA analyses, and written informed consent was obtained from all patients.
Tumor samples. Samples for RNA extraction were snap‐frozen immediately in liquid nitrogen and stored at −80°C for later analysis.
Histologic classification. Neoplastic and corresponding non‐neoplastic regions were categorized according to the Vienna classification of gastrointestinal epithelial neoplasia( 16 , 17 ) by two pathologists (T.O., K.K.), in a blinded manner. Samples for RNA extraction were also histologically confirmed by the pathologists. Those sample classifications that were consistent between the two pathologists were accepted; for inconsistent classifications, the pathologists conferred and agreed on a final classification. The categories used were: 1 = negative for neoplasia/dysplasia; 2 = indefinite for neoplasia/dysplasia; 3 = non‐invasive low‐grade neoplasia (low‐grade adenoma/dysplasia); 4 = non‐invasive high‐grade neoplasia, including high‐grade adenoma/dysplasia and non‐invasive carcinoma (carcinoma in situ); and 5 = invasive neoplasia.
RNA extraction and cDNA synthesis. Total RNA was extracted using RNeasy Mini kits (Qiagen, Hilden, Germany) according to the manufacturer's protocol. All samples were DNase‐treated to remove contaminating DNA, and the yield was measured spectrophotometrically. The RNA quality was assayed on ethidium bromide‐stained 1% agarose gels containing 2.2 M formaldehyde. One microgram of RNA was combined with 0.5 µg oligo(dT)15 primer and used in an ImProm‐II reverse transcription system (Promega, Madison, WI) for the first‐strand synthesis reaction in the presence of 1 U/µL recombinant RNase inhibitor and 6 mM MgCl2.
Real‐time quantitative PCR (RTQ‐PCR) analysis. Real‐time PCR was performed using an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA); data collection and analyses were performed using the accompanying software.
Polymerase chain reaction primers and fluorogenic probes were purchased as TaqMan Gene Expression Assays (Applied Biosystems). A predeveloped TaqMan kit was used for the amplification of a human β‐actin cDNA fragment as an internal control; the assay ID for the B9L kit is Hs00699441_m1. B9L and β‐actin cDNAs were amplified together in duplicate from each sample. All of the experiments were repeated twice. A non‐template control (RNase‐free water) was included in every set of reactions. The thermal cycler conditions were: 2 min at 50°C, 15 min at 95°C, and then 45 cycles of 95°C for 15 s and 60°C for 1 min
The expression level of B9L mRNA was normalized to that of β‐actin using a standard curve, as described by the manufacturer. The B9L expression ratio for a sample was calculated as the ratio between the average B9L signal in the tumor RNA and the signal in RNA from normal adult human colon tissue (Invitrogen, Carlsbad, CA).
Antibodies. Antibodies to B9L were obtained by immunizing rabbits with recombinant GST‐B9L (amino acids 245–564), as described previously.( 15 ) The antibody to β‐catenin was purchased from Transduction Laboratories (San Jose, CA).
Immunohistochemical staining. Serial sections (4 µm‐thick) were cut from formalin‐fixed, paraffin‐embedded tissues and were prepared for hematoxylin‐eosin and immunohistochemical staining. The sections were deparaffinized with xylene and dehydrated through a graded ethanol series. Endogenous peroxidase activity was quenched with 0.3% H2O2‐methanol for 30 min, and the sections were placed in 0.01 M sodium phosphate‐citrate buffer, pH 8.0. For antigen retrieval, the slides were heated in a microwave processor (Energy Beam Science, East Granby, CT) at 95°C for 20 min, then cooled, and rinsed in 0.01 M phosphate‐buffered saline (PBS), pH 7.4. To block non‐specific binding of the primary antibody, the slides were incubated in 10% normal rabbit or mouse serum in PBS for 30 min. The sections were then incubated overnight at 4°C with either a polyclonal antibody to B9L (1:400 dilution) or a monoclonal antibody to β‐catenin (1:2000 dilution). After a thorough wash with Tris‐buffered saline (TBS) containing 0.1% Triton X‐100, the slides were incubated with biotinylated goat antirabbit IgG or horse antimouse IgG for 30 min, followed by incubation with a 1:100 dilution of the avidin‐biotin‐peroxidase complex (Vectastain, Vector Laboratories, Burlingame, CA) for a further 30 min and visualization with 0.02% 3,3′‐diaminobenzidine tetrahydrochloride and 0.005% H2O2 in Tris buffer, pH 7.4. Finally, the sections were counterstained lightly with hematoxylin.
For double staining of β‐catenin and B9L, the labeled streptavidin‐biotin method (for β‐catenin) and the HRP labeled polymer method (Envison) (for B9L) were used, respectively.
Evaluation of immunohistochemical staining. As the nuclear expression of B9L and β‐catenin was not homogeneous but instead varied in both intensity and distribution in the neoplasms, we used the scoring system of Brabletz et al., ( 18 ) with minor modification. This system included an evaluation of both the staining intensity and the percentage of stained cells. The staining intensity was graded as: no staining (as in normal colonic epithelia; value, 0), weak staining (value, 1), moderate staining (value, 2), or strong staining (value, 3). The scores for the percentages of tumor cells with nuclear staining of B9L and β‐catenin were assigned as: 0, 0%; 1, 1–10%; 2, 11–50%; or 3, >50%. The multiplication of the intensity value times the percentage value gave a value from 0 to 9. These values were classified in three groups defined as: low immunoreactivity (values 0 and 1), medium immunoreactivity (values 2 and 3), or high immunoreactivity (values 4, 6, and 9).
Statistical analysis. The statistical analysis was performed using SPSS 11.5J software (SPSS Japan Inc., Tokyo, Japan). The association between B9L or β‐catenin expression and the clinicopathologic parameters was analyzed using either the chi‐squared test or Fisher's exact test. The correlation between B9L or β‐catenin expression and the grade of neoplasia was analyzed by the Kruskal–Wallis test and the chi‐squared test. Values of P < 0.05 were considered to indicate statistical significance.
Results
Histologic classification. The colorectal neoplasms were categorized according to the Vienna classification system (Table 2). Additionally, 16 samples for RTQ‐PCR and 30 samples for immunohistochemistry obtained from non‐neoplastic tissues distant from the colorectal neoplasms were classified as category 1, negative for neoplasia/dysplasia.
Table 2.
Vienna classification of colorectal neoplasias
| Classification | RTQ‐PCR analysis | Immunohistochemical analysis | |||
|---|---|---|---|---|---|
| No. of patients 27 | % | No. of patients 100 | % | ||
| Category 3 | non‐invasive low grade neoplasia | 15 | 56 | 33 | 33 |
| low grade adenoma/dysplasia | |||||
| Category 4 | non‐invasive high grade neoplasia | 9 | 33 | 42 | 42 |
| high grade adenoma/dysplasia, carcinoma in situ | |||||
| Category 5 | invasive neoplasia | 3 | 11 | 25 | 25 |
Real‐time quantitative PCR analysis. First, the mean (±SD) expression level of B9L mRNA in 16 non‐neoplastic samples was 1.13 ± 0.67. B9L mRNA expression was significantly higher than normal in low‐grade neoplasias (2.73 ± 1.96, P < 0.01, 95% confidence interval [CI]: −2.73 to −0.47), high‐grade neoplasias (3.87 ± 2.70, P < 0.02, 95% CI: −4.83 to −0.65), and invasive carcinomas (5.23 ± 2.18, P < 0.08, 95% CI: −9.38 to 1.19; Fig. 1). However, there was no significant difference in the B9L mRNA expression level between low‐grade and high‐grade neoplasias (P < 0.29, 95% CI: −3.37 to 1.09) or between high‐grade neoplasias and invasive carcinomas (P < 0.43, 95% CI: −5.55 to 2.84).
Figure 1.
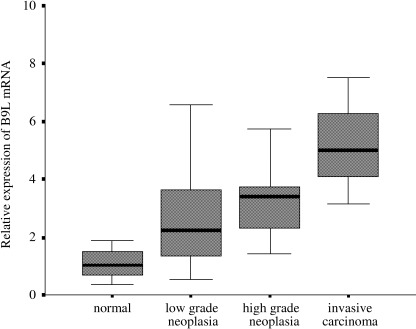
Correlation between B9L mRNA expression and the grade of neoplasia. The B9L mRNA levels were significantly higher in low‐grade neoplasias (2.73 ± 1.96, P < 0.01, 95% CI: −2.73 to −0.47), high‐grade neoplasias (3.87 ± 2.70, P < 0.02, 95% CI: −4.83 to −0.65), and invasive carcinomas (5.23 ± 2.18, P < 0.08, 95% CI: −9.38 to −1.19). There was no significant difference in the B9L mRNA expression level between low‐grade and high‐grade neoplasias (P < 0.29, 95% CI: −3.37 to −1.09) or between high‐grade neoplasias and invasive carcinomas (P < 0.43, 95% CI −5.55 to −2.84).
To compare the B9L expression and clinicopathologic factors, samples were categorized into two groups, high B9L mRNA (B9L expression ratio >2.5) and low B9L mRNA (B9L expression ratio <2.5). Of 27 neoplasias, 14 were categorized as high, and 13 were categorized as low. No statistically significant differences between the groups were observed in clinicopathologic factors including age, gender, location of tumor, size of tumor and morphological type.
Immunohistochemical staining of B9L. All of the samples, 100 neoplasia samples and 30 non‐neoplastic samples, were positive for B9L immunostaining, although the staining intensity and fraction of cells stained varied. The nuclei of both normal glandular cells and neoplastic cells showed staining for B9L, whereas the plasma membrane and cytoplasm did not. Representative samples of B9L immunohistochemical staining are shown in Fig. 2.
Figure 2.
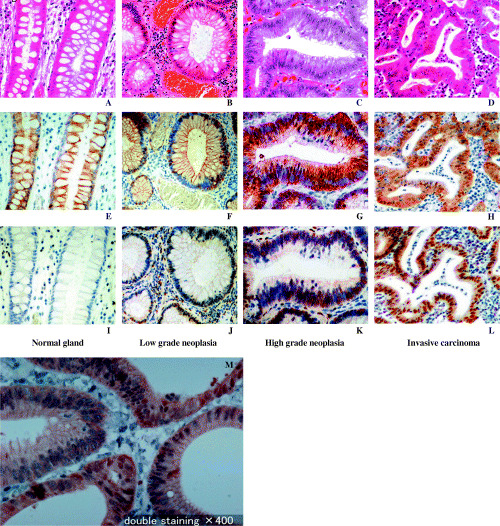
Representative B9L (E–H) and β‐catenin (I–L) immunohistochemical staining. (A, E, I): Normal colonic glandular epithelium; (A) H&E staining; (E) very weak nuclear staining of B9L; (I) discrete membranous staining of β‐catenin. (B, F, J): Non‐invasive low‐grade neoplasia; (B) H&E staining; (F) focal, weak nuclear staining of B9L; (J) discrete membranous staining and weak nuclear staining of β‐catenin in spots. (C, G, K): Non‐invasive high‐grade neoplasia; (C) H&E staining; (G) diffuse heterogeneous nuclear staining of B9L; (K) weak membranous staining and frequent nuclear staining of β‐catenin. (D, H, L): Invasive neoplasia; (D) H&E staining; (H) extensive, strong nuclear staining of B9L; (L) diffuse nuclear staining of β‐catenin. (M): High‐grade neoplasia was double‐stained for β‐catenin (brown) and B9L (blue). Original magnification, ×400.
Both the staining intensity and the fraction of cells stained increased with the progression in the grade of neoplasia. The neoplasias were categorized into three immunoreactivity groups according to B9L immunohistochemistry: 12, 42, and 46 cases were classified as having low, medium, and high levels of B9L immunoreactivity, respectively. The patient characteristics were not significantly different among the three groups. However, nuclear B9L immunoreactivity and the grade of neoplasia were markedly correlated (P < 0.001; Fig. 3). A high level of nuclear immunoreactivity to B9L was observed in 27% of low‐grade neoplasias, in 57% of high‐grade neoplasias, and in 52% of invasive carcinomas, whereas a high level of nuclear immunoreactivity to B9L was not observed in any of the non‐neoplastic samples. Approximately 88% of neoplasias and invasive carcinomas expressed medium or high B9L immunoreactivity levels, whereas this was true for only 23% of non‐neoplastic samples.
Figure 3.
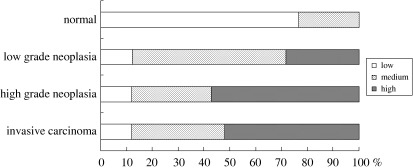
Correlation between nuclear B9L immunoreactivity and the grade of neoplasia. Statistical analysis confirmed a significant correlation (P < 0.001) between nuclear B9L immunoreactivity and the grade of neoplasia.
Immunohistochemical staining of β‐catenin. Immunoreactivity to β‐catenin was observed at the cell membrane and, rarely, in the nucleus in samples negative for neoplasia. In neoplasias, nuclear staining was more prominent and correlated with the grade of neoplasia, whereas membrane and cytoplasmic staining were inconspicuous (Fig. 2). Based on nuclear β‐catenin staining, 38, 23, and 39 cases were classified as having low, medium, and high immunoreactivity, respectively. The patient characteristics were not significantly different among these groups. However, nuclear β‐catenin immunoreactivity and the grade of neoplasia were significantly correlated (P < 0.001; Fig. 4). A high level of nuclear β‐catenin immunoreactivity was seen in 21% of low‐grade neoplasias, in 43% of high‐grade neoplasias, and in 56% of invasive carcinomas. None of the non‐neoplastic samples had a high level of immunoreactivity.
Figure 4.
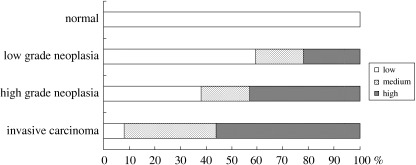
Correlation between nuclear β‐catenin immunoreactivity and the grade of neoplasia. Statistical analysis confirmed a significant correlation (P < 0.001) between nuclear β‐catenin immunoreactivity and the grade of neoplasia.
Correlation of immunoreactivity for B9L and β‐catenin. Immunoreactivity to both nuclear B9L and β‐catenin was observed in 36% of low‐grade neoplasias, 55% of high‐grade neoplasias, and 80% of invasive carcinomas. Double staining of B9L and β‐catenin revealed that B9L and β‐catenin are co‐localized in some high grade neoplasia cells (Fig. 2M). However, within grades of neoplasia, there was no significant correlation between the immunoreactivity of nuclear B9L and β‐catenin.
Discussion
In the present study, we found that B9L expression is enhanced in colorectal neoplasias and invasive carcinomas. The expression of B9L mRNA and immunoreactivity to B9L protein were correlated with the progressive grades of colorectal neoplasias. High nuclear B9L immunoreactivity was observed in 27% of low‐grade neoplasias and in 50% of high‐grade neoplasias and invasive carcinomas. Of particular interest is the fact that B9L immunoreactivity was dramatically increased in low‐grade neoplasias compared with that in non‐neoplastic mucosa.
Most sporadic colorectal cancers are initiated by activating mutations in the Wnt pathway, characterized by the stabilization of β‐catenin and constitutive transcriptional activation by the β‐catenin/TCF‐d complex. The nuclear accumulation and activation of β‐catenin is a critical step in the progression of the adenoma‐carcinoma sequence, leading to the up‐regulation of Wnt target genes such as cyclin D1 in all grades of adenoma.( 19 , 20 ) Various degrees of β‐catenin accumulation in the nuclei of colorectal adenoma cells have been reported. Some studies demonstrated a dependence of nuclear β‐catenin expression on the grade of neoplasia, and others showed a dependence on the size of the colon adenoma.( 18 , 21 ) We have confirmed a link between the nuclear accumulation of β‐catenin and the progression of neoplasia, but a correlation between β‐catenin and the size of the adenoma was not found.
Further, we found that B9L up‐regulation occurred even between the non‐neoplastic state and low‐grade neoplasia.( 15 ) Since B9L has the potential to enhance β‐catenin‐TCF‐mediated transcription, our findings suggest that B9L plays an important role in tumorigenesis induced by aberrant activation of Wnt signaling and may act as a key protein in the progressive dysplasia of neoplasias. Up‐regulation of B9L may activate Wnt signaling even when β‐catenin is not prominently accumulated in the nucleus. The mechanisms of B9L overexpression and degradation and of its interaction with other molecules are still uncharacterized. Also, the mechanism of nuclear translocation of B9L remains to be investigated. Further elucidation of B9L functions may provide insight into the mechanisms of tumorigenesis as well as assist in the development of antitumor reagents and diagnostic methods.
Acknowledgments
We thank Dr T. Nakano for kind suggestions and assistance; Dr Y. Onozato and H. Ishihara of Maebashi Red Cross Hospital, Dr Y. Inui of the Inui Clinic of Internal Medicine, Takasaki, and Dr Y. Togo of Keiaido Hospital, Omama, for supplying samples; and Mr T. Hikino for excellent technical assistance.
This work was supported in part by the Harunasou Foundation Cancer Research Subsidizing Fund, the Kanetsu Chuo Hospital Research Fund, and the Research Fund of the Uchida Clinic in Inamachi, Saitama, the Katoh clinic and the Maebashi Norte hospital in Maebashi, Gunma.
References
- 1. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61: 759–67. [DOI] [PubMed] [Google Scholar]
- 2. Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol 2004; 20: 695–723. [DOI] [PubMed] [Google Scholar]
- 3. Nagase H, Nakamura Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat 1993; 2: 425–34. [DOI] [PubMed] [Google Scholar]
- 4. Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. Beta‐catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci USA 1997; 94: 10330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morin PJ, Sparks AB, Korinek V et al. Activation of beta‐catenin‐Tcf signaling in colon cancer by mutations in beta‐catenin or APC. Science 1997; 275: 1787–90. [DOI] [PubMed] [Google Scholar]
- 6. Liu W, Dong X, Mai M et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta‐catenin/TCF signalling. Nat Genet 2000; 26: 146–7. [DOI] [PubMed] [Google Scholar]
- 7. Akiyama T. Wnt/beta‐catenin signaling. Cytokine Growth Factor Rev 2000; 11: 273–82. [DOI] [PubMed] [Google Scholar]
- 8. Polakis P. Wnt signaling and cancer. Genes Dev 2000; 14: 1837–51. [PubMed] [Google Scholar]
- 9. Giles RH, Van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653: 1–24. [DOI] [PubMed] [Google Scholar]
- 10. Kramps T, Peter O, Brunner E et al. Wnt/wingless signaling requires BCL9/legless‐mediated recruitment of pygopus to the nuclear beta‐catenin‐TCF complex. Cell 2002; 109: 47–60. [DOI] [PubMed] [Google Scholar]
- 11. Willis TG, Zalcberg IR, Coignet LJ et al. Molecular cloning of translocation t (1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood 1998; 91: 1873–81. [PubMed] [Google Scholar]
- 12. Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development 2002; 129: 4089–101. [DOI] [PubMed] [Google Scholar]
- 13. Thompson B, Townsley F, Rosin‐Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol 2002; 4: 367–73. [DOI] [PubMed] [Google Scholar]
- 14. Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD‐finger protein required for Wingless signaling in Drosophila. Development 2002; 129: 2565–76. [DOI] [PubMed] [Google Scholar]
- 15. Adachi S, Jigami T, Yasui T et al. Role of a BCL9‐related beta‐catenin‐binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res 2004; 64: 8496–501. [DOI] [PubMed] [Google Scholar]
- 16. Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol 2001; 36: 445–56. [DOI] [PubMed] [Google Scholar]
- 17. Schlemper RJ, Riddell RH, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T. Expression of nuclear beta‐catenin and c‐myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol 2000; 156: 865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sansom OJ, Reed KR, Van De Wetering M et al. Cyclin D1 is not an immediate target of beta‐catenin following Apc loss in the intestine. J Biol Chem 2005; 280: 28463–7. [DOI] [PubMed] [Google Scholar]
- 20. Brembeck FH, Schwarz‐Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9‐2 in the switch between beta‐catenin's adhesive and transcriptional functions. Genes Dev 2004; 18: 2225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hao X, Tomlinson I, Ilyas M, Palazzo JP, Talbot IC. Reciprocity between membranous and nuclear expression of beta‐catenin in colorectal tumours. Virchows Arch 1997; 431: 167–72. [DOI] [PubMed] [Google Scholar]


