Abstract
Hypersensitivity to mosquito bites is characterized by severe systemic as well as local symptoms, and associated with chronic active EBV infection and NK cell lymphocytosis. In this HEN disease, we investigated the response of PBMC to MSG extracts. PBMC were taken from three defined cases of HEN disease, three borderline cases, five individuals with simple exaggerated reactions to mosquito bites without systemic symptoms (simple responders), and eight healthy donors. PBMC, or purified CD4+, CD8+ or CD56+ cells, were cultured with MSG extracts prepared from each of five mosquito species to examine their proliferation and cytokine secretion. The patients with HEN disease had high stimulation indices with variations in responses to the extracts from Aedes albopictus, Aedes aegypti, Anopheles sinensis and Culex pipiens pallens. However, a non‐Japan‐habitant species Anopheles stephensi did not stimulate the patients’ PBMC. Some borderline or simple responders showed moderate proliferation, and healthy donors had no reactive PBMC. In HEN disease, both CD56+ NK cells (producing IFN‐γ) and CD4+ Th0 cells (producing IL‐4 and IFN‐γ) were increased in the blood. CD4+ cells, but not CD56+ NK cells or CD8+ cells, propagated in response to MSG extracts. However, this response of CD4+ cells and their IL‐4 production were strongly enhanced by coexisting CD56+ cells. We suggest that the CD4+ T cell serving as the primary responder to MSG antigen and the NK cell functioning as the enhancer are both pathogenic in the development of HMB. (Cancer Sci 2005; 96: 519 –526)
Abbreviations
- ConA
concanavalin A
- EBV
Epstein–Barr virus
- EBV‐TR
terminal repetitive (EBV‐TR) sequence of EBV
- FITC
fluoresce inisothiocyanate
- HEN disease
HMB, hypersensitivity to mosquito bites
- HMB‐EBV‐NK
disease
- IFN
interferon
- IL
interleukin
- MSG
mosquito salivary gland
- NK cell
natural killer cell
- PBMC
peripheral blood mononuclear cells
- PE
phyoerythrin
- rIL
recombinant interleukin
- SI
stimulation index.
- TCR
T‐cell receptor.
Hypersensitivity to mosquito bites or mosquito allergy is defined as intense skin reactions to mosquito bites and simultaneous or subsequent various general symptoms, including high fever, lymphadenopathy and hepatosplenomegaly.( 1 ) This disease has been reported mostly in Japanese patients in the first two decades of life. One of the most intriguing features that had made HMB mysterious was the high incidence of malignanat histiocytosis or hemophagocytic syndrome as a fatal outcome.( 2 )
We have found that this enigmatic disorder is associated with chronic active EBV infection( 3 , 4 ) and NK cell lymphocytosis,( 5 ) and thus proposed HMB‐EBV‐NK disease or HEN disease as a clinical entity featured by the triad.( 1 ) Investigation of 58 Japanese cases of HMB revealed that: (i) the onset age of HMB is 0–18 years (median 6.7 years); (ii) the percentage of CD56+ cells is 33.5–67.3% of PBMCs; (iii) more than half of cases died at the age of 10–31 years (median 16.3 years), and death was caused by malignant histiocytosis or hemophagocytic syndrome in 52% of fatal cases, or granular lymphocyte proliferative disorders or malignant lymphoma in 35% cases; and (iv) some patients have hydroa vacciniforme‐like eruptions.( 6 , 7 ) In addition to Japan, HEN disease is present in Taiwan, Korea and Mexico.( 1 , 8 ) The characteristics of circulating NK cells in HEN disease include: (i) large granular lymphocyte morphology; (ii) CD2+ CD3− CD4− CD8− CD11b+ CD16+ CD38+ CD56+ CD57− HLA‐DR+ T‐cell receptor (TCR)− phenotype( 5 ) with the exception of lower numbers of CD56 cases;( 9 ) (iii) high NK activity and antibody‐dependent cellular cytotoxicity; (iv) monoclonal (or oligoclona) EBV infection; (v) secretion of IFN‐γ and IL‐10 but not IL‐2 or IL‐4 (NK1 type); and (vi) high expression of lectin‐type inhibitory receptors (CD94/NKG2 complex) and no expression of immunoglobulin superfamily‐type receptors (NKB1 and NKAT2).( 1 , 10 )
The pathophysiological relationship between HMB, EBV infection and NK cell neoplasma is the most important issue to be clarified in HEN disease. Asada et al.( 11 ) have demonstrated that MSG extracts stimulate PBMC from patients with HEN disease, and the primary responder population is CD4+ T cells. Furthermore, the stimulated CD4+ T cells reactivate EBV that resides in NK cells. Therefore, MSG substances injected intradermally by mosquito bites serve as antigens for T cells, leading to the local and systemic reactions in which NK cells and EBV are involved. The present study was carried out to see how NK cells participate in the T cell response and whether there are differences in PBMC responses to MSG extracts among patients with HEN disease, individuals with simple exaggerated skin reactions to mosquito bites (simple responder), and normal healthy subjects. Results show that CD4+ T cells proliferate vigorously and produce IL‐4 in response to MSG extracts in patients with HEN disease, and this response of CD4+ T cells is enhanced by the coexistence of NK cells.
Materials and Methods
Subjects
This study was carried out with the informed consent of the patients. The subjects enrolled in this study were divided into four groups: (i) three patients with defined HEN disease; (ii) three patients with borderline HEN disease; (iii) five individuals showing simple exaggerated reactions to mosquito bites without general symptoms or NK cell lymphocytosis (simple responders); and (iv) eight normal healthy donors (five men and three women; 12–40 years [mean ± SD, 27.7 ± 7.9]) having normal percentages of circulating lymphocyte populations. The details of the patients are shown in Table 1.
Table 1.
Profiles of patients and controls (eight healthy donors: five men and three women, 12–40 years [mean ± SD, 27.7 ± 7.9])
| Case | Age (years) | Sex | Onset age of HMB (years) | Diagnosis | EBV‐TR Southern blot |
|---|---|---|---|---|---|
| 1 | 16 | F | 2 | HEN disease | Monoconal (PBMC) |
| 2 | 9 | F | 4 | HEN disease | Monoclonal (PBMC) |
| 3 | 16 | M | 3 | HEN disease | Monoclonal (PBMC, skin) |
| 4 | 2 | F | 1 | Borderline HEN † | Oligoclonal (PBMC) |
| 5 | 14 | F | 3 | Borderline HEN ‡ | Monoclonal (PBMC) |
| 6 | 22 | F | 18 | Borderline HEN § | Monoclonal (PBMC, skin) |
| 7 | 2 | M | No HMB | Simple responder | ND |
| 8 | 10 | M | No HMB | Simple responder, SLE | ND |
| 9 | 3 | F | No HMB | Simple responder | ND |
| 10 | 30 | F | No HMB | Simple responder | ND |
| 11 | 36 | F | No HMB | Simple responder | ND |
The antibody titers to EBV in cases 1–6 ranged as follows: VCA‐IgG, 160–5120; VCA‐IgM, <10 to 10; EADR, <10; and EBNA, <10 to 20. The reasons for the diagnosis of ‘borderline HEN disease’ are as follows: †Although the patient had HMB, the Southern blotting of EBV‐TR showed four oligoclonal band, and NK cell lymphocytosis was absent. ‡The patient had a monoclonal EBV‐TR band and NK cell lymphocytosis, but HMB and hydroa vacciniforme subsided spontaneously 4 years previously. §The patient had HMB and cutaneous vasculitis with a monoclonal EBV‐TR band both in PBMC and lesional skin, but the onset age of HMB was relatively high, and NK cell lymphocytosis was absent. ND, not done; SLE, systemic lupus erythematosus.
Southern blot analysis of EBV genomes
Southern blotting was carried out with 32P‐labeled EBV‐TR.( 7 ) The positive control and the negative control were DNA from EBV‐infected tumor cells and placental DNA, respectively.
Reagents and culture medium
Mosquito salivary gland extracts were prepared from each of five mosquito species, including three endemic species in Japan, Aedes albopictus, Culex pipiens pallens and Anopheles sinensis, and two exotic species, Aedes aegypti and Anopheles stephensi. Ten MSG dissected with forceps were homogenized in 10 µL phosphate‐buffered saline (pH 7.4). The concentration of total protein was approximately 2 mg/mL by the Lowry method. The extracts were stored at −70°C until use, and the diluted solutions were filtered with a 45‐µm filter before use. ConA was purchased from Sigma Chemical Co. (St Louis, MO, USA). Human rIL‐2 (Celeuk) was from Takeda Pharmaceutical Co. (Tokyo, Japan).
RPMI‐1640 (Gibco BRL Life Technology, Grand Island, NY, USA) was supplemented with 10% heat‐inactivated fetal calf serum, 2 mM l‐glutamine, 0.05 mM 2‐mercaptoethanol, 0.01 mM sodium pyruvate, 25 mM HEPES, 1% non‐essential amino acids, 100 U/mL penicillin and 100 µg/mL streptomycin (all from Gibco).
Preparation of PBMC, CD4+, CD8+ and NK cells
Peripheral blood mononuclear cells were isolated from patients and control subjects by the standard Ficoll‐Paque method (Pharmacia, Uppsala, Sweden). CD4+ or CD8+ cells were separated from PBMC with anti‐CD4 or anti‐CD8 monoclonal antibody‐conjugated magnetic beads (Dynal, Oslo, Norway) and following DETACHaBEAD (Dynal) according to the manufacturer's directions, with purity of more than 98%. NK cells were separated from PBMC with CD56‐specific monoclonal antibody and antimouse IgG‐conjugated magnetic beads (Dynal), as described previously( 10 ), with purity of more than 96%.
Proliferation of PBMC, CD4+, CD8+ and NK cells to MSG extracts
Triplicate cultures of PBMC, CD4+, CD8+ and NK cells (2 × 105 cells/well) were incubated with MSG extracts at varying dilutions or ConA at 2 µg/mL in a final volume of 150 µL in 96‐well microtiter plates (Corning Glass Works, Corning, NY, USA) for 72 h at 37°C in 5% CO2 in air. Methyl 3H‐thymidine (Amersham, Arlington, IL, USA) was added (1 µCi/well) 14 h before harvest. The cells were collected on glass fiber filters using a cell harvester (Cambridge Technologies, Watertown, MA, USA) and radio‐uptake was measured in a scintillation counter. In some experiments, PBMC were cultured for 10 days in the presence of MSG extracts or rIL‐2 (10 U/mL), and propagating cells were analyzed by flow cytometry.
Preparation of culture supernatants and assay for cytokines
Lymphocytes were cultured in 24‐well plates (2 × 106/1 mL of culture) in the presence or absence of MSG extracts. The culture supernatants were harvested after 72 h cultivation. The amounts of IFN‐γ and IL‐4 present in culture supernatants were measured using enzyme‐linked immunosorbent assay kits (Genzyme, Boston, MA, USA).
Flow cytometric analysis
Hanks’ balanced salt solution containing 0.1% NaN3 and 1% fetal calf serum was used as the staining buffer. Cells were double stained with FITC‐labeled and PE‐labeled monoclonal antibodies for 30 min at 4°C. After three washes, 104 labeled cells were analyzed in a FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, CA, USA). FITC‐conjugated monoclonal antibodies to CD94 (HP‐3D9), NKB1 (DX9) and NKAT2 (DX27) were purchased from PharMingen (Hamburg, Germany). FITC‐conjugated monoclonal antibodies to CD3, CD4 and CD8, and PE‐conjugated monoclonal antibody to CD56 were obtained from Becton Dickinson (San Jose, CA, USA).
Intracellular cytokine staining
Intracellular cytokines were stained according to the protocol of Cytostain (Immunotech, Marseille, France) with a few modifications. Briefly, cells (106 cells/mL) were incubated for 8 h in culture medium with phorbol myristate acetate and calcium ionophore in a 24‐well plate. Brefeldin A was added to each well at a concentration of 10 µg/mL and incubation was prolonged for another 6 h. The cells resuspended in Hanks’ solution were permeabilized with 0.5% saponin for 30 min. They were stained with FITC‐labeled anti‐IFN‐γ and PE‐labeled anti‐IL‐4. Additionally, costaining of CD4 or CD56 was carried out with PerCP‐conjugated monoclonal antibody (PharMingen). Fluorescence profiles were analyzed by three‐color flow‐cytometry in a FACScan. CD4+ or CD56+ cells were gated so that cytokines produced by these populations were seen.
Statistical analysis
The student's t‐test (impaired) was used to determine statistical differences between means.
Results
Background study of patients and control subjects
The patients’ profiles are summarized in Table 1, and the phenotypes of their PBMC are shown in Table 2. As represented by case 1 (Fig. 1), cases 1–3 were typical HEN disease fulfilling the triad, that is, HMB (Fig. 1a), CD56+ CD94+ NKB1− NKAT2− NK cell lymphocytosis (Fig. 1b; Table 2), and monoclonal EBV infection (Fig. 1c; Table 1). Cases 4–6 were designated borderline HEN disease. This was because cases 4 and 6 had HMB and monoclonal or oligoclonal EBV genomes, as assessed by EBV‐TR Southern blot analysis,( 3 , 7 ) but showed no NK cell lymphocytosis, while case 5 had monoclonal EBV infection and NK cell lymphocytosis, but her HMB subsided spontaneously 4 years before. As controls, we chose five individuals showing simple exaggerated reactions to mosquito bites without general symptoms or NK cell lymphocytosis (simple responders, cases 7–11), and eight normal healthy donors. These control individuals had PBMC with normal percentages of each surface molecule.
Table 2.
Phenotypes of the patients’ PBMC
| Case | Diagnosis | Positive cells in PBMC (%) | Positive cells in CD56+ cells (%) | |||||
|---|---|---|---|---|---|---|---|---|
| CD3 | CD4 | CD8 | CD56 † | CD94 † | NKB1 ‡ | NKAT2 ‡ | ||
| 1 | HEN disease | 53 | 49 | 11 | 30 § | 98 | 1 | 1 |
| 2 | HEN disease | 27 | 22 | 6 | 63 | 97 | 1 | 1 |
| 3 | HEN disease | 12 | 9 | 4 | 51 | 99 | 1 | 1 |
| 4 | Borderline HEN | 41 | 31 | 20 | 13 | 80 | 5 | 34 |
| 5 | Borderline HEN | 33 | 18 | 10 | 42 | 93 | 2 | 3 |
| 6 | Borderline HEN | 32 | 17 | 8 | 8 | 73 | 12 | 14 |
| 7 | Simple responder | 65 | 25 | 22 | 14 | 53 | 12 | 29 |
| 8 | Simple responder SLE | 64 | 38 | 20 | 6 | 56 | 11 | 14 |
| 9 | Simple responder | 76 | 46 | 27 | 5 | 43 | 7 | 17 |
| 10 | Simple responder | 51 | 35 | 22 | 12 | 46 | 20 | 21 |
| 11 | Simple responder | 55 | 38 | 22 | 11 | 54 | 8 | 27 |
| 8 healthy donors (mean ± SD) | 62 ± 7.9 | 38 ± 8.2 | 23.3 ± 4.8 | 18.0 ± 5.8 | 49.8 ± 5.6 | 15.5 ± 5.3 | 25.6 ± 8.2 | |
The underlined values are above the mean + 2 SD of the healthy donors.
The underlined numbers are below the mean − 2 SD of the healthy donors.
When the patient was 2–5 years old, the percentage was ∼60%. SLE, systemic lupus erythematosus.
Figure 1.
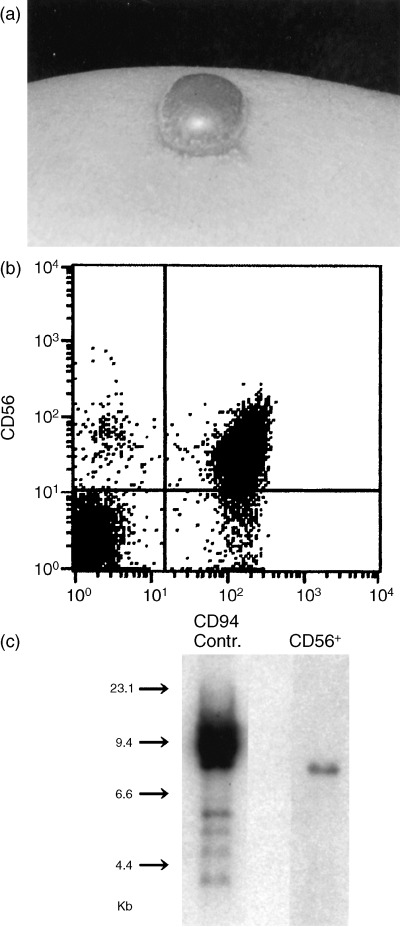
Triad of HEN disease, typically represented by case 1. (a) Bullous intense skin reaction at a mosquito bite site, (b) increased percentage of CD56+ CD94+ cells in PBMC, and (c) monoclonal band for EBV‐TR by Southern blot analysis of CD56+ cells and EBV‐infected control cells.
PBMC from patients with HEN disease proliferate well in response to MSG extracts
Our preliminary study showed that PBMC of case 1 proliferated vigorously in response to Aedes albopictus and moderately to Culex pipiens pallens, but not to Anopheles stephensi. Therefore, we first examined the optimal concentration of MSG extracts by culturing PBMC from case 1 with each of the three extracts at varying dilutions. As shown in Fig. 2, the proliferation reached a nearly comparable level to ConA at 2 µg/mL by the addition of Aedes albopictus extracts at a final dilution of 1:100 or 1:1000 (corresponding to final total protein concentrations of 20 µg/mL or 2 µg/mL, respectively). There was no statistical difference between 1:100 or 1:1000 in Aedes albopictus and Culex pipiens pallens. At 1:10 000, the proliferative responses were reduced by 11% in Aedes albopictus and by 42% in Culex pipiens pallens, when compared to the 1:1000 dilution. Anopheles stephensi yielded only a marginal response even at 1:100. Therefore, we chose a 1:1000 dilution to investigate the ability of MSG extracts to stimulate PBMC in the study.
Figure 2.
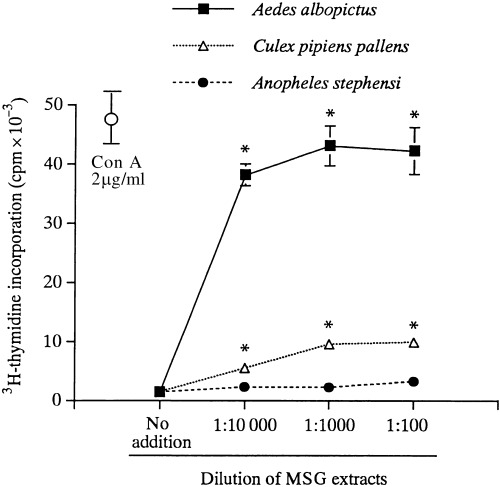
PBMC responses to MSG extracts at varying final concentrations. PBMC from case 1 were cultured for 72 h with Aedes albopictus, Culex pipiens pallens or Anopheles stephenisi at the dilutions indicated, or with ConA at 2 µg/mL. The proliferative responses were measured by 3H‐thymidine incorporation for the last 12 h. *P < 0.05, compared with the no addition group.
Figure 3 shows the PBMC responses to MSG extracts from five species in three patients with HEN disease and one representative healthy donor. Aedes albopictus extracts produced high proliferative responses in all three patients, as did Aedes aegypti but to a lesser degree (Fig. 3a–c). Culex pipiens pallens and Anopheles sinensis stimulated PBMC moderately in case 1 (Fig. 3a) and in cases 1 and 3 (Fig. 3a,c), respectively. No substantial responses were obtained with Anopheles stephensi in any of the patients. A healthy subject exhibited very low or no response to any of the extracts (Fig. 3d).
Figure 3.
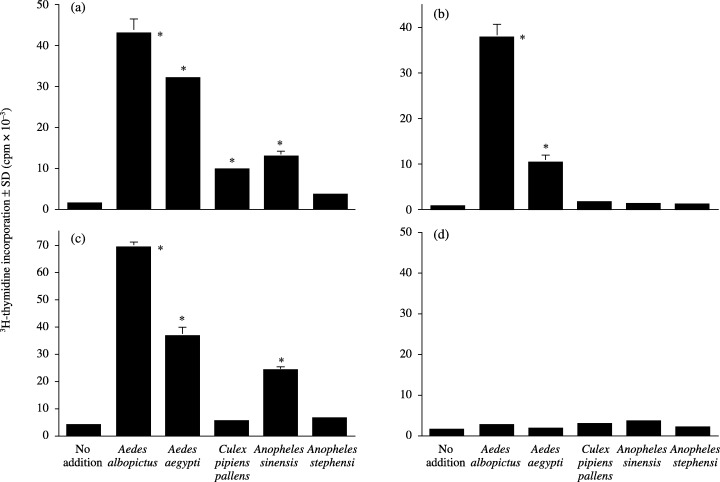
PBMC responses to MSG extracts. PBMC from (a) case 1, (b) case 2 and (c) case 3, and (d) a representative healthy donor were cultured for 72 h with the indicated MSG extracts at a 1:1000 dilution. The proliferative responses were assessed by 3H‐thymidine incorporation. *P < 0.01, compared with the no addition group.
Table 3 summarizes the SI of 11 subjects and eight normal healthy donors. In two of three borderline patients (cases 4–6), Aedes albopictus (cases 4 and 6) and Culex pipiens pallens (case 6) activated PBMC moderately, while these extracts were not stimulatory in case 5. Two of five simple response individuals (cases 7 and 8) had low to moderate responses to Aedes albopictus and Culex pipiens pallens up to 10.8 of SI, but the remaining three cases did not respond to any species. Eight healthy donors were non‐responders for all of the extracts, except for Anopheles sinensis, which produced low but significant responses (2.2 or more of SI).
Table 3.
PBMC responses to MSG extracts
| Case | Diagnosis | Aede albopictus | Aedes aegypti | Culex pipiens pallens | Anopheles sinensis | Anopheles stephensi |
|---|---|---|---|---|---|---|
| 1 | HEN | 29.2 | 21.7 | 6.5 | 8.8 | 2.3 |
| 2 | HEN | 68.9 | 18.5 | 2.5 | 2.0 | 1.5 |
| 3 | HEN | 16.5 | 8.7 | 1.3 | 5.7 | 1.3 |
| 4 | Borderline HEN | 4.4 | ND | 2.2 | ND | 1.6 |
| 5 | Borderline HEN | 1.0 | 1.0 | 1.3 | 1.3 | 1.4 |
| 6 | Borderline HEN | 7.2 | ND | 11.9 | ND | 1.9 |
| 7 | Simple responder | 8.4 | ND | 10.8 | ND | 1.9 |
| 8 | Simple responder SLE | 3.7 | ND | 5.6 | ND | 2.4 |
| 9 | Simple responder | 1.1 | 0.8 | 2.7 | 1.2 | 1.2 |
| 10 | Simple responder | 1.0 | 1.3 | 1.4 | 1.3 | 1.1 |
| 11 | Simple responder | 1.0 | 0.9 | 3.7 | 5.1 | 1.6 |
| 8 healthy donors mean ± SD | 1.5 ± 0.5 | ˙ 1.3 ± 0.2 | 1.5 ± 0.6 | 3.7 ± 1.5 | 1.4 ± 0.5 | |
| (range) | (1.0–2.7) | (1.1–1.7) | (0.9–2.8) | (2.2–7.1) | (1.0–2.6) |
The underlined values are above the mean + 2 SD.
Thus, the results suggest that PBMC from patients with HEN disease have circulating lymphocytes that react well with MSG extracts, and some of the borderline or simple ER individuals have lymphocytes responding at lower levels.
CD4+ T cells are responsible and CD56+ NK cells are helpful for PBMC proliferation in response to MSG extracts
To determine cell populations responsible for PBMC proliferation in response to MSG extracts, we deleted CD4+ cells from PBMC of case 1 using magnetic beads, and examined their response to Aedes albopictus extracts. Figure 4a clearly shows that PBMC deprived of CD4+ cells lost the ability to respond to MSG extracts. When case 1 PBMC were cultured for 10 days in the presence of Aedes albopictus extracts, the cells propagated to occupy more than 10‐fold numbers of the original culture wells. CD4+ cells were expanded preferentially, whereas both CD4+ and CD56+ cells propagated with rIL‐2 instead of the extracts (Fig. 4b). Thus, CD4+ T cells were the predominant responder to MSG extracts.
Figure 4.
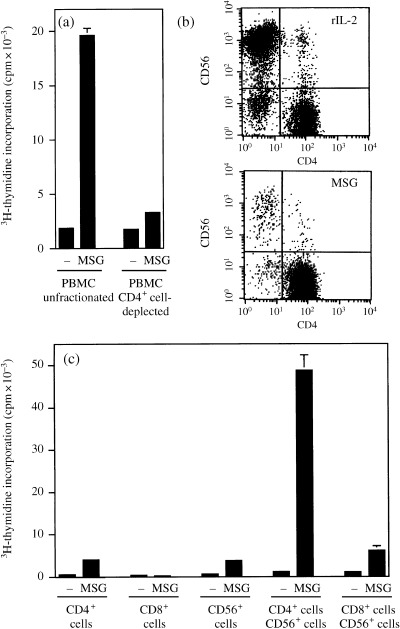
High proliferative responses of case 1 PBMC are attributed to CD4+ T cells but in the presence of CD56+ NK cells. (a) Responses to PBMC unfractionated or deprived of CD4+ cells to Aedes albopictus extracts. (b) Flow cytometry of PBMC cultured for 10 days with IL‐2 or Aedes albopictus extracts. (c) Responses to Aedes albopictus extracts of purified CD4+, CD8+, CD56+, mixture of CD4+ and CD56+, and mixture of CD8+ and CD56+ cells.
To test the cellular interaction in response to MSG extracts, CD4+, CD8+ and CD56+ cells were purified from case 1 PBMC. CD4+ cell and CD56+ cell fractions responded only marginally to Aedes albopictus extracts and the CD8+ cell fraction did not respond at all (Fig. 4c). However, coculture of CD4+ and CD56+ cells resulted in a dramatic proliferative response to MSG extracts. By flow cytometry, the proliferating cells in this coculture were CD4+ cells, as the percentage of CD4+ cells was more than 90% in 5‐day culture. No augmentation by NK cells was found in CD8+ cells. These data suggested that CD4+ T cells were responsible for MSG‐induced PBMC proliferation, and CD56+ NK cells were a strong enhancer for this T cell response by serving as antigen‐presenting or accessory cells or by another mechanism.
CD4+ T cells and CD56+ NK cells are primary sources of IL‐4/IFN‐γ and IFN‐γ, respectively, and NK cells augment IL‐4 production by CD4+ cells
Cytokine production by CD4+ T cells and CD56+ NK cells was examined by stimulation with phorbol myristate acetate and calcium ionophore. In PBMC of case 1, intracytoplasmic cytokine staining revealed that the vast majority of CD4+ T cells elaborated both IL‐4 and IFN‐γ, while CD56+ cells produced IFN‐γ but not IL‐4 (Fig. 5). Therefore, the Th0 cells were the source of IL‐4 and the NK cells were the main source of IFN‐γ.
Figure 5.
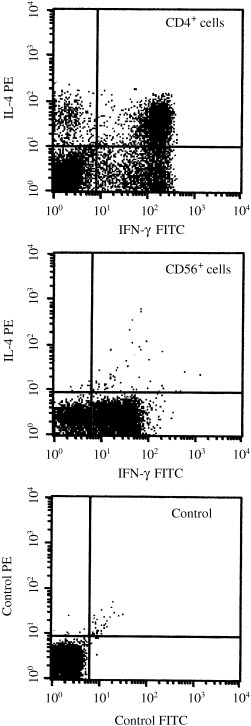
Intracytoplasmic cytokine staining of CD4+ and CD56+ cells. PBMC from case 1 were stimulated with phorbol myristate acetate and Ca ionophore. The cells were triple stained with anti‐IFN‐γ, anti‐IL‐4, and anti‐CD4 or anti‐CD56. CD4+ or CD56+ cells were gated so that cytokine production could be seen in each cell population. The control was carried out with isotype‐matched monoclonal antibodies labeled with FITC and PE.
To test cytokine secretion by a mixture of CD4+ T cells and CD56+ NK cells, these two populations were cocultured at different ratios for 3 days with rIL‐2 or MSG extracts as stimulant (Table 4). In the presence of rIL‐2, the amounts of IFN‐γ and IL‐4 depended on the numbers of NK cells and CD4+ cells, respectively. When cultured with MSG extracts, the production of IFN‐γ again depended on the number of CD56+ cells. However, a higher amount of IL‐4 was paradoxically secreted by the mixture of lower CD4 and higher CD56 numbers. As the source of IL‐4 was CD4+ T cells, this was interpreted to indicate that NK cells supported CD4+ T cells to produce IL‐4 in response to MSG extracts.
Table 4.
Cytokine production by lymphocytes containing various ratios of CD4+/CD56+ cells in response to rIL‐2 or MSG extracts
| Group | No. cells (× 105 cells/well) | Stimulant | IL‐4 (pg/mL) | IFN‐γ (IU/mL) | |
|---|---|---|---|---|---|
| CD4+ | CD56+ | ||||
| A | 20 | 0 | rIL‐2 | 196 | 33 |
| B | 16 | 4 | rIL‐2 | 162 | 647 |
| C | 10 | 10 | rIL‐2 | 76 | 1300 |
| D | 20 | 0 | MSG | 9 | 2 |
| E | 16 | 4 | MSG | 20 | 53 |
| F | 10 | 10 | MSG | 49 | 73 |
Purified CD4+ and CD56+ cells were prepared from case 1. These two fractions were mixed at the indicated ratio, and the total number of cells was always 2 × 106 cells/well. They were cultured in the presence of rIL‐2 (10 IU/mL) or MSG extracts (1:1000) for 3 days. The supernatants were subjected to enzyme‐linked immunosorbent assay for quantification of IFN‐γ and IL‐4.
Discussion
Epstein–Barr virus is known to activate NK and T cells by promoting cytokine‐related genes such as EBV‐induced gene 3 encoding a widely expressed IL‐12p40‐related protein.( 12 ) EBV‐carrying NK cells in HEN disease express the activation‐associated molecules MHC class II and CD94 at high levels.( 10 ) Our study aimed to investigate the responses of these potentially activated NK and T cells to mosquito bites in HEN disease. Until our previous( 11 ) and present studies, it had been predicted that EBV‐infected NK cells are the primary responder to mosquito substance. However, CD4+ T cells, but not NK cells, proliferated in response to MSG extracts, and NK cells enhanced this T cell response dramatically. The mechanism by which NK cells assist T cells remains uncharacterized in this study. As highly purified CD4+ T cells per se did not proliferate in response to MSG extracts,( 11 ) the T cell proliferation seems to be a conventional TCR‐mediated response to MSG antigenic peptide. It is therefore considered that NK cells serve as antigen‐presenting cells because they possess MHC class II molecules, and moreover, NK cells may play an additional significant accessory role.( 13 )
The activation of CD4+ T cells by MSG extracts plus NK cells was also confirmed by their cytokine production. In the in vitro system, CD4+ T cells produced IL‐4 upon stimulation with MSG extracts. This finding is in accordance with the high serum IgE levels observed in most of the patients with HEN disease.( 1 ) Notably, the vast majority of CD4+ T cells in the patient's PBMC produced IFN‐γ as well as IL‐4. Although the presence of a Th0 population in EBV‐infected patients has not been reported, there have been several reports demonstrating an increase in Th0 cells in human immunodeficiency virus‐positive patients,( 14 , 15 , 16 ) which results from a Th1 to Th2 shift promoted by the virus.( 14 ) Such a Th2 skewing also might occur in EBV‐infected patients.
Interferon‐γ and IL‐4 are representative Th1 and Th2 cytokines, respectively, and generally are viewed as mutual antagonists. It may therefore be anticipated that the mosquito antigen‐specific IL‐4‐producing T cell and the IFN‐γ‐producing NK cell interact negatively with each other. However, recent mounting evidence has suggested that IFN‐γ leads to Th2 cytokine production,( 17 , 18 ) as IFN‐γ enhances CD4+ T cell priming for IL‐4 production.( 17 ) Conversely, IL‐4 induces IFN‐γ production by NK cells,( 19 , 20 ) in particular, synergistically with IL‐2 or IL‐12.( 19 ) Furthermore, NK cells in HEN disease were found to be NK1 cells producing IFN‐γ and IL‐10.( 10 ) This cytokine profile of NK cells has been supported by the clinical observation that these two cytokines are elevated in sera from patients with hemophagocytic syndrome.( 21 , 22 ) IL‐10 might be beneficial for promoting the Th1 to Th0 shift in the CD4+ cells, as observed in T regulatory 1 cells whose differentiation is induced by IFN‐γ and IL‐10.( 23 )
Among the five mosquito species tested, Aedes albopictus, an endemic species in Japan, exerted the highest T cell response. This is in contrast to a non‐Japanese habitant, Anopheles stephensi, which induced very low to no T cell response. Aedes aegypti is another exotic species, but it rather highly stimulated the patients’ T cells, suggesting that there is crossreactivity between members of the Aedes family. The two Japanese endemic species, Anopheles sinensis and Culex pipiens pallens, stimulated PBMC moderately in some patients. Taken together, these results suggest that it is likely that patients with HEN disease have T cells immunized with endemic mosquito antigens. In two of three patients with borderline HEN and two of five simple responders, Aedes albopictus and/or Culex pipiens pallens induced moderate response levels. Given that both the sensitized T cell and the EBV‐infected NK cell are both required for effective response of PBMC to MSG extracts, the patients with borderline HEN and the simple responders were not fully equipped in terms of these two lymphocyte populations. More specifically, simple responders may have MSG‐specific CD4+ T cells but lack the enhancer NK cells.
Asada et al. have shown that coculturing NK cells and MSG extract‐activated CD4+ T cells induces the expression of EBV lytic‐cycle proteins in NK cells.( 11 ) Furthermore, the expression of BZLF1, a viral lytic‐cycle transactivator, was detectable at the skin lesion induced by scratch patch testing with MSG extracts. Thus, mosquito‐antigen‐specific CD4+ T cells play a key role in reactivation of latent EBV infection in NK cells. Considering that NK cells assist in CD4+ T cell proliferation, there is a mutual interaction between the CD4+ T cell and the NK cell in response to mosquito antigen. Although the patients usually have NK cell lymphocytosis, the present and former( 11 ) studies conversely imply that MSG extract‐stimulated CD4+ T cells increase in number and lead to lysis of NK cells and a resultant decrease in NK cell number. However, because mosquito bites only occur occasionally, the patients usually have a high number of EBV‐infected NK cells. Upon the bite of a mosquito, CD4+ T cells may propagate and transiently increase in number with a reduced number of NK cells, but they may be again restored after recovery from the mosquito bite episode. It is suggested that the activation of CD4+ T cells and the reactivation of NK cell‐EBV contribute to the pathogenesis of systemic symptoms of HMB in HEN disease.
References
- 1. Tokura Y, Ishihara S, Tagawa S, Seo S, Ohshima K, Takigawa M. Hypersensitivity to mosquito bites as the primary clinical manifestation of Epstein–Barr virus‐associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol 2001; 45: 569–78. [DOI] [PubMed] [Google Scholar]
- 2. Hidano A, Kawakami M, Yago A. Hypersensitivity to mosquito bite and malignant histiocytosis. Jpn J Exp Med 1982; 52: 303–6. [PubMed] [Google Scholar]
- 3. Ishihara S, Ohshima K, Tokura Y et al. Hypersensitivity to mosquito bites conceals clonal lymphoproliferation of Epstein–Barr viral DNA‐positive natural killer cells. Jpn J Cancer Res 1997; 88: 82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishihara S, Yabuta R, Tokura Y, Ohshima K, Tagawa S. Hypersensitivity to mosquito bites is not an allergic disease, but an Epstein–Barr virus‐associated lymphoproliferative disease. Int J Hematol 2000; 72: 223–8. [PubMed] [Google Scholar]
- 5. Tokura Y, Tamura Y, Takigawa M et al. Severe hypersensitivity to mosquito bites associated with natural killer cell lymphocytosis. Arch Dermatol 1990; 126: 362–8. [PubMed] [Google Scholar]
- 6. Iwatsuki K, Ohtsuka M, Harada H, Han G, Kaneko F. Clinicopathologic manifestations of Epstein–Barr virus‐associated cutaneous lymphoproliferative disorders. Arch Dermatol 1997; 133: 1081–6. [PubMed] [Google Scholar]
- 7. Tokura Y, Ishihara S, Ohshima K et al. Severe mosquito hypersensitivity, natural killer cell leukaemia, latent or chronic active Epstein–Barr virus infection and hydroa vacciniforme‐like eruption. Br J Dermatol 1998; 138: 905–6. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz‐Maldonado R, Parilla FM, De La Luz Orozco‐Covarrubias M, Ridaura C, Tamayo Sanchez L, Duran McKinster C. Edematous, scarring vasculitis: a new multisystemic disease with malignant potential. J Am Acad Dermatol 1995; 32: 37–44. [DOI] [PubMed] [Google Scholar]
- 9. Adachi A, Horikawa T, Kunisada M et al. Hypersensitivity to mosquito bites in association with chronic Epstein–Barr virus infection and natural killer (NK) leukaemia/lymphoma with expansion of NK cells expressing a low level of CD56. Br J Dermatol 2002; 147: 1036–7. [DOI] [PubMed] [Google Scholar]
- 10. Seo N, Tokura Y, Ishihara S, Takeoka Y, Tagawa S, Takigawa M. Disordered expression of inhibitory receptors on the NK1‐type natural killer (NK) leukaemic cells from patients with hypersensitivity to mosquito bites. Clin Exp Immunol 2000; 120: 413–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asada H, Miyagawa S, Sumikawa Y et al. CD4+ T‐lymphocyte‐induced Epstein–Barr virus reactivation in a patient with severe hypersensitivity to mosquito bites and Epstein–Barr virus‐induced NK cell lymphocytosis. Arch Dermatol 2003; 139: 1601–7. [DOI] [PubMed] [Google Scholar]
- 12. Devergne O, Birkenbach M, Kieff E. Epstein–Barr virus‐induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA 1997; 28: 12 041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanna J, Gonen‐Gross T, Fitchett J et al. Novel APC‐like properties of human NK cells directly regulate T cell activation. J Clin Invest 2004; 114: 1612–23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Maggi E, Mazzetti M, Ravina A et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science 1994; 265: 244–8. [DOI] [PubMed] [Google Scholar]
- 15. Meyaard L, Otto SA, Keet IP, Van Lier RA, Miedema F. Changes in cytokine secretion patterns of CD4+ T‐cell clones in human immunodeficiency virus infection. Blood 1994; 84: 4262–8. [PubMed] [Google Scholar]
- 16. Autran B, Legac E, Blanc C, Debre PA. Th0/Th2‐like function of CD4+ CD7− T helper cells from normal donors and HIV‐infected patients. J Immunol 1995; 154: 1408–17. [PubMed] [Google Scholar]
- 17. Bocek P Jr, Foucras G, Paul WE. Interferon‐γ enhances both in vitro and in vivo priming of CD4+ T cells for IL‐4 production. J Exp Med 2004; 199: 1619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral‐induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol 2004; 5: 337–43. [DOI] [PubMed] [Google Scholar]
- 19. Bream JH, Curiel RE, Yu C‐R et al. IL‐4 synergistically enhances both IL‐2‐ and IL‐12‐induced IFN‐γ expression in murine NK cells. Blood 2003; 102: 207–14. [DOI] [PubMed] [Google Scholar]
- 20. Noble A, Kemeny DM. Interleukin‐4 enhances interferon‐γ synthesis but inhibits development of interferon‐γ‐producing cells. Immunology 1995; 85: 357–63. [PMC free article] [PubMed] [Google Scholar]
- 21. Lay JD, Tsao CJ, Chen JY, Kadin ME, Su IJ. Upregulation of tumor necrosis factor‐α gene by Epstein–Barr virus and activation of macrophages in Epstein–Barr virus‐infected T cells in the pathogenesis of hemophagocytic syndrome. J Clin Invest 1997; 100: 1969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osugi Y, Hara J, Tagawa S et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood 1997; 89: 4100–3. [PubMed] [Google Scholar]
- 23. Levings MK, Sangregorio R, Galbiati F, Squadrone S, De Waal Malefyt R, Roncarolo MG. IFN‐α and IL‐10 induce the differentiation of human type 1 T regulatory cells. J Immunol 2001; 166: 5530–9. [DOI] [PubMed] [Google Scholar]


