Abstract
Signal transducer and activator of transcription 3 (STAT3) is a central cytoplasmic transcription factor. Abnormal activation of STAT3 plays a critical role in oncogenesis and has been found frequently in a wide variety of human tumors including pancreatic cancer. In this study, we elucidated the significance of the STAT3 signaling pathway on metastatic potentials of pancreatic cancer. We found that phosphorylated STAT3 (p‐STAT3) protein levels were significantly higher in highly metastatic SW1990 cells compared to the poorly metastatic CaPan‐2 cells, which expressed weak levels of this protein. Furthermore, a Janus kinase (JAK) specific inhibitor, AG490, significantly inhibited the expression of p‐STAT3, and subsequently reduced invasion and adhesion potential of SW1990 cells compared to the cells treated with vehicle only. Finally, inhibition of the STAT3 signaling pathway by AG490 also led to a decrease in matrix metalloproteinase‐2 and vascular endothelial growth factor expression at the protein and mRNA levels. These results demonstrate that activation of the STAT3 signaling pathway plays an important role in the progression of pancreatic cancer and that inhibition of this pathway may be useful for an anti‐invasive therapeutic option in pancreatic cancer. (Cancer Sci 2006; 97: 1417–1423)
Pancreatic cancer remains a widespread and difficult disease to treat with an overall 5‐year survival of less than 5%. Thus, it represents one of the leading causes of cancer deaths in industrialized countries despite advances in medical therapy and surgical techniques.( 1 , 2 ) Because of the aggressive natural history of this disease, in which most patients have local or metastatic spread at the time of presentation, less than 10% of these cases constitute candidates for surgical resection at the time of diagnosis. Even among patients undergoing a potentially curative resection, the long‐term outcome remains unsatisfactory due to early recurrence and metastatic disease.( 3 ) Unfortunately, effective systemic therapy capable of reversing the aggressive nature of this disease is currently not available. Thus, understanding the molecular mechanisms underlying pancreatic cancer is a most important issue at this time.
Signal transducer and activator of transcription 3 (STAT3), a member of the Janus kinase (JAK)/STAT signaling pathway, is a central cytoplasmic transcription factor that is activated by phosphorylation of a conserved tyrosine residue (Tyr705) in response to extracellular signals and oncogenes. Once the tyrosine is phosphorylated, two STAT3 monomers form dimers through reciprocal phosphotyrosine–SH2 interactions, translocate to the nucleus where they bind to STAT3‐specific DNA‐response elements of target genes, and induce gene transcription.( 4 , 5 ) Accumulating evidence has demonstrated that elevated STAT3 activity is frequently found in a wide variety of human tumors including pancreatic cancer.( 6 , 7 , 8 , 9 ) It has been shown previously that STAT3 regulates a number of pathways important in tumorigenesis including cell cycle progression, apoptosis, tumor angiogenesis, and tumor cell evasion of the immune system.( 10 , 11 )
Recently, it was demonstrated that overexpression of p‐STAT3 correlated with the invasion and metastasis of colorectal adenocarcinoma and cutaneous squamous cell carcinoma.( 12 , 13 ) Furthermore, disrupted STAT3 signaling has been reported to lead to cell invasion through decreasing cell‐to‐cell homotypic adhesions and increasing cell motility and scattering.( 14 ) These studies suggest that the activation of STAT3 might play an important role in the invasion and metastasis of carcinomas. However, it is unknown whether STAT3 activation critically regulates the invasive and metastatic behavior of pancreatic tumors.
In the present study, we sought to determine whether the STAT3 signaling pathway regulates the invasive and metastatic potential of pancreatic cancer cells. We found that p‐STAT3 activity correlates with the invasion capacity of the pancreatic cancer cell lines. Furthermore, we found that inhibition of activated STAT3 by AG490 not only significantly suppressed matrix metalloproteinase (MMP)‐2 and vascular endothelial growth factor (VEGF) expression, but also reduced invasiveness of human pancreatic cancer cells with a high metastatic potential. Our results demonstrate that activation of the STAT3 signaling pathway may be critical for invasive and metastatic behavior of pancreatic tumors and that inhibition of this pathway may offer a novel strategy for pancreatic cancer intervention.
Materials and Methods
Cells and reagents. Human pancreatic cancer cell lines SW1990 and CaPan‐2 were obtained from American Type Culture Collection (ATCC). Tumor cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 100 units/mL penicillin, and 100 µg/mL streptomycin in a humidified incubator with an atmosphere of 5% CO2, 95% air at 37°C. The JAK‐specific inhibitor, AG490 (Calbiochem, San Diego, CA, USA) was dissolved in 100% dimethyl sulfoxide (DMSO; Sigma, St Louis, MO, USA) as a ×1000 stock solution and then diluted with the culture medium for experiments. The final concentration of DMSO for all treatments was maintained at 0.1%.
MTT assay. Cell viability was determined by 3‐(4,5‐dimethylthiazole‐2‐yl)‐2.5‐diphenyltetrazolium bromide (MTT) assay. Briefly, exponentially growing pancreatic cancer cells were seeded in 96‐well culture plates in culture medium at an optimal density. After 24 h, the medium was changed to fresh culture medium containing either vehicle alone (0.1% DMSO) or the indicated dose of AG490. MTT assays were performed after 1, 2 and 3 days of AG490 treatment. At the time of the assay, the cells were stained with 20 µL MTT (5 mg/mL) (Sigma) at 37°C for 4 h and subsequently made soluble in 150 µL of DMSO. Absorbance was measured at 570 nm using a microtiter plate reader (Wako, Osaka, Japan). The results were calculated as mean values of eight wells per treatment group.
Electrophoretic mobility shift analysis. Nuclear protein extracts were prepared with Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA). Protein concentrations were determined using an assay kit (Bio‐Rad, Hercules, CA, USA). STAT3 transcription factor activities were assessed by chemiluminescent EMSA Kit (Pierce, Rockford, IL, USA), according to the manufacturer's protocol. Briefly, nuclear protein extracts (10 µg) were incubated in a final volume of 20 µL of ×10 binding buffer, 50% glycerol, 100 mM MgCl2, 1 µg/µL poly (dIdC), 1% NP‐40 with the biotin end‐labeled or unlabeled double‐stranded STAT3 consensus‐binding motif 5′‐GAT CCT TCT GGG AAT TCC TAG ATC‐3′ (Sangon, Shanghai, China) for 20 min at room temperature. The protein‐DNA complexes were electrophoresed on a 6% polyacrylamide gel in ×0.5 tris‐boracic acid electrophoretic buffer (TBE) buffer and transferred onto nylon membranes by semidry blotting. After cross‐linking transferred DNA to nylon membranes with UV‐light cross‐linker (Pfizer Pharmacia, Cambridge, MA, USA), biotin‐labeled DNA was detected by chemiluminescence.
Invasion assay. Invasion assay was performed using a specialized invasion chamber that included a 24‐well tissue culture plate with 12 cell‐culture inserts (Chemicon, Temecula, CA, USA). The inserts contained an 8 µm pore size polycarbonate membrane with a precoated thin layer of basement membrane matrix (ECMatrix). Briefly, media supplemented with 10% fetal bovine serum was poured into the lower chamber as a hemo‐attractant. After reaching 60–70% subconfluence, pancreatic cancer cells were trypsinized, re‐suspended in DMEM (1 × 106 cells/mL), and 0.3 mL re‐seeded into the upper chambers. Cells were cultured in medium containing either vehicle alone (control) or indicated doses of AG490. After 48 h incubation at 37°C, non‐invasive cells were removed from the upper surface of the membrane using a moist cotton‐tipped swab. Invasive cells on the lower surface of the membrane, which had invaded the ECMatrix and had migrated through the polycarbonate membrane, were stained with the staining solution for 20 min and rinsed with distilled water several times. Invasiveness was quantitated by dissolving stained cells in 10% acetic acid and an equal amount of the dye/solution mixture was transferred onto a 96‐well plate for colorimetric reading of optical density at 560 nm.
Adhesion assay. Cell adhesiveness to the extracellular matrices was measured by an adhesion assay. The 96‐well culture plates were precoated with 10 µg (30 µL) Matrigel and dried overnight at 4°C. The wells were then rehydrated and blocked for 1 h at 37°C with 2% bovine serum albumin (BSA) in phosphate‐buffered saline (PBS). Cells were cultured with various concentrations of AG490 or vehicle alone (control) for 48 h before the harvest. After pretreatment, cells were trypsinized, washed three times with serum‐free medium, checked for cell viability by tripan blue, and seeded at a density of 1 × 105 cells/well on the Matrigel‐coated 96‐well plates. Cells were allowed to adhere for 1 h at 37°C, and nonadherent cells were carefully removed by washing gently with PBS three times. Adherent cells were stained for 4 h at 37°C with 50 µL MTT (1 mg/mL), made soluble in 200 µL of DMSO and measured at 570 nm using a microtiter plate reader (Wako). The results were calculated as mean values of eight wells per treatment group.
Reverse transcription‐polymerase chain reaction (RT‐PCR). Total RNA extraction from pancreatic cancer cells was performed with Trizol Reagent (Life Technologies, Rockville, MD, USA). Then, 2 µg of total RNA was reverse transcribed with the First Strand cDNA Synthesis Kit (Promega, Madison, WI, USA) to synthesize cDNA samples. Subsequently, 2 µL of cDNA product was then subjected to PCR amplification with Taq DNA polymerase (Sangon) on a thermal cycler using the following primers. The oligo‐nucleotide primers for MMP‐2, VEGF and β‐actin were constructed on the basis of published sequences.
The PCR primers used to detect each factor were as follows: MMP‐2, sense strand 5′‐GTGCTGAAGGACACACTAAAGAAGA‐3′, antisense strand 5′‐TTGCCATCCTTCTCAAAGTTGTAGG‐3′, with a product length of 605 bp;( 15 ) VEGF, sense strand 5′‐CCTGGTGGACATCTTCCAGGAGTACC‐3′, antisense strand 5′‐GAAGCTCATCTCTCCTATGTGCTGGC‐3′, with a product length of 196 bp;( 16 ) and β‐actin, sense strand 5′‐ATCTGGCACCACACCTTCTACAATGAGCTGCG‐3′, antisense strand 5′‐CGTCATACTCCTGCTTGCTGATCCACATCTGC‐3′, with a product length of 838 bp.( 17 ) The PCR conditions were as follows: one cycle of denaturing at 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 1 min, before a final extension at 72°C for 10 min. The PCR products were loaded onto 2% agarose gels and visualized with ethidium bromide under UV light.
Western blot. Whole‐cell protein extracts and nuclear protein extracts from pancreatic cancer cells were prepared with RIPA Lysis Buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Nuclear Extract Kit (Active Motif), respectively, according to the manufacturers’ instructions. Protein concentrations were determined using an assay kit (Bio‐Rad). Lysates containing 100 µg of protein were mixed with loading‐buffer with 5%β‐mercaptoethanol, and heated for 5 min at 100°C. Samples were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto nitrocellulose membranes by semidry blotting. Membranes were incubated in blocking buffer (tris buffered saline [TBS], 0.1% Tween 20, and 5% non‐fat dry milk) for 1 h at room temperature, followed by hybridization with anti‐p‐STAT3 [tyr‐705] antibody (Cell Signaling Technology, Beverly, MA, USA; 1:1000 dilution), anti‐STAT3 antibody (Cell Signaling Technology; 1:1000 dilution), anti‐MMP‐2 antibody (Santa Cruz Biotechnology; 1:500 dilution), anti‐VEGF antibody (Santa Cruz Biotechnology; 1:500 dilution) or anti β‐actin antibody (Lab Vision, Fremont, CA, USA; 1:100 dilution), at 4° overnight. After three washes in TBS/0.1% Tween 20, the membranes underwent hybridization with a horseradish peroxidase‐conjugated secondary antibody rabbit IgG (Santa Cruz Biotechnology; 1:5000 dilution) for 1 h at room temperature. After three washes in TBS/0.1% Tween 20, signals were detected by chemiluminescence using the western blotting luminol reagent (Santa Cruz Biotechnology).
Statistical analysis. The densitometric analysis of autoradiograms was carried out using a NIH image program (version 1.61). All assays were conducted three times and found to be reproducible. Numeric data are presented as mean ± standard deviation of the three experiments. Student's t‐test was used for comparing the differences between groups. Statistical significance was assigned if P < 0.05.
Results
p‐STAT3 protein expression is associated with in vitro invasiveness of human pancreatic cancer cells. We compared the levels of p‐STAT3 protein and STAT3 DNA‐binding activity between SW1990 and CaPan‐2 cell lines. As shown in Fig. 1A, p‐STAT3 protein levels were significantly higher in SW1990 cells compared to the CaPan‐2 cells. Furthermore, EMSA indicated significant amounts of STAT3 DNA‐binding activity in SW1990 cells, while weak binding activity was observed in CaPan‐2 cells (Fig. 1B). We also compared the invasion ability between SW1990 and CaPan‐2 cell lines. SW1990 cells were 8.5‐fold more invasive than CaPan‐2 cells (Fig. 1C,D). Thus, p‐STAT3 protein levels as well as STAT3 DNA‐binding activity positively correlated with cancer cell metastatic potential.
Figure 1.
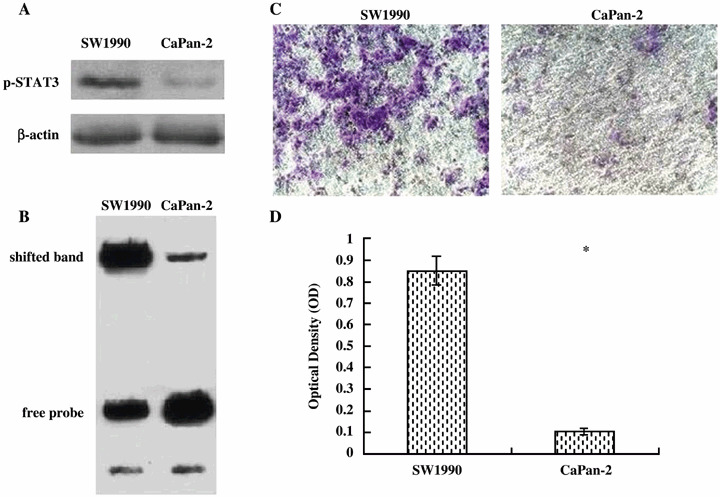
STAT3 phosphorylation and DNA‐binding activity and invasion ability in SW1990 and CaPan‐2 cells. (A) Western blot analysis, showing p‐STAT3 protein levels are significantly higher in SW1990 cells than in CaPan‐2 cells. (B) EMSA, showing STAT3 DNA‐binding activity is significantly higher in SW1990 cells than in CaPan‐2 cells. (C, D) Invasion assays, showing SW1990 cells have greater levels of invasiveness than CaPan‐2 cells. The blue‐stained cells are those that invaded the ECMatrix and migrated through the polycarbonate membrane to the lower surface of the membrane (original magnification ×200). Bars indicate mean ± SD of triplicate experiments; *P < 0.05 vs SW1990 cells.
Effect of AG490 on viability of pancreatic cancer cells. Because activation of STAT3 was positively associated with invasive potential, we next measured the cell viability of two pancreatic cancer cell lines in the presence of different concentrations of AG490, a specific JAK inhibitor. After incubating cells with 5, 10 or 20 µM of AG490 for 2 days, we observed that the cell numbers were not affected in any of the two cell lines compared to the vehicle‐treated cells (Fig. 2). However, cell viability was significantly suppressed by 40, 80 and 160 µM of AG490 in SW1990 cells and 80 and 160 µM of AG490 in CaPan‐2 cells after 2 days of incubation. Because of these results, cell invasion assay was performed with doses lower than 20 µM AG490 to remove its influence on cell viability.
Figure 2.
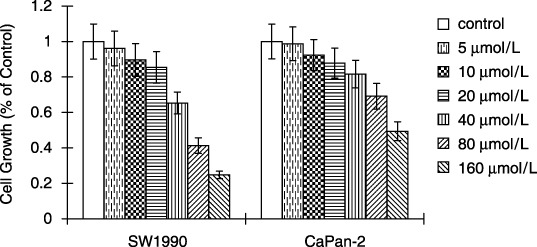
Effect of AG490 on pancreatic cancer cell viability. Cells growing in 96‐well plates were treated with AG490 for 2 days, and the MTT assay revealed that the cell viability was significantly suppressed by 40, 80 and 160 µM AG490 in SW1990 cells and 80 and 160 µM AG490 in CaPan‐2 cells. Cell viability was not significantly affected by 5, 10 and 20 µM AG490 after 2 days of incubation. Data are mean ± SD of eight wells. Bars indicate mean ± SD of triplicate experiments.
Effect of AG490 on invasion of pancreatic cancer cells. To evaluate the effects of inhibition of STAT3 activity on pancreatic cancer invasion, we performed an in vitro invasion assay using AG490 (Fig. 3). An invasion system was set up as described in Materials and Methods and the solution of AG490 was added into upper wells at a final concentration of 5, 10 or 20 µM. AG490 markedly reduced invasion of SW1990 cells at concentrations of 10 and 20 µM. Specifically, 10 µM of AG490 inhibited invasion by 35% in SW1990 cells, which was significant (P < 0.05) compared to the vehicle‐treated cells. In contrast, the invasion ability of CaPan‐2 cells was not significantly reduced by AG490 even when used at concentrations as high as 20 µM.
Figure 3.

Effect of AG490 on invasion of pancreatic cancer cells. Invasion assay was performed using a specialized invasion chamber that included a 24‐well tissue culture plate with 12 cell‐culture inserts. Invasion assay indicated AG490 significantly inhibited the ability of SW1990 cells to invade in a dose‐dependent manner. In contrast, CaPan‐2 cells were not significantly affected by AG490. Bars indicate mean ± SD of triplicate experiments.
Effect of AG490 on adhesion of pancreatic cancer cells. Cell adhesive ability to Matrigel (a recombinant extracellular matrix) was estimated by adhesion assay. In SW1990 cells, the number of cells adherent to Matrigel was significantly reduced in a dose‐dependent fashion in the presence of AG490 (Fig. 4). 10 µM of AG490 inhibited adhesion by 36% in SW1990 cells, which was significant (P < 0.05) compared to the vehicle‐treated cells. Although a decrease of the adhesion was also observed in CaPan‐2 cells, the inhibition rate did not achieve a statistically significant difference probably due to the low rate of decrease.
Figure 4.

Effect of AG490 on adhesion of pancreatic cancer cells. Cell adhesiveness to the extracellular matrices was measured by an adhesion assay, which indicated that AG490 significantly inhibited SW1990 cells, but not CaPan‐2 cells, in a dose‐dependent manner. Bars indicate mean ± SD of triplicate experiments; *P < 0.05; **P < 0.01.
Effect of AG490 on STAT3, p‐STAT3 protein level and STAT3 DNA‐binding activity of SW1990 cells. To examine the effects of AG490 on p‐STAT3 protein level and STAT3 DNA‐binding activity of SW1990 cells, western blotting and EMSA were carried out. The results indicated that AG490 did not affect total STAT3 protein levels; however, at 10 µM it suppressed p‐STAT3 protein levels and STAT3 DNA‐binding activity to 34% and 30%, respectively, of the levels of those treated with DMSO control in SW1990 cells (Fig. 5). Furthermore, the inhibition of p‐STAT3 protein levels and STAT3 DNA‐binding activity by AG490 was dose‐dependent such that the relative expressions at 20 µM were lower those that at 10 µM.
Figure 5.

Effect of AG490 on STAT3 activation. (A) Protein samples extracted from SW1990 cells treated for 48 h with AG490 were subjected to western blotting for p‐STAT3 and β‐actin proteins. AG490 significantly decreased p‐STAT3 protein expression in SW1990 cells in a dose‐dependent manner after 48 h incubation. The levels of β‐actin expression were determined as a control for equivalent protein loading. (B) Nuclear protein samples extracted from SW1990 cells after the treatments with AG490 for 48 h were subjected to EMSA for STAT3 DNA‐binding activity. AG490 significantly inhibited STAT3 DNA‐binding activity in a dose‐dependent manner in SW1990 cells after 48 h incubation.
Effect of AG490 on expression of VEGF and MMP‐2 in SW1990 cells. STAT3 activation contributes to oncogenesis through regulation of its target genes, which have recently been proved to be VEGF and MMP‐2. Therefore, we detected the effect of AG490 on expression of VEGF and MMP‐2. As shown in Fig. 6A, 48 h incubation with AG490 reduced MMP‐2 and VEGF protein expression in a dose‐dependent manner in SW1990 cells. RT‐PCR, which was also carried out at this time point, demonstrated that the expression of MMP‐2 and VEGF mRNAs in SW1990 cells were significantly down‐regulated by 20 µM of AG490 (Fig. 6B). Densitometric analysis revealed that the relative expression decreased to 11.78 ± 2.79% for MMP‐2, and 30.39 ± 3.96% for VEGF as compared to the controls (P < 0.05). The inhibitory effect of AG490 was dose‐dependent such that the relative expressions at 20 µM were lower those that at 10 µM.
Figure 6.
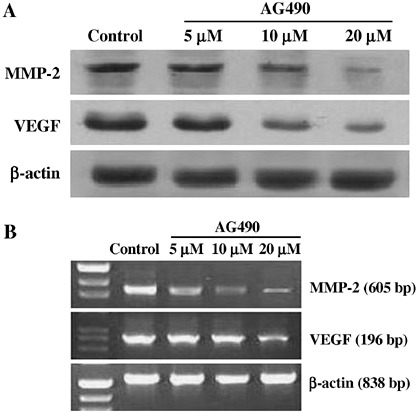
Effect of AG490 on expression of MMP‐2 and VEGF in SW1990 cells. (A) Protein samples extracted from SW1990 cells treated for 48 h with AG490 were subjected to western blotting for MMP‐2, VEGF, and β‐actin proteins. AG490 significantly decreased MMP‐2 and VEGF protein expression in SW1990 cells in a dose‐dependent manner. The levels of β‐actin expression were determined as a control for equivalent protein loading. (B) RNA samples extracted from SW1990 cells treated for 48 h with AG490 were subjected to RT‐PCR for MMP‐2, VEGF, and β‐actin. AG490 also significantly decreased the expressions of MMP‐2 and VEGF mRNAs in SW1990 cells, and the inhibitory effect was dose‐dependent. RT‐PCR for β‐actin was performed in parallel to show an equal amount of total RNA in the sample.
Discussion
During malignant transformation, STAT3 is frequently overexpressed and constitutively activated by tyrosine phosphorylation. Previous studies have demonstrated that activated STAT3 is overexpressed in human pancreatic cancer tissues and cell lines.( 18 , 19 , 20 ) Inappropriate and constitutive activation of STAT3 may be responsible for pancreatic cancer progression through regulating the expressions of target genes, such as c‐Myc, Bcl‐xL, p21WAF1 and cyclinD1, and functional inactivation of STAT3 by dominant‐negative STAT3 or AG490 could inhibit the proliferation and promote the apoptosis of pancreatic cancer cells.( 18 , 21 ) Despite the clear importance of STAT3 in cell proliferation and survival in human pancreatic cancer, its potential contribution to pancreatic cancer invasion and metastasis still remains hypothetical.
We have previously reported that moderate to strong expression of p‐STAT3 was observed in >70% of pancreatic cancers and the p‐STAT3 expression was significantly related to the clinical stage and lymph node metastasis, whereas normal pancreatic ductal epithelium lacks p‐STAT3 protein.( 19 ) Although this clinical study with human pancreatic cancer tissues strongly suggested that overexpression of p‐STAT3 may play a role in aggressive behaviors of pancreatic cancers, the precise role of STAT3 in pancreatic cancer cell invasion and metastasis was not fully examined. In an effort to elucidate the role of STAT3 in pancreatic cancer, we utilized a JAK‐specific inhibitor, AG490, and found that it suppressed in vitro invasion in pancreatic cancer cell lines harboring high STAT3 activity.
Previous studies showed that STAT3 was activated in a few human pancreatic cancer cells and BxPc‐3, FG, PANC‐1, and SW1990 cell lines had strong STAT3 DNA‐binding activity.( 22 ) In the present study, we first compared the levels of p‐STAT3 protein and STAT3 DNA‐binding activity between SW1990 and CaPan‐2 cell lines. We found that p‐STAT3 protein levels were significantly higher in SW1990 cells than in CaPan‐2 cells, which expressed weak levels of this protein. A similar trend was seen in STAT3 DNA‐binding activity as assessed by EMSA. Strong STAT3 DNA‐binding activity was observed in SW1990 cells, while weak STAT3 DNA‐binding activity was found in CaPan‐2 cells. To determine whether p‐STAT3 protein and increased DNA‐binding activity correlated with the biological activity of the pancreatic cancer cell lines used, we examined the invasion ability of these cells with a cell invasion assay kit. We found that SW1990 cells showed greater levels of invasiveness than the CaPan‐2 cells. Thus, p‐STAT3 protein levels as well as STAT3 DNA‐binding activity positively correlated with their metastatic potential. Taken together, these results demonstrate a strong relationship between STAT3 activity and the invasive ability of human pancreatic cancer cells with differing metastatic potentials.
These initial observations prompted us to further question whether modulation of STAT3 could affect the invasive ability of pancreatic cancer cells. STAT3 is a downstream transcription factor of JAK, and JAK inhibitor could inhibit STAT3 signal pathway.( 23 , 24 ) In the present study, we demonstrated that AG490, a JAK‐specific inhibitor, strongly suppressed STAT3 activity and invasion of the SW1990 cell line, which exhibited high STAT3 activity, but not the CaPan‐2 cell line, which exhibited minimal STAT3 activity.
The invasiveness of tumor cells is a multistep process and cell adhesion to the extracellular matrix is an important element.( 25 ) Thus, we examined the effect of AG490 on adhesion capability of pancreatic cancer cells. Adhesion assay indicated that SW1990 cells’ ability to adhere was attenuated in a dose‐dependent manner when cells were treated with AG490. In contrast, the adhesion ability of CaPan‐2 cells was not significantly reduced by AG490. Therefore, blocking the STAT3 pathway with AG490 could suppress invasion of pancreatic cancer cells by decreasing cell adhesion to the extracellular matrix.
The mechanism by which AG490 inhibits the invasion is considered to be via down‐regulation of genes related to proteolysis and angiogenesis. Synthesis, secretion, activation and use of proteases to degrade various components of the basement membrane and extracellular matrix are believed to be indispensable aspects of the invasive phenotype during the cancer invasion process.( 26 , 27 ) According to studies using clinical samples of pancreatic cancer and pancreatic cancer cell lines, MMPs play important roles in tumor cell invasion and metastasis through degrading components of the basement membranes and extracellular matrix.( 28 , 29 , 30 ) Recently, some studies have found that STAT3 signaling directly regulates MMP‐2 expression, tumor invasion, and metastasis in metastatic melanoma cells and proved MMP‐2 to be a target gene of STAT3.( 31 , 32 ) In our present study, the use of AG490 also markedly reduced the mRNA and protein expression of MMP‐2 in SW1990 cells through blocking the STAT3 signaling pathway.
Cancer cells need to acquire phenotypes of angiogenesis in addition to proteolysis for invasion and metastasis in vivo. VEGF is known to be a potent angiogenic mitogen that plays an important role in tumor angiogenesis and metastasis.( 33 ) Accumulating evidence demonstrates that VEGF expression is associated with poor survival and prognosis for pancreatic cancer and that increased VEGF expression is correlated with increased microvessel density, local invasion, liver metastasis, and early recurrence after curative resection.( 34 , 35 , 36 , 37 ) Furthermore, VEGF is a downstream target gene of STAT3,( 38 ) and a recent study has reported that stable transfection of dominant‐negative STAT3 decreases VEGF expression in the pancreatic cancer cell line FG.( 22 ) In the present study, we also found that AG490 significantly decreased the mRNA and protein expression of VEGF in SW1990 cells.
Although the upstream signals of STAT3 are not completely understood, JAK is responsible for the tyrosine phosphorylation of STAT3 in response to extracellular signals and oncogenes. Previous studies demonstrated that JAK/STAT3 signals played an important role in the progression of pancreatic cancer and a JAK‐specific inhibitor could markedly inhibit JAK/STAT3 signals in pancreatic cancer cells.( 18 , 21 ) In the present study, we showed that a JAK‐specific inhibitor, AG490, reduced the expression of p‐STAT3 protein and STAT3 DNA‐binding activity in human pancreatic cancer cell lines. These results suggest that JAK may be one of the key upstream activators of STAT3 in pancreatic cancer. AG490 strongly inhibited the cell invasion of SW1990 cells, which have high STAT3 activity. This could be based on inhibition of STAT3 as AG490 did not significantly suppress invasion of CaPan‐2 cells, which have faint STAT3 activity. These results suggest that the inhibitory mechanism of AG490 could be based on inhibition of STAT3 activity.
In conclusion, JAK/STAT3 signals are responsible for the invasion and metastasis of human pancreatic cancer via regulation of cell adhesion ability and the expression of genes related to proteolysis and angiogenesis. Blocking the JAK/STAT3 pathway may provide a novel modality in preventing the invasion and metastasis of pancreatic cancer.
References
- 1. Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002; 2: 897–909. [DOI] [PubMed] [Google Scholar]
- 2. Li DH, Xie KP, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363: 1049–57. [DOI] [PubMed] [Google Scholar]
- 3. Neoptolemos JP, Cunningham D, Friess H et al. Adjuvant therapy in pancreatic cancer: historical and current perspectives. Ann Oncol 2003; 14: 675–92. [DOI] [PubMed] [Google Scholar]
- 4. Darnell JE Jr. STATs and gene regulation. Science 1997; 277: 1630–5. [DOI] [PubMed] [Google Scholar]
- 5. Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene 2000; 19: 2474–88. [DOI] [PubMed] [Google Scholar]
- 6. Grandis JR, Drenning SD, Zeng Q et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo . Proc Natl Acad Sci 2000; 97: 4227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhir R, Ni Z, Lou W, Demiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate 2002; 51: 241–6. [DOI] [PubMed] [Google Scholar]
- 8. Niu G, Bowman T, Huang M et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002; 21: 7001–10. [DOI] [PubMed] [Google Scholar]
- 9. Scholz A, Heinze S, Detjen KM et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology 2003; 125: 891–905. [DOI] [PubMed] [Google Scholar]
- 10. Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2005; 2: 315–24. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh FC, Cheng G, Lin J. Evaluation of potential Stat3‐regulated genes in human breast cancer. Biochem Biophys Res Commun 2005; 335: 292–9. [DOI] [PubMed] [Google Scholar]
- 12. Kusaba T, Nakayama T, Yamazumi K et al. Expression of p‐STAT3 in human colorectal adenocarcinoma and adenoma: correlation with clinicopathological factors. J Clin Pathol 2005; 58: 833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suiqing C, Min Z, Lirong C. Overexpression of phosphorylated‐STAT3 correlated with the invasion and metastasis of cutaneous squamous cell carcinoma. J Dermatol 2005; 32: 354–60. [DOI] [PubMed] [Google Scholar]
- 14. Rivat C, De Wever O, Bruyneel E, Mareel M, Gespach C, Attoub S. Disruption of STAT3 signaling leads to tumor cell invasion through alterations of homotypic cell–cell adhesion complexes. Oncogene 2004; 23: 3317–27. [DOI] [PubMed] [Google Scholar]
- 15. Uchima Y, Sawada T, Nishihara T, Maeda K, Ohira M, Hirakawa K. Inhibition and mechanism of action of a protease inhibitor in human pancreatic cancer cells. Pancreas 2004; 29: 123–31. [DOI] [PubMed] [Google Scholar]
- 16. Brown KJ, Maynes SF, Bezos A, Maguire DJ, Ford MD, Parish CR. A novel in vitro assay for human angiogenesis. Laboratory Invest 1996; 75: 539–55. [PubMed] [Google Scholar]
- 17. Zhu Z, Yao J, Wang F, Xu Q. TNF‐alpha and the phenotypic transformation of human peritoneal mesothelial cell. Chin Med J (Engl) 2002; 115: 513–7. [PubMed] [Google Scholar]
- 18. Scholz A, Heinze S, Detjen KM et al. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology 2003; 125: 891–905. [DOI] [PubMed] [Google Scholar]
- 19. Qiu ZJ, Liu C, Hu HH, Cao J. Expressions of STAT3 and Cyclin D1 in pancreatic carcinoma and their clinical significance. Chin J Pancreatol 2005; 5: 24–7. [Google Scholar]
- 20. Liu C, Qiu ZJ, Sun HC, Hu HH, Huang KJ. Expressions of pSTAT3 and VEGF in pancreatic carcinoma and their clinical significance. Chin J Hepatobiliary Surg 2006; 12: 471–3. [Google Scholar]
- 21. Toyonaga T, Nakano K, Nagano M et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription‐3 pathway inhibits growth of human pancreatic cancer. Cancer Lett 2003; 201: 107–16. [DOI] [PubMed] [Google Scholar]
- 22. Wei D, Le X, Zheng L et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 2003; 22: 319–29. [DOI] [PubMed] [Google Scholar]
- 23. Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res 2000; 60: 1225–8. [PubMed] [Google Scholar]
- 24. Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: Direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther 2004; 3: 11–20. [PubMed] [Google Scholar]
- 25. Liotta LA, Kohn E. Cancer invasion and metastases. JAMA 1990; 263: 1123–6. [PubMed] [Google Scholar]
- 26. Stetler‐Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993; 9: 541–73. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen TH. Mechanisms of metastasis. Clinics Dermatol 2004; 22: 209–16. [DOI] [PubMed] [Google Scholar]
- 28. Bloomstom M, Zeros EE, Rosemurgy AS 2nd. Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol 2002; 9: 668–74. [DOI] [PubMed] [Google Scholar]
- 29. Matsuyama Y, Takao S, Aikou T. Comparison of matrix metalloproteinase expression between primary tumors with or without liver metastasis in pancreatic and colorectal carcinomas. J Surg Oncol 2002; 80: 105–10. [DOI] [PubMed] [Google Scholar]
- 30. Tan X, Egami H, Ishikawa S et al. Involvement of matrix metalloproteinase‐7 in invasion‐metastasis through induction of cell dissociation in pancreatic cancer. Int J Oncol 2005; 26: 1283–9. [PubMed] [Google Scholar]
- 31. Xie TX, Wei D, Liu M et al. Stat3 activation regulates the expression of matrix metalloproteinase‐2 and tumor invasion and metastasis. Oncogene 2004; 23: 3550–60. [DOI] [PubMed] [Google Scholar]
- 32. Xie TX, Huang FJ, Aldape KD et al. Activation of Stat3 in human melanoma promotes brain metastasis. Cancer Res 2006; 66: 3188–96. [DOI] [PubMed] [Google Scholar]
- 33. Grunstein J, Roberts WG, Mathieu‐Costello O et al. Tumor‐derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res 1999; 59: 1592–8. [PubMed] [Google Scholar]
- 34. Itakura J, Ishiwata T, Friess H et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 1997; 3: 1309–16. [PubMed] [Google Scholar]
- 35. Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 2000; 88: 2239–45. [DOI] [PubMed] [Google Scholar]
- 36. Niedergethmann M, Hildenbrand R, Wostbrock B et al. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas 2002; 25: 122–9. [DOI] [PubMed] [Google Scholar]
- 37. Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas 2003; 26: 344–9. [DOI] [PubMed] [Google Scholar]
- 38. Niu G, Wright KL, Huang M et al. Constitutive Stat3 activity up‐regulates VEGF expression and tumor angiogenesis. Oncogene 2002; 21: 2000–8. [DOI] [PubMed] [Google Scholar]


