Abstract
The WW‐domain‐containing oxidoreductase (WWOX) gene spans the common chromosomal fragile site FRA16D (16q23.2) and is believed to be a tumor suppressor in various human malignancies. We have previously shown frequent down‐modulation of Wwox expression in pancreatic carcinoma (PC); however, biological function of Wwox in pancreatic duct carcinogenesis remains unknown. In PANC‐1 (Wwox‐negative) PC‐derived cells, restoration of recombinant WWOX gene expression with adenoviral gene delivery (Ad‐WWOX) effectively increased the number of cells with subG1 DNA contents in a multiplicity of infection‐dependent manners: Ad‐WWOX infection up‐regulated caspase‐3 activity and reduced procaspase‐3 and procaspase‐8 levels. We also confirmed that restoration of WWOX gene suppressed cell growth in vitro and tumorigenicity in vivo. In addition, transduction of wild‐type WWOX‐expressing vector inhibited PANC‐1 colony formation; however, substitution of Y33 of Wwox with arginine did not lead to inhibition of colony formation, suggesting the biological significance of the WW1 domain of Wwox for its tumor‐suppressing activity. In PC tissue samples, abundant cytoplasmic Wwox expression was detected in the normal pancreatic duct epithelium, whereas Wwox expression was frequently reduced not only in a large fraction of PC but also in precancerous lesions in accord with the pancreatic intraepithelial neoplasia (PanIN) grade, which was closely correlated with patients’ poorer outcome. Interestingly, the existence of Wwox expression was associated with elevated mothers against decapentaplegic homolog 4 (Smad4) protein levels in vitro and in vivo. These findings suggest that down‐modulation of Wwox expression is an early event and may be associated with the down‐regulation of Smad4 protein levels during pancreatic duct carcinogenesis. (Cancer Sci 2008; 99: 1370–1376)
Pancreatic carcinoma (PC) is among the most aggressive and lethal human diseases, with a very poor prognosis; even with complete surgical resection and adjuvant chemotherapy, the 5‐year survival rate is less than 20%.( 1 ) Infiltrating carcinomas of the exocrine pancreas arise from histologically identifiable intraductal precursors that undergo a series of architectural and cytologic changes. These intraductal lesions of the pancreas are also known as pancreatic intraepithelial neoplasias (PanIN), and they progress from flat to papillary without atypia to papillary with atypia to carcinoma in situ.( 2 , 3 ) Multiple genetic and epigenetic alterations have been documented in PC: numerous alterations in KRAS,( 4 ) HER‐2,( 5 ) CDKN2A,( 4 , 6 ) BRCA2,( 7 ) TP53,( 8 ) and SMAD4,( 9 ) have been described in a variety of PanIN using both genetic and immunohistochemical analyses, and some in situ lesions eventually progress to infiltrating carcinoma.( 3 ) But much remains unknown about development and progression of pancreatic duct lesions.
Common chromosome fragile sites in the human genome are particularly susceptible to damage by environmental carcinogens. Common fragile sites have been observed at or near structural chromosome defects recognized in various cancers, and such instability contributes to neoplasia by virtue of altered expression of the associated genes. For instance, homozygous deletion and chromosomal breakage at FRA3B (3p14.2) have been shown to result in a loss of fragile histidine triad (FHIT) gene expression in many human cancers.( 10 , 11 ) Restoration of the FHIT gene in cancer cells effectively induces caspase‐dependent apoptosis with a suppression of the Akt–survivin pathway,( 12 , 13 ) confirming the role of Fhit as a tumor suppressor. Similarly, the WW‐domain‐containing oxidoreductase (WWOX) gene encompasses FRA16D (16q23.2). Frequent loss of heterozygosity (LOH) at the WWOX locus, hypermethylation of the WWOX promoter and resultant loss of Wwox expression has been reported in breast,( 14 ) esophageal,( 15 ) and other human malignancies.( 16 , 17 , 18 )
Wwox protein contains two WW domains (WW1 and WW2) at the N‐terminus and a central short‐chain dehydrogenase/reductase (SDR) domain that has amino acid sequence homology with steroid oxidoreductases.( 19 , 20 , 21 ) Recent studies have revealed the tumor suppressor effects of Wwox in human cancers: restoration of the WWOX gene by adenoviral gene delivery was found to suppress tumor growth and increase the number of cells with subG1 DNA content.( 21 , 22 ) Interestingly, the WW1‐domain‐specific proline‐rich ligand, the PPXY motif, has been identified as critical for Wwox function as a regulator of the subcellular localization of p73,( 23 ) and AP‐2 γ transcription factors.( 24 ) In particular, the substitution of Y33 with arginine diminished Wwox interaction with v‐erb‐a erythroblastic leukemia viral oncogene homolog 4 (ErB4),( 25 ) and Jun,( 26 ) suggesting that Y33 within the WW1 domain, a physiological phosphorylation target for Src tyrosine kinase, is critical for Wwox‐mediated protein–protein interactions. Furthermore, osteosarcomas in juvenile Wwox−/– mice and lung papillary carcinoma in adult Wwox+/– mice occurred spontaneously,( 27 ) and treatment with ethylnitrosourea and N‐nitrosomethylbenzylamine effectively induced lung tumors/lymphomas,( 27 ) and forestomach tumors in Wwox+/– mice,( 28 ) respectively, confirming the Wwox tumor‐suppressor effects in vivo.
We have previously demonstrated the possible function of Wwox in PC: hypermethylation‐mediated down‐regulation of Wwox expression levels was frequently detected in PC‐derived cells and PC tissue samples. In addition, transfection of WWOX inhibited colony formation of PC cell lines by triggering apoptosis.( 29 ) In this study, we further investigated the effect of Wwox in the regulation of cell growth and apoptosis in PC‐derived cells using adenoviral gene delivery. Moreover, we investigated the expression of Wwox in PC tissue samples and adjacent PanIN to find out the role of Wwox in pancreatic duct carcinogenesis.
Materials and Methods
Cell culture and tissue samples. Human PC‐derived cell line PANC‐1 (Wwox‐negative,( 29 ) American Type Culture Collection, Manassas, VA, USA) was routinely maintained in Roswell Park Memorial Institute media (RPMI)‐1640 supplemented with 10% fetal bovine serum. QBI‐HEK 293CymR cells (Qbiogene, Irvine, CA, USA) were used for generation, amplification and titration of recombinant adenoviruses.( 12 , 13 ) In total, 32 formalin‐fixed and paraffin‐embedded sporadic PC specimens surgically removed at Kobe University Hospital (Kobe, Japan) were employed. Informed consent was obtained from all patients and the study was approved by the Kobe University Institutional Review Board. None of these cases had received adjuvant chemotherapy or radiotherapy before surgery. Histological examination was performed according to the General Rules for the Study of Pancreatic Cancer,( 30 ) along with tumor–lymph nodes–metasteses (TNM) classification.( 31 ) Non‐neoplastic ducts including normal pancreatic ducts and PanIN( 32 ) adjacent to PC were included in the analyses.
Immunoblot. Samples were extracted in cell lysis buffer containing 50 mM TRIS‐HCl (pH 7.4), 125 mM NaCl, 0.1% Triton X‐100, 5 mM ethylenediamine tetraacetic acid (EDTA), 1% (v/v) protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and 1% (v/v) phosphatase inhibitor cocktail (Sigma). Total protein (20 µg) was denatured and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by electrotransfer to Hybond‐C membrane (GE Healthcare, Piscataway, NJ, USA). Membranes were probed with the following antibodies: anti‐Wwox,( 21 ) anti‐procaspase‐3 (Cell Signaling, Beverly, MA, USA), anti‐procaspase‐8 (Cell Signaling), anti‐Smad4 (Cell Signaling), anti‐Smad2/3 (Cell Signaling), anti‐green fluorescent protein (GFP; MBL, Nagoya, Japan) and anti‐β‐actin (Sigma). After probing with appropriate secondary antibodies, signals were detected by chemiluminescence substrate.
Gene transduction. Adenoviruses carrying human recombinant wild‐type WWOX (Ad‐WWOX) and GFP (Ad‐GFP) were prepared, amplified, and titrated as described elsewhere.( 21 ) Ad‐GFP virus was used as a non‐specific control for gene transfer. PANC‐1 cells were incubated with adenoviral aliquots at a desired multiplicity of infection (MOI) for 4 h before addition of culture medium (>25 × volume of virus inoculum). Transduction efficiency was assessed by visualization of GFP‐expressing cells using fluorescence microscopy. Human WWOX complementary DNA (cDNA) was subcloned into the mammalian expression vector pRcCMV (Invitrogen, Carlsbad, CA, USA) to generate pWWOX vector. Also, site‐directed mutagenesis was performed using pWWOX‐Y33R, as well as the SDR domain‐defective mutant WWOX‐expressing vectors pΔWWOX and pΔWWOX‐Y33R. Each plasmid was transfected into PANC‐1 cells using Lipofectamine (Invitrogen).
Flow cytometric analysis and caspase‐3 activity assay. To analyze cellular DNA content, PANC‐1 cells were collected and fixed in 70% methanol, treated with RNase A and stained with propidium iodide. The analysis was performed with a fluorescence‐activated cell sorting (FACS) Calibur cytometer (BD Biosciences, San Jose, CA, USA). The activity of caspase‐3 was evaluated by the caspase‐3 colorimetric substrate/inhibitor (Sigma). Briefly, PANC‐1 cells (5 × 106 cells/dish) infected with Ad‐WWOX or Ad‐GFP (MOI 0–50) were suspended in cell lysis buffer (50 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid [HEPES], 5 mM 3,3‐cholamidopropyl‐dimethylammonio‐1‐propanesulfonate [CHAPS] and 1 mM dithiothreitol [DTT]). In total, 100 µL of assay buffer (20 mM HEPES, 0.1% CHAPS, 5 mM DTT and 2 mM EDTA) and 10 µL of acetyl‐Asp‐Glu‐Val‐Asp p‐nitroanilide (Ac‐DEVD‐pNA) substrate were added to triplicate culture wells in 96‐microwell plates. N‐Ac‐Asp‐Glu‐Val‐Asp‐Asp‐CHO (Ac‐DEVD‐CHO), an aldehyde caspase‐3‐specific inhibitor, was also used to test the specificity of caspase‐3. The plates were then incubated at 37°C for 2 h and caspase‐3 activity in the lysate was determined by absorbance at 405 nm on a microwell plate reader.
Cell growth test, colony formation assay and tumorigenicity tests. For the cell growth test, PANC‐1 cells were seeded in a 100‐mm plate at a density of 1 × 105 cells and infected with Ad‐WWOX and Ad‐GFP (MOI 25). Viable cells were counted. For colony formation assay, PANC‐1 cells (1.0 × 104) in 100‐mm plates in triplicate were transfected with 6 µg wild‐type and mutant WWOX‐expressing vectors using Lipofectamine (Invitrogen). Forty‐eight hours after transfection, cells were fixed with methanol and stained with Giemsa, and visible colonies (>0.5 mm in diameter) were counted. For tumorigenicity test, viable PANC‐1 cell numbers were counted 24 h after infection by Ad‐WWOX or Ad‐GFP (MOI 25). Cells were then collected, and 1 × 106 cells were injected into the flanks of 10‐week‐old female nude mice (Japan Clea, Tokyo, Japan), five mice per group. Five control mice were injected with the same number of uninfected PANC‐1 cells. Animals were monitored daily and subcutaneous (s.c.) tumor sizes were measured twice a week. At endpoint (7 weeks), animals were sacrificed, tumors were weighed and tumor volumes were calculated using the formula V = (the shortest diameter)2 × (the longest diameter).
Immunohistochemistry. A modified version of the immunoglobulin enzyme bridge technique with labeled streptavidin biotin (LSAB) kit (Dako, Glostrup, Denmark) was used.( 33 ) Briefly, deparaffinized and rehydrated sections were autoclaved in a citrate buffer. After blocking of endogenous peroxidase activity and non‐specific reactions, the primary antibodies against Wwox,( 21 ) and Smad4 (Cell Signaling) were applied to sections and were subsequently incubated with biotinylated monkey antirabbit immunoglobulin G (IgG). Streptavidin conjugated to horseradish peroxidase was used to immerse with 3,3‐diaminobenzidine tetrahydrochloride. Immunohistochmical results were evaluated by two pathologists (S.N. and S.S.) who were blinded to clinical information. Immunoreactivities of Wwox and Smad4 were graded according to the number of stained cells and the staining intensity in individual cells as follows: (–), almost no positive cells; (+), 5–50% of tumor cells showed weak immunoreactivity; (++), >50% of tumor cells showed weak immunoreactivity or tumor cells showed intense immunoreactivity. Similarly, Smad4 immunoreactivity was graded as follows: (–), negative; (+), positive.
Statistical analysis. Statistical analysis was conducted using χ2 test to evaluate the relationship between Wwox immunoreactivity and clincopathologic characters. Student's two‐sided t‐test was used to compare values of test and control samples. Survival curves were drawn according to the Kaplan–Meier method and differences between the curves were analyzed by applying the log‐rank test. P‐values less than 0.05 were considered statistically significant.
Results
Restoration of WWOX induces caspase‐dependent apoptosis and suppresses tumor growth in vitro and in vivo. The tumor‐suppressing and apoptosis‐inducing effects of Wwox were investigated in order to characterize the functions of Ad‐WWOX in PC cells. In PANC‐1 cells, transduction of Ad‐WWOX, but not that of Ad‐GFP, induced apoptosis; 48 h after infection, Ad‐WWOX led to a MOI‐dependent elevation in the number of cells with subG1 DNA contents (Fig. 1a,b). Although restoration of the WWOX gene with Ad‐WWOX increased the number of cells with subG1 DNA contents in PK‐1 (Wwox‐negative) cells, Ad‐WWOX infection was less effective in PK‐9 (Wwox‐positive) cells (data not shown). Activation of caspase‐3 was also MOI‐dependent, which was confirmed by detection of reduced levels of procaspase‐3 and procaspase‐8 expression (Fig. 1c). We also evaluated the growth‐suppressing effect of Wwox and found that Ad‐WWOX effectively suppressed cell growth in vitro (Fig. 2a) and tumorigenicity in vivo (Fig. 2b).
Figure 1.
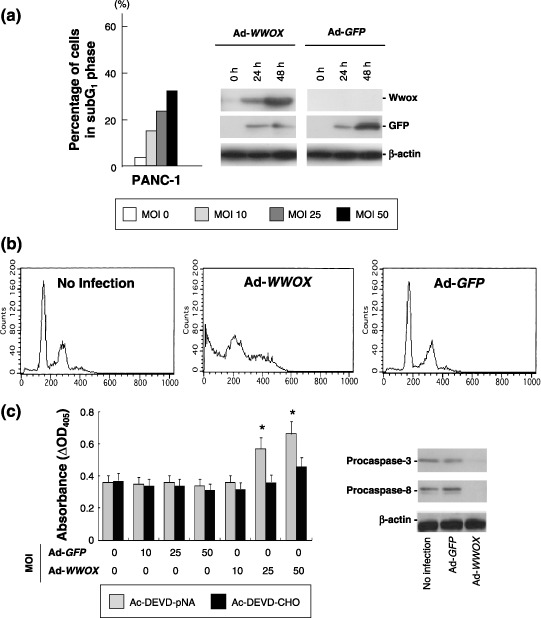
Restoration of WW‐domain‐containing oxidoreductase (Wwox) expression induces caspase‐dependent apoptosis in PANC‐1 cells. (a) Percentage of cells in subG1 DNA content for adenoviral gene delivery (Ad)‐WWOX infection. Ad‐antigreen fluorescent protein (GFP) did not increase the fraction of subG1 DNA content more than 5% (data not shown). Recombinant Wwox protein was detected by immunoblot. (b) Representative results of flow cytometric analysis. Cells were collected 48 h after Ad‐WWOX or Ad‐GFP infection (multiplicity of infection [MOI] 25). (c) Increased activity of caspase‐3 by infection of Ad‐WWOX. Decrease of procaspase‐3 and procaspase‐8 determined by immunoblot are also shown. *P < 0.05.
Figure 2.
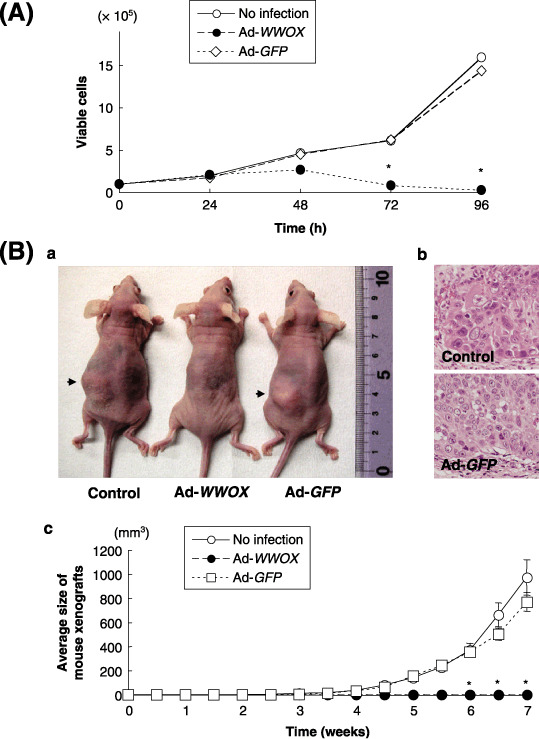
Growth suppression by restoration of the WW‐domain‐containing oxidoreductase (WWOX) gene in PANC‐1 cells in vitro and in vivo. (A) Cell growth test after transduction of the WWOX gene with adenoviral (Ad)‐WWOX (multiplicity of infection [MOI] 25). (B) Tumorigenicity of Ad‐WWOX infected PANC‐1 cells. (a) Representative mouse subcutaneous (s.c.) tumors at 7 weeks. (b) Histological examination of the s.c. tumors (hematoxylin & eosin (HE). staining). (c) Restoration of Wwox expression inhibited s.c. tumor formation. *P < 0.05.
Since the physiological phosphorylation site Y33 of Wwox is essential for Wwox‐mediated tumor suppression,( 23 , 24 , 25 , 26 ) the biological significance of Y33 in the WW1 domain was confirmed, as compared with the significance of the C‐terminus SDR domain (Fig. 3a,b). Interestingly, the Y33 mutant Wwox did not lead to inhibition of colony formation; thus, the Y33 mutant Wwox was much less effective than the SDR‐deleted mutant in terms of suppressing colony formation (Fig. 3c,d).
Figure 3.
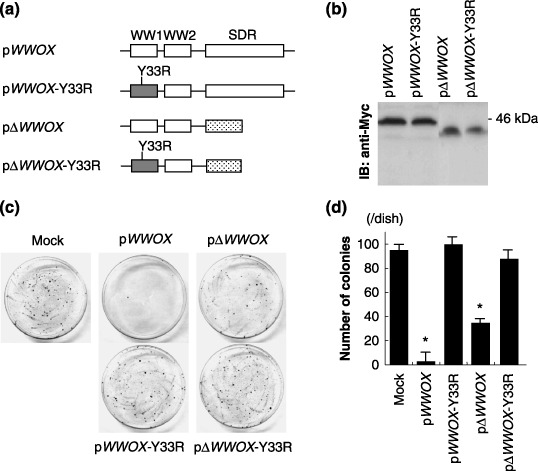
Biological significance of the WW1 domain in PANC‐1 cells. (a) A scheme of the wild‐type and mutant WW‐domain‐containing oxidoreductase (WWOX)‐expressing vector constructs. Substitution of Y33 with arginine was conducted to generate Y33R mutants, and the short‐chain dehydrogenase/reductase (SDR)‐domain deletion mutants were also designed. (b) Detection of recombinant Wwox protein by immunoblot. The N‐termini of these proteins were tagged with Myc. (c) Representative plates for colony formation assay. (d) Quantification of colonies per plate from 100‐mm plates, for pcDNA3 (mock), pWWOX, pWWOX‐Y33R, pΔWWOX and pΔWWOX‐ Y33R‐transfected PANC‐1 cells. *P < 0.05.
Reduction of Wwox expression during pancreatic duct carcinogenesis. The expression levels of Wwox in non‐neoplastic pancreatic duct and PC tissue samples were determined by immunohistochemistry. Abundant cytoplasmic Wwox expression was detected in the normal pancreatic duct epithelium, whereas Wwox expression was reduced in precancerous lesions in accord with the PanIN grade (Fig. 4a). In PC, absent (–) or low (+) Wwox expression was frequently detected (Fig. 4b), with a significant correlation with frequent incidence of lymph node metastasis (P = 0.026; Table 1). We also performed microsatellite analysis,( 34 ) and methylation‐specific polymerase chain reaction (MSP),( 17 ) to investigate the genetic and epigenetic backgrounds of these Wwox‐negative PCs. The frequencies of LOH at D16S3029 within the WWOX locus and hypermethylation of the WWOX promoter CpG (cytosine and guanine separated by a phosphate) island were 22% and 20%, respectively; however, hypermethylation of the WWOX exon 1 CpG island was detected in 83% of Wwox‐negative PC (data not shown).( 17 ) Among 32 patients with PC who underwent curative surgery and who received follow‐up care at Kobe University Hospital, a significant difference in survival rates was detected depending on Wwox level: the absent (–) and low (+) Wwox expression was significantly associated with shorter survival times, as compared with tumors expressing high (++) levels of Wwox expression (P = 0.024; Fig. 4c).
Figure 4.
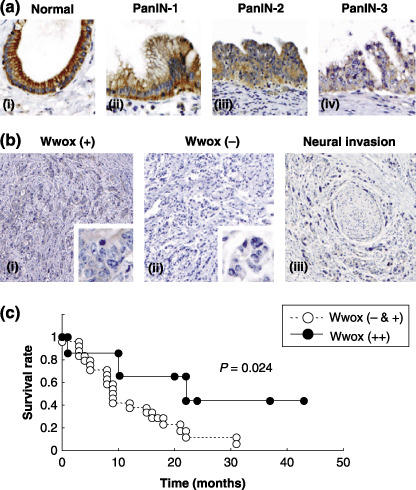
WW‐domain‐containing oxidoreductase (Wwox) expression in pancreatic carcinoma (PC) tissue samples. (a) Wwox expression in‐non‐neoplastic pancreatic duct epithelia (i) and pancreatic intraepithelial neoplasia (PanIN) (ii, PanIN‐1; iii, PanIN‐2; and iv, PanIN‐3). (b) Wwox expression in PC. Representative tumors with weak (i) and negative Wwox expression (ii & iii) are shown. (c) Kaplan–Meier curves of the overall survival of PC patients whose cancer samples are positive (++), and negative or weak (– & +) for Wwox expression.
Table 1.
Results of WW‐domain‐containing oxidoreductase (Wwox) expression in pancreatic carcinoma (PC): association with clinicopathological findings
| Wwox expression † | P‐value* | |||
|---|---|---|---|---|
| Total n (%) | (– & +) n (%) | (++) n (%) | ||
| Total | 32 (100%) | 25 (79%) | 7 (21%) | |
| Histology ‡ | ||||
| Moderately diff. type | 29 (91%) | 23 (72%) | 6 (19%) | 0.887 |
| Poorly diff. type | 3 (9%) | 2 (6%) | 1 (3%) | |
| Tumor size ‡ | ||||
| TS1 + TS2 | 14 (44%) | 10 (31%) | 4 (13%) | 0.351 |
| TS3 + TS4 | 18 (56%) | 15 (47%) | 3 (9%) | |
| Type of tumor growth ‡ | ||||
| INFβ | 27 (84%) | 21 (66%) | 6 (18%) | 0.704 |
| INFγ | 5 (16%) | 4 (13%) | 1 (3%) | |
| Lymphatic vessels infiltration | ||||
| Negative | 3 (9%) | 2 (6%) | 1 (3%) | 0.881 |
| Positive | 29 (91%) | 23 (72%) | 6 (19%) | |
| Venous vessels infiltration | ||||
| Negative | 5 (16%) | 2 (7%) | 3 (9%) | 0.057 |
| Positive | 27 (84%) | 23 (71%) | 4 (13%) | |
| Lymph node metastasis | ||||
| Negative | 7 (22%) | 3 (9%) | 4 (13%) | 0.026 |
| Positive | 25 (78%) | 22 (69%) | 3 (9%) | |
| Clinicopathological stage ‡ | ||||
| I + II + III | 10 (31%) | 7 (22%) | 3 (9%) | 0.885 |
| IV | 22 (69%) | 18 (56%) | 4 (13%) | |
P‐values less than 0.05 were considered to be statistically significant.
Evaluation of Wwox expression was described in the text.
Wwox restores Smad4 levels in PC cells. Inactivating mutations in components of the transforming growth factor beta (TGF‐β)–Smad signaling pathway results in resistance to the antiproliferative effects of TGF‐β in PC cells.( 35 , 36 , 37 ) To gain a better understanding of the role of Wwox on the TGF‐β–Smad signaling pathway, we investigated the association between Wwox and Smad4 expression. Loss of Smad4 expression tended to associate with low Wwox levels (Fig. 5a,b). In PANC‐1 cells, restoration of the Wwox expression elevated Smad4 protein levels 48 h after pWWOX transfection (Fig. 5c). No alteration was found at the messenger RNA (mRNA) levels of Smad4 expression (data not shown).
Figure 5.
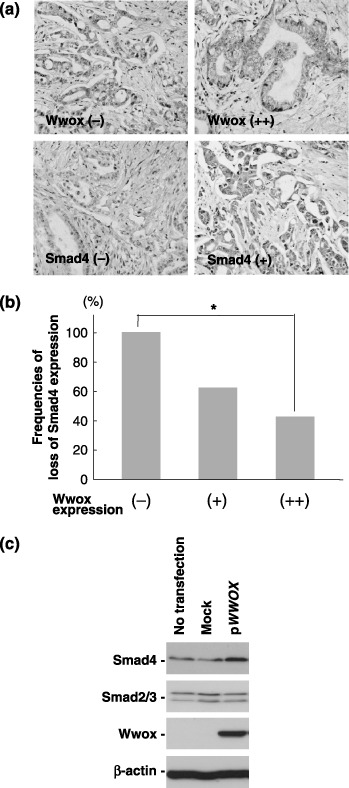
Association between WW‐domain‐containing oxidoreductase (Wwox) and mothers against decapentaplegic homolog 4 (Smad4) expression in pancreatic carcinoma (PC) cells. (a) Representative immunohistochemistry results for Wwox and Smad4. (b) Frequencies of loss of Smad4 expression in PC samples. (c) Wwox modulated Smad4 expression at the protein level in PANC‐1 cells. pWWOX was transfected into PANC‐1 cells and the cells were collected 48 after transfection.
Discussion
Carcinoma cell lines and primary tumors exhibit hemizygous or homozygous deletion with endpoints within fragile regions of the human genome, especially within the most active common fragile site, FRA3B, which is encompassed by the FHIT gene.( 11 ) As has been observed with the FHIT gene and other fragile‐site‐related genes, deletion at a certain locus and loss of protein expression has been shown to promote cell transformation and immortalization in various human malignancies; this is likewise the case with the WWOX (FRA16D) locus and Wwox expression.( 38 , 39 , 40 ) In this study, decreased Wwox expression has been detected in PC cells and tissues, as compared with that of normal pancreatic tissue. Since the WWOX gene is encompassed by FRA16D, and loss of Wwox expression was frequently detected in many human malignancies, we hypothesized that a reduction in Wwox expression may be closely associated with a deletion at this locus; LOH at D16S3029 was, however, infrequent.
Conversely, hypermethylation at WWOX regulatory sites was frequently detected, suggesting that epigenetic regulation of WWOX transcripts, particularly by hypermethylation at the exon 1 CpG island, may play an important role in pancreatic tumorigenesis.( 29 ) According to previous studies, hypermethylation at both the promoter and exon 1 CpG islands of the WWOX gene is a relatively common feature in lung and breast cancers,( 41 , 42 ) whereas hypermethylation at either of these sites does not appear to be associated with bladder cancer.( 17 ) In heterochromatin, most candidate CpG sites expand from the promoter region to the exon 1 region and the methylated CpG sites are bound by methyl‐cytosine‐binding protein complexes, which exclude transcription factor complexes.( 43 ) We assessed the importance of the exon 1 CpG island in the regulation of WWOX expression in PC and confirmed that demethylation at the WWOX regulatory sites resulted in restoration of Wwox protein expression in MIA PaCa‐2 cells (from undifferentiated human pancreatic carcinoma cell line; supplementary Fig. S1). Recently, post‐transcriptional regulation of Wwox protein levels has been documented: phosphorylation of Wwox at Y287 by Ack1, an activated Cdc42‐associated kinase, promotes Wwox degradation, consequently stimulating prostate tumorigenesis.( 44 ) Further investigation will be needed to clarify the mechanism by which Wwox expression levels may be down‐regulated.
Molecular genetic analyses have provided a convincing line of evidence that pancreatic duct lesions are the precursors to infiltrating PC. Almost all of the genetic alterations that have been identified in infiltrating PC have also been detected in these ductal lesions and, remarkably, the prevalence of these genetic alterations increases as the degree of cytological and architectural atypia in the duct lesions increases.( 32 ) Interestingly, in PanIN, reduced Wwox expression was detected in accord with the progression of morphological features. This finding suggests that suppression of Wwox expression may accelerate the accumulation of genetic changes in oncogenic (KRAS and HER‐2) and tumor suppressor proteins (CDKN2A, TP53 and SMAD4), consequently leading to the transformation of pancreatic duct lesions. Also, loss of Wwox expression was found to be correlated with the grade of PC malignancy, as determined by the incidence of lymph node metastasis and poorer outcome. These findings suggest the significance of reduced Wwox expression during pancreatic duct carcinogenesis and progression of PC.
Here we noted that restoration of WWOX expression induced caspase‐dependent apoptosis and suppressed cell growth in vitro and in vivo. The WWOX gene encodes a 46‐kDa protein that contains two WW domains (WW1 and WW2), which are involved in protein–protein interactions, and an SDR domain, which may be involved in sex‐steroid metabolism.( 45 ) Ludes‐Meyers et al.( 46 ) identified the specific proline‐rich ligand for Wwox as PPXY motif and demonstrated the N‐terminal WW1 domain is responsible for this interaction. Various transcription factors have been identified as Wwox‐interacting proteins: p73, the p53 family protein, at 482PPPPY488 motif,( 23 ) Ap‐2 γ at 56PPPYFPPPY64 motif,( 24 ) and Jun proto‐oncogene, at 67PPVY70 motif physically interact with Wwox,( 26 ) respectively, inhibiting nuclear transport and resulting in down‐regulation of their transcriptional activity. In this study, pWWOX transfection increased Smad4 expression at the protein level, but not at the mRNA level, suggesting the Wwox‐mediated post‐transcriptional regulation of Smad4 levels. The SMAD4 gene (also referred to DPC4, for deleted in pancreatic carcinoma locus 4) shares characteristics with typical tumor suppressor genes,( 47 ) about half of PC contain either homozygous deletions of the SMAD4 locus or inactivating mutations in one allele associated with LOH, and a resultant loss of Smad4 expression is frequently detected in PC.( 9 , 47 ) Indeed, in the context of TGF‐β signaling, Smad4 plays a central role in the nuclear transport of Smad2 and Smad3 proteins; Smad4 forms complexes with Smad2 and Smad3 after activation of TGF‐β receptor, which results in tumor suppression by the up‐regulation of the cyclin‐dependent kinase inhibitors p15 and p21, as well as the up‐regulation of other cell‐cycle and cell‐death regulators.( 48 ) Therefore, Wwox may exhibit such tumor suppressor effects through TGF‐β–Smad signaling by increasing Smad4 levels. Smad4 can be proteasomally degraded after polyubiquitination by Smurf1 and Smurf2 E3 ligase complexes, which possess the WW domain.( 49 , 50 ) Although these Smurfs cannot bind to Smad4 directly, the PPXY motif within Smad2 and Smad7 enable the formation of complexes with Smad4 and Smurfs, which subsequently leads to Smad4 degradation. Further investigation will be necessary to elucidate the mechanism by which Smad4 levels can be increased by restoration of the WWOX gene.
Supporting information
Fig. S1. Supplemental data 1. Hypermethylation‐mediated reduction of WW‐domain‐containing oxidoreductase (Wwox) expression in MIA PaCa‐2 cells. Human pancreatic carcinoma (PC)‐derived cell line MIA PaCa‐2 (American Type Culture Collection, Manassas, VA, USA) was routinely maintained in Roswell Park Memorial Institute media (RPMI)‐1640 supplemented with 10% fetal bovine serum. (A) The effect of 5‐aza‐2′‐deoxycytidine (5‐aza‐dC; Sigma, St. Louis, MO, USA), trichostatin A (TSA; Sigma), and a combination of the two drugs on the WWOX methylation status in MIA PaCa‐2 cells. For methylation‐specific polymerase chain reaction (MSP), genomic DNA was treated with sodium bisulfite (Qiagen, Hilden, Germany) and was analyzed by MSP using primer sets within CpG (cytosine and guanine separated by a phosphate) sites in the WWOX promoter and exon 1.( 12 ) Polymerase chain reaction (PCR) samples were resolved by electrophoresis on a 2% agarose gel. For treatment with 5‐aza‐dC and TSA, cells were seeded in a 100‐mm plate at a density of 1 × 106 cells. After 24 h, cells were treated with 5‐aza‐dC (5 µM) and/or TSA (1 µM). Genomic DNA and cell lysate were isolated 5 days after addition of 5‐aza‐dC and/or TSA treatments.( 22 ) M, methylated; U, unmethylated. (B) Restoration of Wwox protein by treatment with 5‐aza‐dC and TSA in MIA PaCa‐2 cells. Relative intensity of these bands are visualized.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
This work was supported by Grant‐in‐Aid for Scientific Research (C‐19590347) from the Japan Society for Promotion of Science, the Terry Fox Run Foundation for Cancer Research, Sidney Kimmel Foundation for Cancer Research grant, Ohio Cancer Research Associates grant, USPHS National Cancer Institute grant (CA120516), and the Charlotte Geyer Foundation.
References
- 1. Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–65. [DOI] [PubMed] [Google Scholar]
- 2. Brat DJ, Lillemoe KD, Yeo CJ, Warfield PB, Hruban RH. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol 1998; 22: 163–9. [DOI] [PubMed] [Google Scholar]
- 3. Wilentz RE, Iacobuzio‐Donahue CA, Argani P et al . Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2001; 60: 2000–6. [PubMed] [Google Scholar]
- 4. Moskaluk CA, Hruban RH, Kern SE. p16 and K‐ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res 1997; 57: 2140–3. [PubMed] [Google Scholar]
- 5. Day JD, DiGiuseppe JA, Yeo C et al . Immunohistochemical evaluation of HER‐2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol 1996; 27: 119–24. [DOI] [PubMed] [Google Scholar]
- 6. Wilentz RE, Geradts J, Maynard R et al . Inactivation of the p16 (INK4A) tumor‐suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res 1998; 58: 4740–4. [PubMed] [Google Scholar]
- 7. Goggins M, Schutte M, Lu J et al . Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res 1996; 56: 5360–4. [PubMed] [Google Scholar]
- 8. DiGiuseppe JA, Hruban RH, Goodman SN et al . Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol 1994; 101: 684–8. [DOI] [PubMed] [Google Scholar]
- 9. Wilentz RE, Su GH, Dai JL et al . Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J Pathol 2000; 156: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohta M, Inoue H, Cotticelli MG et al . The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma‐associated t (3;8) breakpoint, is abnormal in digestive tract cancers. Cell 1996: 84: 587–97. [DOI] [PubMed]
- 11. Huebner K, Croce CM. Cancer and the FRA3B/FHIT fragile locus: it's a HIT. Br J Cancer 2003; 88: 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishii H, Dumon KR, Vecchione A et al . Effect of adenoviral transduction of the fragile histidine triad gene into esophageal cancer cells. Cancer Res 2001; 61: 1578–84. [PubMed] [Google Scholar]
- 13. Semba S, Trapasso F, Fabbri M et al . Fhit modulation of the Akt–survivin pathway in lung cancer cells: Fhit‐tyrosine; 114 (Y114): is essential. Oncogene 2006; 25: 2860–72. [DOI] [PubMed] [Google Scholar]
- 14. Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene 2002; 21: 1832–40. [DOI] [PubMed] [Google Scholar]
- 15. Kuroki T, Trapasso F, Shiraishi T et al . Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res 2002; 62: 2258–60. [PubMed] [Google Scholar]
- 16. Paige AJ, Taylor KJ, Taylor C et al . WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA 2001; 98: 11417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iliopoulos D, Guler G, Han SY et al . Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene 2005; 24: 1625–33. [DOI] [PubMed] [Google Scholar]
- 18. Finnis M, Dayan S, Hobson L et al . Common chromosomal fragile site FRA16D mutation in cancer cells. Hum Mol Genet 2005; 14: 1341–9. [DOI] [PubMed] [Google Scholar]
- 19. Chang NS, Pratt N, Heath J et al . Hyaluronidase induction of a WW domain‐containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276: 3361–70. [DOI] [PubMed] [Google Scholar]
- 20. Bednarek AK, Keck‐Waggoner CL, Daniel RL et al . WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res 2001; 61: 8068–73. [PubMed] [Google Scholar]
- 21. Fabbri M, Iliopoulos D, Trapasso F et al . WWOX gene restoration prevents lung cancer growth in vitro and in vivo . Proc Natl Acad Sci USA 2005; 102: 15611–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin HR, Iliopoulos D, Semba S et al . A role for the WWOX gene in prostate cancer. Cancer Res 2006; 66: 6477–81. [DOI] [PubMed] [Google Scholar]
- 23. Aqeilan RI, Pekarsky Y, Herrero JJ et al . Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA 2004; 101: 4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP‐2γ transcription factor. Cancer Res 2004; 64: 8256–61. [DOI] [PubMed] [Google Scholar]
- 25. Aqeilan RI, Donati V, Palamarchuk A et al . WW domain‐containing proteins, WWOX and YAP, compete for interaction with ErbB‐4 and modulate its transcriptional function. Cancer Res 2005; 65: 6764–72. [DOI] [PubMed] [Google Scholar]
- 26. Gaudio E, Palamarchuk A, Palumbo T et al . Physical association with WWOX suppresses c‐Jun transcriptional activity. Cancer Res 2006; 66: 11 585–9. [DOI] [PubMed] [Google Scholar]
- 27. Aqeilan RI, Trapasso F, Hussain S et al . Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA 2007; 104: 3949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM. Inactivation of the Wwox gene accelerates forestomach tumor progression in vivo . Cancer Res 2007; 67: 5606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuroki T, Yendamuri S, Trapasso F et al . The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenensis. Clin Cancer Res 2004; 10: 2459–65. [DOI] [PubMed] [Google Scholar]
- 30. Japan Pancreas Society . Classification of Pancreatic Cancer, 5th edn. Tokyo: Kanehara Co. Ltd, 2002. [Google Scholar]
- 31. Sobin LH, Wittekind CH. UICC TNM Classification of Malignant Tumor, 5th edn. New York: John Wiley and Sons, Co. Ltd, 1997. [Google Scholar]
- 32. Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res 2000; 6: 2969–72. [PubMed] [Google Scholar]
- 33. Semba S, Itoh N, Ito M et al . Down‐regulation of PIK3CG, a catalytic subunit of phosphatidylinositol 3‐OH kinase, by CpG hypermethylation in human colorectal carcinoma. Clin Cancer Res 2003; 8: 3824–31. [PubMed] [Google Scholar]
- 34. Hasuo T, Semba S, Li D et al . Assessment of microsatellite instability status for the prediction of metachronous recurrence after initial endoscopic submucosal dissection for early gastric cancer. Br J Cancer 2007; 96: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riggins GJ, Kinzler KW, Vogelstein B, Thiagalingam S. Frequency of Smad gene mutations in human cancers. Cancer Res 1997; 57: 2578–80. [PubMed] [Google Scholar]
- 36. Jonson T, Gorunova L, Dawiskiba S et al . Molecular analyses of the 15q and 18q SMAD genes in pancreatic cancer. Genes Chromosomes Cancer 1999; 24: 62–71. [DOI] [PubMed] [Google Scholar]
- 37. Massaguè J, Blain SW, Lo RS. TGF‐β signaling in growth control, cancer, and heritable disorders. Cell 2000; 103: 295–309. [DOI] [PubMed] [Google Scholar]
- 38. Matsuyama A, Croce CM, Huebner K. Common fragile genes. Eur J Histochem 2004; 48: 29–36. [PubMed] [Google Scholar]
- 39. Iliopoulos D, Guler G, Han SY et al . Roles of FHIT and WWOX fragile genes in cancer. Cancer Lett 2006; 232: 27–36. [DOI] [PubMed] [Google Scholar]
- 40. Ramos D, Aldaz CM. WWOX, a chromosomal fragile site gene and its role in cancer. Adv Exp Med Biol 2006; 587: 149–59. [DOI] [PubMed] [Google Scholar]
- 41. Cantor JP, Iliopoulos D, Rao AS et al . Epigenetic modulation of endogenous tumor suppressor expression in lung cancer xenografts suppresses tumorigenicity. Int J Cancer 2007; 120: 24–31. [DOI] [PubMed] [Google Scholar]
- 42. Iliopoulos D, Fabbri M, Druck T, Qin HR, Han SY, Huebner K. Inhibition of breast cancer cell growth in vitro and in vivo. effect of restoration of Wwox expression. Clin Cancer Res 2007; 13: 268–74. [DOI] [PubMed] [Google Scholar]
- 43. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002; 3: 415–28. [DOI] [PubMed] [Google Scholar]
- 44. Mahajan NP, Whang YE, Mohler JL, Earp HS. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis. role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res 2005; 65: 10514–23. [DOI] [PubMed] [Google Scholar]
- 45. Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain‐containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res 2000; 60: 2140–5. [PubMed] [Google Scholar]
- 46. Ludes‐Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM. WWOX binds the specific proline‐rich ligand PPXY. identification of candidate interacting proteins. Oncogene 2004; 23: 5049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hahn SA, Schutte M, Hoque AT et al . DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996; 271: 350–3. [DOI] [PubMed] [Google Scholar]
- 48. Heldin CH, Miyazono K, Ten Dijke P. TGF‐β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–71. [DOI] [PubMed] [Google Scholar]
- 49. Morèn A, Imamura T, Miyazono K, Heldin CH, Moustakas A. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 2005; 280: 22115–23. [DOI] [PubMed] [Google Scholar]
- 50. Morèn A, Hellman U, Inada Y, Imamura T, Heldin CH, Moustakas A. Differential ubiquitination defines the functional status of the tumor suppressor Smad4. J Biol Chem 2003; 278: 33571–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Supplemental data 1. Hypermethylation‐mediated reduction of WW‐domain‐containing oxidoreductase (Wwox) expression in MIA PaCa‐2 cells. Human pancreatic carcinoma (PC)‐derived cell line MIA PaCa‐2 (American Type Culture Collection, Manassas, VA, USA) was routinely maintained in Roswell Park Memorial Institute media (RPMI)‐1640 supplemented with 10% fetal bovine serum. (A) The effect of 5‐aza‐2′‐deoxycytidine (5‐aza‐dC; Sigma, St. Louis, MO, USA), trichostatin A (TSA; Sigma), and a combination of the two drugs on the WWOX methylation status in MIA PaCa‐2 cells. For methylation‐specific polymerase chain reaction (MSP), genomic DNA was treated with sodium bisulfite (Qiagen, Hilden, Germany) and was analyzed by MSP using primer sets within CpG (cytosine and guanine separated by a phosphate) sites in the WWOX promoter and exon 1.( 12 ) Polymerase chain reaction (PCR) samples were resolved by electrophoresis on a 2% agarose gel. For treatment with 5‐aza‐dC and TSA, cells were seeded in a 100‐mm plate at a density of 1 × 106 cells. After 24 h, cells were treated with 5‐aza‐dC (5 µM) and/or TSA (1 µM). Genomic DNA and cell lysate were isolated 5 days after addition of 5‐aza‐dC and/or TSA treatments.( 22 ) M, methylated; U, unmethylated. (B) Restoration of Wwox protein by treatment with 5‐aza‐dC and TSA in MIA PaCa‐2 cells. Relative intensity of these bands are visualized.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


