Abstract
The aim of this study is to clarify the benefit of combination chemotherapy in gastric cancer based on a cell‐signal inhibitor and an anticancer drug. Two scirrhous gastric cancer cell lines and two non‐scirrhous gastric cancer cell lines were used. Five anticancer drugs (5‐fluorouracil [5FU], paclitaxel, oxaliplatin, irinotecan, and gemcitabine) and four cell‐signal inhibitors, mammalian target of rapamycin (mTOR) inhibitor, glycogen synthase kinase 3β, p38αβMAPK, and cyclin‐dependent kinase, were used. The proliferation of cancer cells was examined by MTT assay and in vivo study. The apoptosis of cancer cells and the expression of apoptosis‐related molecules were examined by flow cytometry, real‐time PCR, and immunostaining. mTOR inhibitors with 5FU showed a synergistic antiproliferative effect in scirrhous gastric cancer, whereas the other signal inhibitors showed no synergistic effect with any anticancer drugs. mTOR inhibitor decreased the IC50 of 5FU and increased the apoptosis rate in scirrhous gastric cancer cells, but not in non‐scirrhous gastric cancer cells. The pan‐caspase inhibitor, zVAD‐fmk, inhibits apoptosis induced in combination with 5FU and mTOR inhibitor. mTOR inhibitor decreased dihydropyrimidine dehydrogenase, thymidylatesynthase, and bcl‐2expression, and increased caspase‐3 and p21 expression of scirrhous gastric cancer cells, but did not affect those of non‐scirrhous gastric cancer cells. In an in vivo study, mTOR inhibitor significantly enhanced the therapeutic efficacy of S1, an analog of 5FU. These findings suggest that mTOR inhibitor interacts with 5FU in a synergistic manner in scirrhous gastric cancer cells by the activation of the apoptosis signal. Therefore, mTOR inhibitor is a promising therapeutic agent in combination with 5FU in scirrhous gastric cancer. (Cancer Sci 2009; 100: 2402–2410)
Gastric carcinoma remains one of the major causes of cancer deaths around the world.( 1 , 2 ) Most patients with advanced gastric cancer need chemotherapy. Among the chemotherapeutic agents for gastric cancer, a 5FU analog, S1, has recently become the first line chemotherapy for gastric cancer patients in Japan, while several new drugs, including the taxanes such as PTX, the third‐generation platinum derivative OXA, the topoisomerase‐I inhibitor SN38, and the pyrimidine analog GEM, have emerged. These agents provide a better prognosis for patients with advanced gastric cancer.( 3 , 4 , 5 , 6 ) Even so, the response rate remains low.
Cell‐signal inhibitors are also emerging as a potential therapy for cancers, because signal pathways play an important role in the progression of various types of carcinomas. The proliferation of cancer cells mainly involves the PI3K signal pathway and the MAPK signal. The PI3K/Akt signal and the MAPK are proposed to be a critical integrator of various signaling transduction systems.( 7 ) mTOR and GSK3β are located downstream of the PI3K/Akt signal. mTOR inhibitor has been reported to show the proliferation inhibitory effect against various kinds of tumor cells through the inhibition of Akt signaling.( 8 , 9 ) p38MAPK is a member of the MAPK signaling cascade. CDK, a group of protein kinases, were originally described as key regulators of the cell cycle.
Combination chemotherapy with an anticancer drug and a cell‐signal inhibitor might achieve a better response rate, thus exceeding the efficacy of single treatment. In gastric cancer there are only a few published reports of the combination effect of a cell‐signal inhibitor and an anticancer drug.( 10 , 11 ) In this study, we examined the combination effects of these signal inhibitors with anticancer drugs on the proliferation of gastric cancer cells.
Materials and Methods
Chemicals and anticancer drugs. We used five cell‐signal inhibitors and five anticancer drugs. As inhibitors of the cell‐signal pathway, mTOR inhibitors rapamycin (Sigma, St Louis, MO, USA) and rapamycin analog CCI‐779 (Wyeth Pharmaceuticals, Collegeville, PA, USA), GSK3β inhibitor AR‐A014418, p38αβMAPK inhibitor SB239063, and CDK inhibitor SU9516 (all Calbiochem, Darmstadt, Germany) were used. Anticancer drugs 5FU (Kyowa Hakko, Tokyo, Japan), PTX (Bristol‐Myers, Wallingford, CT), OXA (Yakult, Tokyo, Japan), irinotecan active metabolite SN38 (Yakult), and GEM (Eli Lilly, Kobe, Japan), were used. For the in vivo study, S1 (Taiho Pharmaceutical, Tokyo, Japan) a 5FU analog, was used. All reagents were formulated as recommended by their suppliers.
Cell culture and cell lines. The human gastric cancer cell lines OCUM‐2M,( 12 ) OCUM‐8,( 13 ) MKN‐45, and MKN‐74 were used in this study. OCUM‐2M and OCUM‐8 were derived from a scirrhous gastric adenocarcinoma, and MKN‐45 and MKN‐74 were derived from a non‐scirrhous gastric adenocarcinoma.
Cell growth assays. Cancer cells (1.0 × 104) were placed in each well of a 96‐well plate. The plates were incubated with or without the addition of a cell‐signal inhibitor, and with or without an anticancer drug. After incubation for 72 h, 20 mL MTT (Sigma) was added in each wells. The formazan product of MTT was measured as absorbance at 570 nm using a microtiter plate reader (PM2004; Wako, Osaka, Japan). The potential synergy between the anticancer drugs and mTOR inhibitors, rapamycin (20 nM or 100 nM) or CCI‐779 (5 nM or 50 nM), was evaluated using Drewinko’s fraction method.( 14 ) The synergistic, additive, or antagonistic interactions were determined when the value was less than the expected value, more than the expected value, but less than the drugs’ value, or more than the drugs’ value, respectively. The expected value of the combined effects (%) = the effects of the anti‐cancer drug/control × the effects of cell‐signal inhibitor/control × 100 was calculated.
Flow cytometry. Apoptosis was detected using flow cytometry by staining cells with annexin V‐FITC and propidium iodide (BD Pharmingen, San Diego, CA, USA) labeling. OCUM‐2M and OCUM‐8 cells were seeded at a density of 1.0 × 105 cells/mL in six 100‐mm plates. With or without the addition of 5FU at the concentration of IC50, and with or without rapamycin (20 nM) or CCI‐779 (5 nM), the plates were incubated for 72 h. We also examined the effect of zVAD‐fmk (Sigma), a pan‐caspase inhibitor, on the apoptosis rate of cancer cells. zVAD‐fmk (50 μM) was added 1 h before treatment, and the plates were incubated for 72 h. Using an apoptosis kit, cells were stained with annexin V‐FITC and propidium iodide according to the instructions of the manufacturers, and immediately analyzed by FACScan flow cytometry (Becton Dickinson, Mountain View, CA, USA). We evaluated the synergistic effect of apoptosis by the combination of mTOR inhibitor and 5FU in scirrhous gastric cancer. The survival rate was calculated as follows: survival rate (%) = 100 × (100 − apoptotic rate)/(100 − apoptotic rate of the control). The synergistic interactions were determined when the value was less than the expected value (Exp). The expected value (%) = (survival rate of the anticancer drug) × (survival rate of mTOR inhibitor) × 100 was calculated.
Quantitative real‐time RT‐PCR. We examined the expression at the mRNA level of genes, including TS, DPD, caspase‐3, bcl‐2, and p21, as previously reported.( 15 ) Quantitative real‐time RT‐PCR was done on the ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA) using the commercially available gene expression assay for TS, DPD, p21, bcl‐2 and caspase‐3 (Hs00426591, Hs00559278, Hs01121172, Hs00099010, and Hs00234387, respectively). The threshold cycle (C t) values were used to calculate the relative expression ratios between control and treated cells using the formula described by Pfaffl.( 16 ) Quantitative PCR reactions were done in triplicate.
Animal models. BALB/c nude mice (Clea Japan, Shizuoka, Japan) were used. All experiments with nude mice were carried out in accordance with guidelines for animal experiments of Osaka City University Medical School. Xenografts were established by injecting 1 × 107 OCUM‐2M cells into the flanks of mice at 4 weeks of age. Mean tumor volume was observed to be 100 mm3 at 6 days after inoculation. Accordingly, 3 mg/kg/day of rapamycin, or 20 mg/kg/day of CCI‐779, and/or 10 mg/kg/day of S1 was given for 5 days per week for 2 weeks, except in the control. Rapamycin and CCI‐779 were intraperitonealy injected, and S1 was given orally. Tumor volumes (V) were determined at each time point by measuring length (l) and width (w), then calculating the volume (V = lw 2/2). Medication‐defined groups were CMC (control group; n = 6), rapamycin (3 mg/kg/day; n = 6), S1 (10 mg/kg/day; n = 6), rapamycin combined with S1 (n = 6), CCI‐779 (20 mg/kg/day; n = 6), and CCI‐779 combined with S1 (n = 6). After mice were killed, the specimens were fixed in 10% formalin for paraffin sectioning. Sections were stained immunohistochemically using anticaspase3 antibody (Cell Signaling, Danvers, USA), anti‐p21 antibody (Dako, Glostrup, Denmark), and an in situ apoptosis detection kit (Takara, Shiga, Japan).
Statistical methods. Comparisons among datasets were made with the Kruskal–Wallis one‐way anova by ranks followed by Dunn’s multiple comparison test. Probability values of P < 0.05 were regarded as statistically significant. All statistical tests were two‐sided.
Results
Combination effect of signal inhibitors with anticancer drugs. We investigated the antiproliferative effect of signal inhibitors, including mTOR inhibitor, GSK3β inhibitor, p38αβMAPK inhibitor, and CDK inhibitor, in combination with an anticancer drug, including 5FU, PTX, OXA, SN38, or GEM (Table 1). A synergistic antiproliferative effect was found only for the combination of the mTOR inhibitors rapamycin and CCI‐779 with 5FU in scirrhous gastric cancer OCUM‐2M and OCUM‐8 cells. In contrast, GSK3β inhibitor, p38αβMAPK inhibitor, and CKD inhibitor showed no synergistic effect when combined with any anticancer drug. An additive effect was found for the combination of CDK inhibitor with 5FU, SN38, or GEM in both scirrhous gastric cancer cell lines. An antagonistic effect was found in the other combinations of signal inhibitors with anticancer drugs in OCUM‐2M or OCUM‐8 cells. We then investigated the effect of the combination of 5FU and mTOR inhibitor in detail.
Table 1.
Growth inhibitory effect of signal inhibitors in combination with anticancer drugs in two scirrhous gastric cancer cell lines
| Anticancer drug | OCUM‐2M | OCUM‐8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rapamycin (mTOR) | CCI‐779 (mTOR) | AR‐A014418 (GSK3β) | SB239063 (p38αβMAPK) | SU9516 (CDK) | Rapamycin (mTOR) | CCI‐779 (mTOR) | AR‐A014418 (GSK3β) | SB239063 (p38αβMAPK) | SU9516 (CDK) | |
| 5FU | Synergistic | Synergistic | – | – | Additive | Synergistic | Synergistic | – | – | Additive |
| PTX | – | – | – | – | – | Additive | Additive | – | – | Additive |
| OXA | Additive | – | Additive | Additive | – | – | – | – | – | – |
| SN38 | – | – | Additive | – | Additive | – | – | – | – | Additive |
| GEM | – | – | – | – | Additive | – | Additive | – | – | Additive |
–, antagonistic; 5FU, 5‐fluorouracil; CDK, cyclin‐dependent kinase; GEM, gemcitabine; GSK, glycogen synthase kinase; mTOR, mammalian target of rapamycin; OXA, oxaliplatin; PTX, paclitaxel; SN38, irinotecan.
Synergistic effects of mTOR inhibitor with 5FU in gastric cancer cell lines. The proliferation of OCUM‐2M and OCUM‐8 cells was significantly decreased by rapamycin and CCI‐779. 5FU was added to cancer cell cultures at the IC75 for each cell line. The proliferation effect for OCUM‐2M and OCUM‐8 cells with the combination of mTOR inhibitors with 5FU was lower than the expected additive effects (Fig. 1a,b), suggesting that the combination of rapamycin or CCI‐779 with 5FU shows a synergistic effect. In contrast, in MKN‐45 and MKN‐74 cells, the proliferation effect of the combination of mTOR inhibitor with 5FU was higher than the expected additive effect (Fig. 1c,d).
Figure 1.
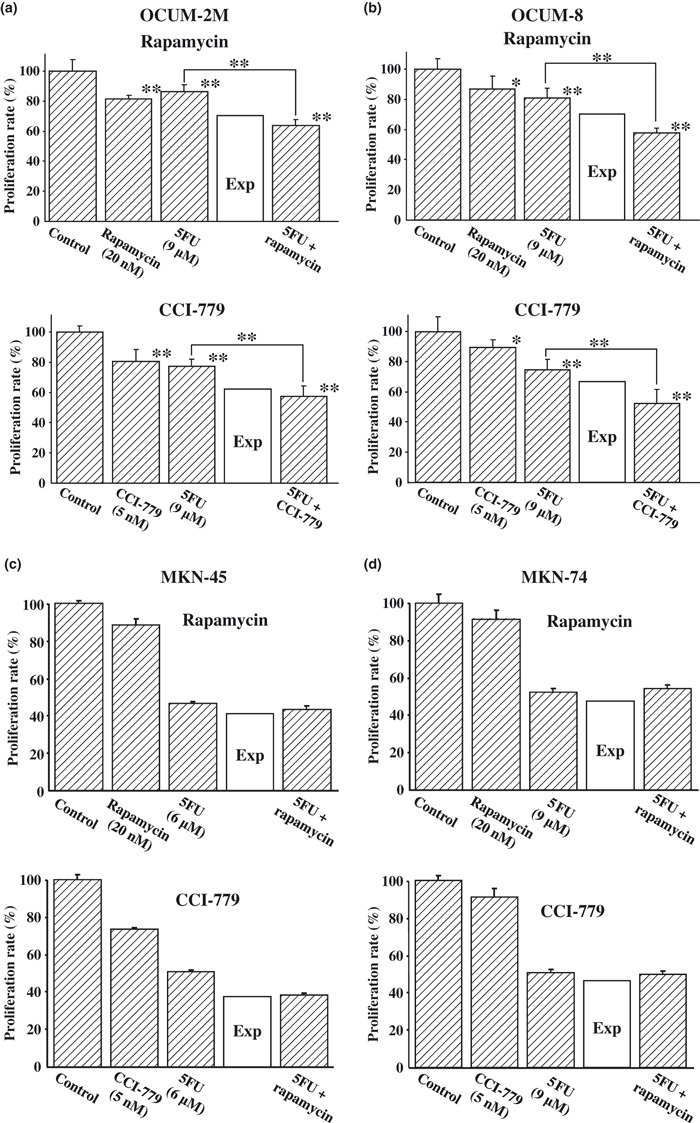
Synergistic effects of mammalian target of rapamycin (mTOR) inhibitor in combination with 5‐fluorouracil (5FU) by MTT assay. A synergistic antiproliferative effect in response to a combination of mTOR inhibitor with 5FU was observed in both OCUM‐2M (a) and OCUM‐8 (b) scirrhous gastric cancer cells, but not in MKN‐45 (c) or MKN‐74 (d) non‐scirrhous gastric cancer cells. The results are presented as the mean of three independent experiments, and the bars indicate the SD. *P < 0.05 and **P < 0.01 compared with each anticancer drug alone. Exp, an expected additive value.
mTOR inhibitor increased the efficiency of anticancer drug. Figure 2 shows the IC50 of 5FU with or without mTOR inhibitor. The IC50 of 5FU was decreased in combination with rapamycin or CCI‐779 in scirrhous gastric cancer cells, OCUM‐2M and OCUM‐8. In contrast, the IC50 of 5FU was increased by the combination with rapamycin in non‐scirrhous gastric cancer cells, MKN‐45 and MKN‐74.
Figure 2.
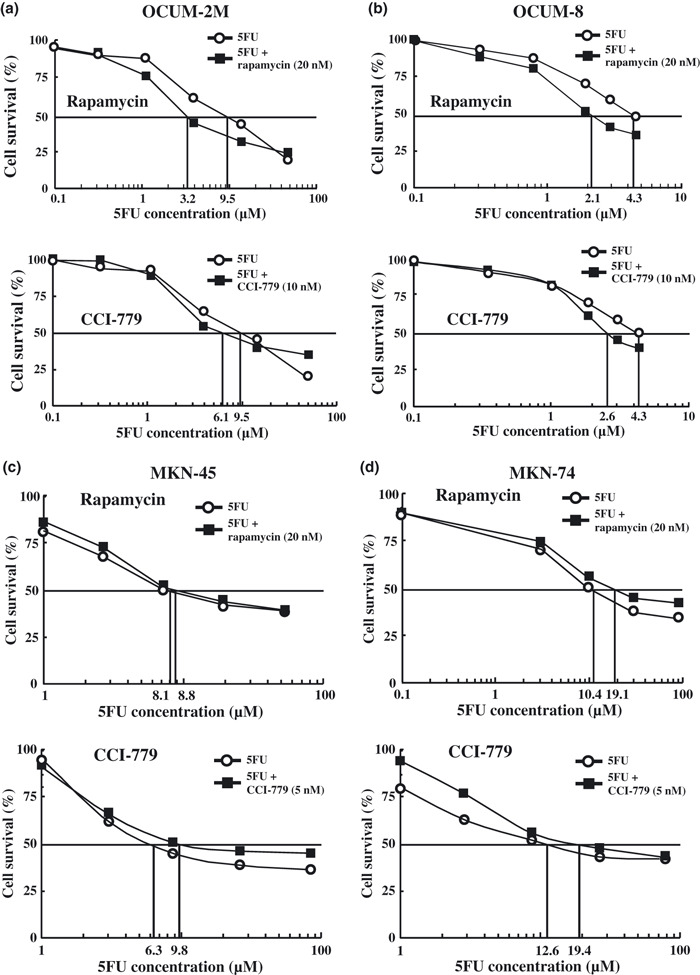
IC50 of 5‐fluorouracil (5FU) in combination with mammalian target of rapamycin (mTOR) inhibitor. The IC50 of 5FU was decreased when combined with rapamycin or CCI‐779 in OCUM‐2M (a) and OCUM‐8 (b) scirrhous gastric cancer cells. In contrast, the IC50 of 5FU was increased when combined with rapamycin or CCI‐779 in MKN‐45 (c) and MKN‐74 (d) non‐scirrhous gastric cancer cells.
mTOR inhibitor increased apoptosis induced by anticancer drug. Figure 3 shows the rates of apoptosis induced by the combined exposure of OCUM‐2M, OCUM‐8, MKN‐45, and MKN‐74 cells to mTOR inhibitor and/or 5FU in the absence or presence of the pan‐caspase inhibitor zVAD‐fmk. The latter was used to clarify the effect of caspase on apoptosis by mTOR inhibitors. The mTOR inhibitors increased apoptosis induced by 5FU in OCUM‐2M and OCUM‐8 cells, but not in MKN‐45 or MKN‐74 cells. Figure 3a shows flow cytometric analysis of OCUM‐2M cells. 5FU, rapamycin, and CCI‐779 increased the apoptosis rate of OCUM‐2M cells compared with the control. The combined exposure to the combination of 5FU with rapamycin or 5FU with CCI‐779 significantly (P < 0.05) increased the apoptosis rate of cancer cells, compared to the control and to those treated with 5FU alone and rapamycin alone. zVAD‐fmk (50 μM) decreased the rate of apoptosis induced in combination with 5FU and mTOR inhibitor in OCUM‐2M cells. No significant difference of apoptosis rate was found between the combinations of 5FU with mTOR inhibitor and 5FU alone in the zVAD‐fmk group. Figure 3b shows flow cytometric analysis of OCUM‐8 cells. 5FU, rapamycin, or CCI‐779 increased apoptosis compared with the control. In addition, the apoptosis rates induced by the combination of rapamycin or CCI‐779 with 5FU were significantly (P < 0.05) increased compared to the control, 5FU, and mTOR inhibitor alone. In contrast, no significant difference of apoptosis rate was found between the combinations of 5FU with mTOR inhibitor and 5FU alone in the zVAD‐fmk group. We evaluated the synergistic effect of apoptosis by combining mTOR inhibitor and 5FU in OCUM‐2M and OCUM‐8 cells (Fig. 3c). The survival rate for OCUM‐2M cells treated with 5FU, rapamycin, or CCI‐779 were 87%, 95%, and 96%, respectively. Survival rates after exposure to the combination of 5FU and rapamycin or 5FU and CCI‐779 were 73% and 78%, respectively. The survival rate for OCUM‐2M cells treated with the combination of mTOR inhibitors was lower than the expected value (82% and 83%, respectively), indicating that the combination of 5FU with rapamycin and CCI‐779 shows a synergistic effect. Survival rates of OCUM‐8 cells after treatment with 5FU, rapamycin, or CCI‐779 were 85%, 80%, and 87%, respectively. In response to the combination of 5FU and rapamycin or 5FU and CCI‐779, the survival rates were 67% and 72%, respectively. The survival rate for OCUM‐8 cells following treatment with mTOR inhibitors was lower than the expected value (68% and 73%, respectively), evaluating that the combination of 5FU with rapamycin or CCI‐779 shows a synergistic effect in OCUM‐8 cells. Figure 3d shows flow cytometric analysis of MKN‐45 and MKN‐74 cells. 5FU, rapamycin, and CCI‐779 increased the apoptosis rate of MKN‐45 and MKN‐74 cells compared with the control. The combined exposure to the combination of 5FU with rapamycin increased the apoptosis rate of cancer cells compared to those treated with 5FU alone, but not significantly.
Figure 3.
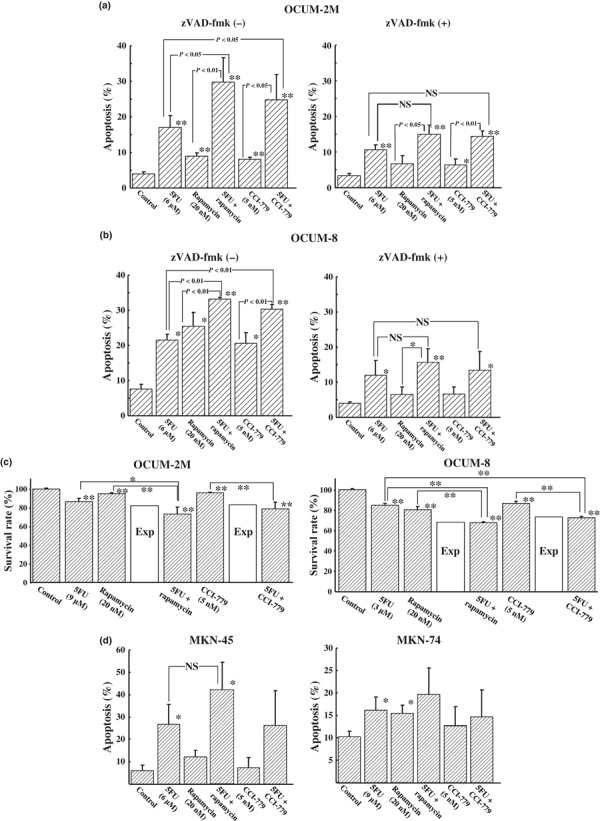
Effects of 5‐fluorouracil (5FU) and/or mammalian target of rapamycin (mTOR) inhibitor on apoptosis induction and gene expression. 5FU, rapamycin, and CCI‐779 increased the apoptosis rate of OCUM‐2M (a) and OCUM‐8 (b) scirrhous gastric cancer cells compared with the control. The combined exposure to the combination of 5FU with rapamycin or CCI‐779 significantly (P < 0.05) increased the apoptosis rate of cancer cells, compared to the control and to those treated with 5FU, rapamycin, or CCI‐779 alone. Pan‐caspase inhibitor zVAD‐fmk (50 μM) decreased the rate of apoptosis induced by combination with 5FU and mTOR inhibitor. No significant difference of apoptosis rate was found between the combinations of 5FU with mTOR inhibitor and 5FU alone in the zVAD‐fmk group. (c) The survival rate for OCUM‐2M and OCUM‐8 non‐scirrhous gastric cancer cells following exposure to the combination of mTOR inhibitors was lower than the expected value, evaluating that the combination of 5FU with rapamycin or CCI‐779 shows a synergistic effect in both cell lines. In MKN‐45 and MKN‐74 cells (d), the combination of 5FU with rapamycin or CCI‐779 did not a show significant increase in apoptosis, compared to 5FU alone. *P < 0.05 and **P < 0.01 versus control. Exp, an expected additive value; NS, not significant.
Effect of mTOR inhibitor on gene expression by real‐time PCR. mTOR inhibitors decreased the expression of DPD, TS, and bcl‐2 genes in scirrhous gastric cancer OCUM‐2M and OCUM‐8 cells compared to the control. Caspase‐3 mRNA expression was significantly increased by the combination of 5FU and mTOR inhibitor in OCUM‐2M and OCUM‐8 cells compared to the single treatment. In OCUM‐8 cells, the expression of p21 was significantly increased by the combination treatment compared to the single treatment. In contrast, mTOR inhibitor did not affect the expression of those genes in non‐scirrhous gastric cancer MKN‐45 and MKN‐74 cells, as shown by real‐time PCR (Fig. 4).
Figure 4.
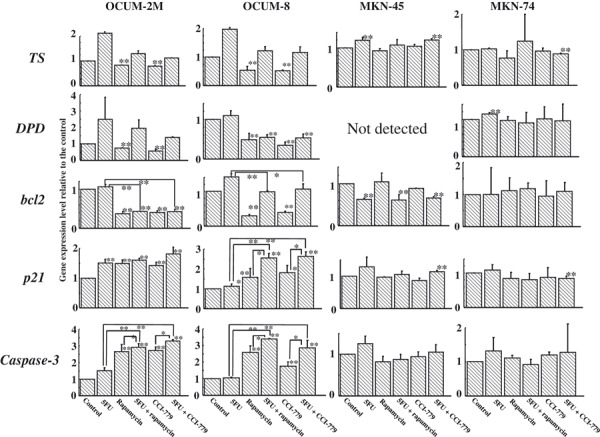
Effects of 5‐fluorouracil (5FU) and/or mammalian target of rapamycin (mTOR) inhibitor on gene expression. Dihydropyrimidine dehydrogenase (DPD), thymidylatesynthase (TS), and bcl‐2 mRNA expression were significantly decreased by mTOR inhibitor in OCUM‐2M and OCUM‐8 scirrhous gastric cancer cells. p21 and caspase‐3 mRNA expression levels were higher after exposure to 5FU and/or mTOR inhibitor than the control in OCUM‐2M and OCUM‐8 cells. In contrast, mTOR inhibitor did not affect gene expression levels in MKN‐45 or MKN‐74 non‐scirrhous gastric cancer cells. *P ≤ 0.05 and **P ≤ 0.01 versus control.
Effect of mTOR inhibitor on tumor development in vivo. The mean volumes of the subcutaneous tumor of the control, S1, rapamycin, S1 plus rapamycin, CCI‐779, and S1 plus CCI‐779 groups were 92.2, 56.4, 67.5, 25.0, 50.6, and 22.5 mm3, respectively. The size of tumors in mice receiving the combination of S1 with the mTOR inhibitors rapamycin or CCI‐779 was significantly smaller than in those receiving either S1, rapamycin, or CCI‐779 alone (Fig. 5a). The combination of mTOR inhibitors with S1 significantly (P < 0.05) decreased the number of mitosis positive cells. Rapamycin significantly (P < 0.01) increased the number of apoptotic cells, compared to the control. The combination of rapamycin with S1 significantly (P < 0.01) increased the number of apoptotic cells, compared to those receiving S1 alone. CCI‐779 increased the number of apoptotic cells. Taken together, S1 with CCI‐779 significantly (P < 0.05) increased the number of apoptotic cells compared to S1 alone (Fig. 5b). The immunohistochemical study showed that the expressions of caspase‐3 and p21 were enhanced in the S1 with mTOR inhibitor‐treated group compared to the control, 5FU, or mTOR inhibitor groups (Fig. 5c).
Figure 5.
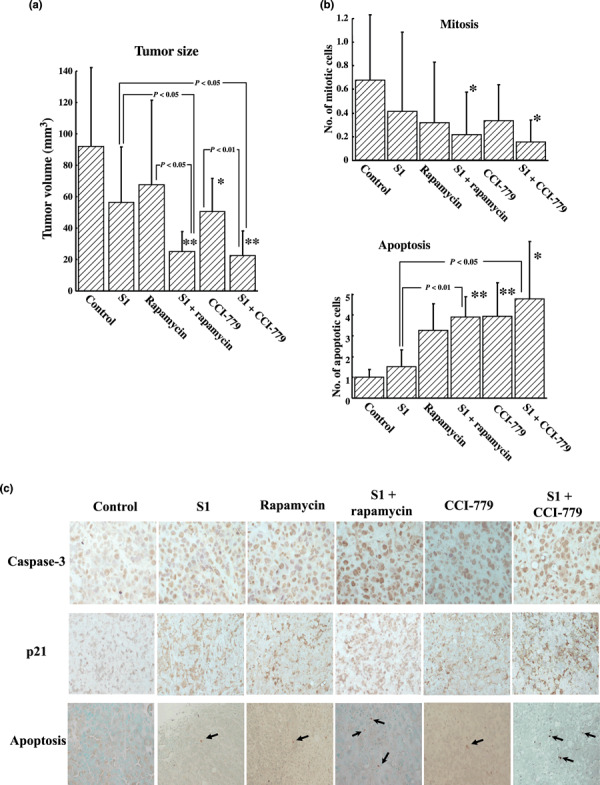
Effect of mammalian target of rapamycin (mTOR) inhibitor on the proliferation of xenografted tumor in vivo. (a) The tumor volume was significantly (P < 0.05) decreased by S1 plus rapamycin compared to the volume resulting from S1 or rapamycin treatment alone. In addition, the tumor volume was significantly decreased by S1 plus CCI‐779 compared to the volume resulting from S1 (P < 0.05) or CCI‐779 (P < 0.01) treatment alone. *P ≤ 0.05 and **P ≤ 0.01 vs control. (b) The number of mitotic or apoptotic cells in xenograft tumors. The number of mitotic cells was significantly (P < 0.05) decreased by the combination of S1 with rapamycin (0.21 ± 0.35) or CCI‐779 (0.15 ± 0.18) compared to the control (0.66 ± 0.54). The number of apoptotic cells was increased by rapamycin alone (3.22 ± 1.25) and by S1 with rapamycin (3.86 ± 0.96) compared to the control (0.98 ± 0.38). S1 with rapamycin significantly (P < 0.01) increased the number of apoptotic cells compared to S1 alone (1.48 ± 0. 8). S1 with CCI‐779 (4.7 ± 2.7) also significantly (P < 0.05) increased the number of lapoptotic cells compared to S1 alone. *P ≤ 0.05 and **P ≤ 0.01 vs control. (c) Expression of caspase‐3, p21 and apoptotic cells in xenograft tumors. S1 with mTOR inhibitor, rapamycin, or CCI‐779 enhanced the expression of caspase‐3, p21, and apoptotic cells in xenograft tumors compared to the control. Arrows indicate the apoptotic cells.
Discussion
Scirrhous gastric carcinoma, a diffusely infiltrating type of gastric carcinoma also known as linitis plastica‐type gastric carcinoma, carries a poor prognosis compared to other types of gastric carcinomas, with 5‐year survival rates in the range of 10–15%.( 17 ) Most patients with scirrhous gastric cancer are diagnosed at an advanced stage( 17 ) and treated with S1, (18) a 5FU analog. In this study, synergistic antiproliferative effects of the mTOR inhibitors, rapamycin and CCI‐779, were found in combination with 5FU in the scirrhous gastric cancer cell lines OCUM‐2M and OCUM‐8. We first investigated the antiproliferative effect of four types of signal inhibitors, mTOR inhibitor, GSK3β inhibitor, p38αβMAPK inhibitor, and CDK inhibitor, in combination with five anticancer drugs, 5FU, PTX, OXA, SN38, and GEM, in gastric cancer cell lines. The synergistic antiproliferative effects of mTOR inhibitors were observed only in combination with 5FU in scirrhous gastric cancer cell lines, but no synergistic effect of GSK3β inhibitor, p38αβMAPK inhibitor, or CDK inhibitor was found when combined with any anticancer drugs. Then we investigated the effect of mTOR inhibitor on the IC50 of 5FU in four gastric cancer cell lines, COUM‐2M, OCUM‐8, MKN‐45 and MKN‐74. We found that mTOR inhibitors decreased the IC50 of 5FU in scirrhous gastric cell lines, but not in the non‐scirrhous gastric cancer cell lines MKN‐45 and MKN‐74. Moreover, mouse xenografts of OCUM‐2M cells revealed that treatment with mTOR inhibitor significantly enhanced the therapeutic efficacy of S1, a 5FU analog. These findings suggest that the mTOR inhibitor is a promising chemotherapeutical agent in combination with the anticancer drug 5FU for patients with the scirrhous types of gastric cancer, and support further clinical evaluation of mTOR inhibitor in combination with 5FU.
A phase II clinical test of the mTOR inhibitor RAD001 is ongoing in gastric cancer. Kamata et al. ( 10 ) reported that the combination rapamycin with cisplatin increased the expression of Bax, a proapoptotic protein, and significantly suppressed the proliferation of gastric cancer. Cejka et al. ( 11 ) reported that the combination of mTOR inhibitor with cyclophosphamide resulted in synergistic antitumor activity against gastric cancer. In contrast, our study showed that an antagonistic effect was found for the combination of mTOR inhibitors with anticancer drugs other than 5FU in both scirrhous gastric cancer cell lines, OCUM‐2M and OCUM‐8. These findings suggested that anticancer drugs combined with mTOR inhibitor should be classified according to the histologic type of gastric cancer.
Rapamycin and its derivatives are generally regarded as having cytostatic effects because these drugs arrest cells in the G1 phase but do not generally induce apoptosis.( 19 ) In our study, the combination of mTOR inhibitor and 5FU significantly increased apoptotic cancer cells in comparison to mTOR inhibitor or 5FU alone in both scirrhous gastric cancer cell lines in vitro. Also, the combination of rapamycin with S1, a 5FU analog, significantly increased the number of apoptotic cells in xenografted tumors made up of OCUM‐2M cells compared to S1 alone. mTOR inhibitor might induce differential apoptosis responses when given as a single exposure vs in combination with 5FU.
To understand the implication of caspases in the apoptosis responses of combined 5FU and mTOR inhibitor, the effect of zVAD‐fmk, a pan‐caspase inhibitor, was investigated. The apoptosis rates were significantly high in the 5FU and mTOR inhibitor combination, compared to 5FU alone or mTOR alone. zVAD‐fmk inhibited apoptosis induced in combination with 5FU and mTOR inhibitor in both OCUM‐2M and OCUM‐8 cell lines. These results suggest that the apoptosis induced by the combination of 5FU and mTOR inhibitor might be related with caspase‐dependent pathways. Caspase‐3 mRNA expression was significantly increased by the combination treatment 5FU and mTOR inhibitor compared to the single treatment. We investigated the expression of caspase‐3 in protein level (Fig. S1). The immunohistochemical study also showed that the expressions of caspase‐3 were enhanced in the combination of S1 with mTOR inhibitor treatment group compared to the single treatment group. Caspase‐3 might be associated with apoptotic signals by the combination of 5FU and mTOR inhibitor.
p21 and bcl‐2 are also associated with apoptosis.( 20 , 21 ) mTOR inhibitors upregulated the expression of p21 mRNA, and downregulated the expression of bcl‐2 mRNA in scirrhous gastric cancer cell lines, compared with the control. Overall, the immunostaining study of p21 using xenografted tumors confirmed these mRNA results. The antiproliferative effects induced by mTOR inhibitor were reported to correlate with the arrest in the G1 phase of the cell cycle through the regulation of cellular p21 or p53 levels.( 22 , 23 ) These findings suggested that alteration of apoptosis genes, including p21 and bcl‐2 by mTOR inhibitor might be one of the reasons for the synergistic effects induced by the combination of 5FU and mTOR inhibitor. In OCUM‐8 cells, apoptosis stimulated by the single treatment of mTOR inhibitor is decreased by zVAD‐fmk. bcl2 mRNA expression was decreased more by the single treatment of mTOR inhibitor than by the combination treatment in OCUM‐8 cells. These results might suggest that the bcl2 signaling is associated with apoptosis induced by not only the combination treatment but also the single treatment of mTOR inhibitor in OCUM‐8 cells.
The suppression of TS and DPD expression by mTOR inhibitor might be one of the reasons for the synergistic effects induced by the combination of mTOR inhibitor and 5FU. Although 5FU and GEM are both pyrimidine analogs, the antitumor effect was different between the two when combined with mTOR inhibitor. DPD is the key enzyme in the 5FU catabolic pathway; 5FU is inactivated by DPD.( 24 ) mTOR inhibitor suppressed DPD expression that was synergistically decreased in combination with mTOR inhibitor and 5FU. 5FU inhibits DNA synthesis by the inhibition of TS activity.( 25 ) The TS expression level was decreased by mTOR inhibitor, and was synergistically decreased in combination with mTOR inhibitor and 5FU. In contrast, GEM is inactivated by deoxycytidine deaminase‐mediated conversion to difluorodeoxyuridine.( 6 ) GEM undergoes complex intracellular conversion to the nucleotides GEM diphosphate and triphosphate responsible for its cytotoxic actions on DNA synthesis.( 26 ) These differences of catabolic pathway between 5FU and GEM might be responsible for different antitumor effects between 5FU and GEM when combined with mTOR inhibitor.
In this study, a synergistic antiproliferative effect in response to a combination of mTOR inhibitors with 5FU was observed in scirrhous gastric cancer cells, but not in non‐scirrhous gastric cancer cells. The mTOR inhibitors decreased the expression levels of TS and DPD, key enzymes in the 5FU catabolic pathway in scirrhous gastric cancer cells, but not in non‐scirrhous gastric cancer cells. These results might explain one of the reasons for the different effects of mTOR inhibitors on scirrhous and non‐scirrhous gastric cancer.
In conclusion, our data suggest that mTOR inhibitor can increase the chemosensitivity of 5FU by apoptosis induction in scirrhous gastric cancer cells. The synergistic effect of mTOR inhibitor might be mediated by the regulation of TP, DPD, Caspase‐3, p21 and bcl‐2 expression. mTOR inhibitor is a promising chemotherapeutic agent, in combination with the anticancer drug 5FU, for patients with scirrhous gastric cancer.
Abbreviations
- 5FU
5‐fluorouracil
- CDK
cyclin‐dependent kinase
- DPD
dihydropyrimidine dehydrogenase
- GEM
gemcitabine
- GSK
glycogen synthase kinase
- mTOR
mammalian target of rapamycin
- OXA
oxaliplatin
- PI3K
phosphatidylinositol‐3‐OH kinase
- PTX
paclitaxel
- SN38
irinotecan
- TS
thymidylatesynthase
Supporting information
Fig. S1. Western blot analysis of caspase‐3. The expression of active‐type caspase‐3 (19 kDa) of OCUM‐2M scirrhous gastric cancer cells was increased by 5‐fluorouracil (5FU) alone, mammalian target of rapamycin (mTOR) inhibitor alone, or the combination of 5FU with rapamycin, compared to the control. Moreover, caspase‐3 expression was higher after exposure to the combination of 5FU and rapamycin than by 5FU or mTOR inhibitor alone.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
This study was partially funded by Grants‐in Aid for Scientific Research (18591475, 20591073, and 18390369) from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
References
- 1. Jemal A, Tiwari RC, Murray T et al. Cancer statistics, 2004. CA Cancer J Clin 2004; 54: 8–29. [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Correale P, Fulfaro F, Marsili S et al. Gemcitabine (GEM) plus oxaliplatin, folinic acid, and 5‐fluorouracil (FOLFOX‐4) in patients with advanced gastric cancer. Cancer Chemother Pharmacol 2005; 56: 563–8. [DOI] [PubMed] [Google Scholar]
- 4. Koutras AK, Gerolymos MK, Kontogeorgou E et al. Phase II study of irinotecan plus leucovorin and bolus 5‐fluorouracil as first‐ or second‐line chemotherapy in patients with advanced gastric or esophageal‐gastric junction adenocarcinoma. J Chemother 2007; 19: 724–30. [DOI] [PubMed] [Google Scholar]
- 5. Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol 2008; 43: 256–64. [DOI] [PubMed] [Google Scholar]
- 6. Hironaka S, Zenda S, Boku N, Fukutomi A, Yoshino T, Onozawa Y. Weekly paclitaxel as second‐line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 2006; 9: 14–8. [DOI] [PubMed] [Google Scholar]
- 7. Krasilnikov MA. Phosphatidylinositol‐3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry (Mosc) 2000; 65: 59–67. [PubMed] [Google Scholar]
- 8. Huang S, Shu L, Dilling MB et al. Sustained activation of the JNK cascade and rapamycin‐induced apoptosis are suppressed by p53/p21(Cip1). Mol Cell 2003; 11: 1491–501. [DOI] [PubMed] [Google Scholar]
- 9. Mondesire WH, Jian W, Zhang H et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy‐induced cytotoxicity in breast cancer cells. Clin Cancer Res 2004; 10: 7031–42. [DOI] [PubMed] [Google Scholar]
- 10. Kamata S, Kishimoto T, Kobayashi S, Miyazaki M, Ishikura H. Possible involvement of persistent activity of the mammalian target of rapamycin pathway in the cisplatin resistance of AFP‐producing gastric cancer cells. Cancer Biol Ther 2007; 6: 1036–43. [DOI] [PubMed] [Google Scholar]
- 11. Cejka D, Preusser M, Fuereder T et al. mTOR inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo . Anticancer Res 2008; 28: 3801–8. [PubMed] [Google Scholar]
- 12. Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M. Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer 1995; 72: 1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takemura S, Yashiro M, Sunami T, Tendo M, Hirakawa K. Novel models for human scirrhous gastric carcinoma in vivo . Cancer Sci 2004; 95: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drewinko B, Green C, Loo TL. Combination chemotherapy in vitro with cis‐dichlorodiammineplatinum(II). Cancer Treat Rep 1976; 60: 1619–25. [PubMed] [Google Scholar]
- 15. Zhang X, Yashiro M, Ren J, Hirakawa K. Histone deacetylase inhibitor, trichostatin A, increases the chemosensitivity of anticancer drugs in gastric cancer cell lines. Oncol Rep 2006; 16: 563–8. [PubMed] [Google Scholar]
- 16. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otsuji E, Kuriu Y, Okamoto K et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg 2004; 188: 327–32. [DOI] [PubMed] [Google Scholar]
- 18. Sakuramoto S, Sasako M, Yamaguchi T et al. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med 2007; 357: 1810–20. [DOI] [PubMed] [Google Scholar]
- 19. Mabuchi S, Altomare DA, Cheung M et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin‐induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res 2007; 13: 4261–70. [DOI] [PubMed] [Google Scholar]
- 20. Osaki M, Tatebe S, Goto A, Hayashi H, Oshimura M, Ito H. 5‐Fluorouracil (5‐FU) induced apoptosis in gastric cancer cell lines: role of the p53 gene. Apoptosis 1997; 2: 221–6. [DOI] [PubMed] [Google Scholar]
- 21. Lin HL, Liu TY, Wu CW, Chi CW. 2‐Methoxyestradiol‐induced caspase‐3 activation and apoptosis occurs through G(2)/M arrest dependent and independent pathways in gastric carcinoma cells. Cancer 2001; 92: 500–9. [DOI] [PubMed] [Google Scholar]
- 22. Huang S, Liu LN, Hosoi H, Dilling MB, Shikata T, Houghton PJ. p53/p21(CIP1) cooperate in enforcing rapamycin‐induced G(1) arrest and determine the cellular response to rapamycin. Cancer Res 2001; 61: 3373–81. [PubMed] [Google Scholar]
- 23. Law M, Forrester E, Chytil A et al. Rapamycin disrupts cyclin/cyclin‐dependent kinase/p21/proliferating cell nuclear antigen complexes and cyclin D1 reverses rapamycin action by stabilizing these complexes. Cancer Res 2006; 66: 1070–80. [DOI] [PubMed] [Google Scholar]
- 24. Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5‐fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res 1987; 47: 2203–6. [PubMed] [Google Scholar]
- 25. Spiegelman S, Sawyer R, Nayak R, Ritzi E, Stolfi R, Martin D. Improving the anti‐tumor activity of 5‐fluorouracil by increasing its incorporation into RNA via metabolic modulation. Proc Natl Acad Sci USA 1980; 77: 4966–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feliu J, Mel R, Borrega P et al. Phase II study of a fixed dose‐rate infusion of gemcitabine associated with uracil/tegafur in advanced carcinoma of the pancreas. Ann Oncol 2002; 13: 1756–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Western blot analysis of caspase‐3. The expression of active‐type caspase‐3 (19 kDa) of OCUM‐2M scirrhous gastric cancer cells was increased by 5‐fluorouracil (5FU) alone, mammalian target of rapamycin (mTOR) inhibitor alone, or the combination of 5FU with rapamycin, compared to the control. Moreover, caspase‐3 expression was higher after exposure to the combination of 5FU and rapamycin than by 5FU or mTOR inhibitor alone.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


