Abstract
Polo‐like kinase 1 (PLK1) is centrally involved in the regulation of mitosis in normal and malignant cells. It is known that inhibition of PLK1 expression in vitro and in vivo leads to mitotic arrest, induction of apoptosis and suppression of tumor growth. In the present study, expression of PLK1 was investigated in paraffin tissue of 135 cases of gastric adenocarcinoma and in 46 corresponding lymph node metastases by immunohistochemistry. Expression data were correlated with clinicopathological parameters and patient survival. Seventy‐three (54.1%) of 135 carcinomas showed an overexpression of PLK1 compared to normal gastric mucosa. Overexpression of PLK1 correlated positively with tumor stage, nodal status and diffuse growth pattern. PLK1 expression in the primary tumor did not differ from PLK1 expression in the corresponding lymph node metastases. PLK1 expression was a significant prognostic factor in univariate but not in multivariate survival analysis. As a conclusion, upregulated PLK1 expression in gastric cancer correlates with a malignant tumor phenotype and has impact on patient prognosis. These data underscore the importance of PLK1 in gastric carcinogenesis and present a translational link for functional data into potential clinical use by defining PLK1 as an attractive protein for novel targeted chemotherapeutic approaches in gastric cancer. (Cancer Sci 2006; 97: 271 –276)
Gastric cancer, although steadily declining in incidence over the last 20 years, still represents one of the major cancer types in the western world. In 2005, 21 860 new cases and 11 550 cancer‐related deaths were estimated for the USA.( 1 ) In Asian countries gastric cancer still represents one of the most important solid tumors with respect to both incidence and mortality.( 2 ) Five‐year survival, which is fairly high for patients with localized disease (58%), is dramatically reduced for those patients with tumor spread to regional lymph nodes (23%) or distant organ sites (3%). Unfortunately, at the time of diagnosis, 64% of all patients present with such regional or distant disease.( 1 )
Surgical removal of the stomach with or without accompanying adjuvant chemotherapy is still the only treatment that promises a complete cure in the early stages.( 3 ) For patients with advanced tumor stages, therapeutic options are limited due to relative ineffectiveness of chemotherapy and radiotherapy. In those patients, unstoppable disease progression usually leads to tumor‐related death within 1 year.( 3 )
Based on these epidemiological data it is obvious that there is an urgent need for novel molecular‐targeted therapeutic approaches in the treatment of gastric cancer. An old idea that has lately been revitalized owing to advances in molecular biology techniques is to focus on the inhibition of uncontrolled tumor‐cell proliferation in order to control disease progression. In this context, proteins involved in the regulation of mitosis are of interest.
Polo‐like kinase (PLK) is a serine/threonine kinase family whose first member Polo was described by Sunkel and Glover in Drosophila in 1988.( 4 ) Since then, homologs of this protein have been found in every species investigated.( 5 ) In humans, to date four homologs have been described, with PLK1 being the most intensively studied member of this kinase family.( 6 ) Functional data, accumulated mainly over the last 5 years, have proved a pivotal role for PLK1 in the regulation of mitosis in normal and malignant cells.( 7 , 8 , 9 ) Recently, several groups have shown that inhibition of PLK1 in vitro and in vivo leads to a dramatic inhibition of tumor growth.( 10 , 11 , 12 , 13 ) In addition, PLK1 overexpression has been observed in a broad variety of solid human tumors,( 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 ) further intensifying the interest in PLK1 as a target for novel chemotherapeutic approaches.
In the present study, we aimed to investigate the expression patterns of PLK1 in primary gastric adenocarcinomas and in corresponding lymph node metastases. Furthermore, we studied the linkage of PLK1 expression with clinicopathological data and patient survival in this tumor entity.
Materials and Methods
Patients
Between 1995 and 2002, a total of 217 consecutive patients who were diagnosed for gastric cancer at the Institute of Pathology, Charité University Hospital (Berlin, Germany) were identified. From these patients, only those with presurgically untreated primary gastric adenocarcinomas and without other known malignancies were included in this study.
Using these criteria, 135 cases (patients’ age: 25–93 years, median 63.4 years) were included to form the study cohort. Clinical follow‐up data were available for all patients in the study cohort. The median follow‐up time of patients still alive at the endpoint of analysis was 32.7 months. Eighty‐seven (64.4%) patients died during follow up. Median survival time in this group was 9.1 months. Histological diagnosis and tumor grade were established on standard hematoxylin and eosin‐stained sections according to the guidelines of the World Health Organization.( 24 ) The distribution of clinicopathological parameters in the study population is given in Table 1.
Table 1.
Overall expression of polo‐like kinase 1 (PLK1) in gastric carcinoma and the distribution of PLK1 expression in the study population stratified for selected tumor parameters
| Characteristic | All cases | PLK1 negative | PLK1 positive | P‐value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| All cases | 135 | 100 | 62 | 45.9 | 73 | 54.1 | |
| Age | 0.016 † | ||||||
| ≤65 years | 74 | 54.8 | 41 | 55.4 | 33 | 44.6 | |
| >65 years | 61 | 45.2 | 21 | 34.4 | 40 | 65.6 | |
| Tumor stage | 0.001 § | ||||||
| T1 | 8 | 5.9 | 4 | 50 | 4 | 50 | |
| T2 | 64 | 47.4 | 40 | 62.5 | 24 | 37.5 | |
| T3 | 49 | 36.3 | 15 | 30.6 | 34 | 69.4 | |
| T4 | 14 | 10.4 | 3 | 21.4 | 11 | 78.6 | |
| Nodal status | 0.003 § | ||||||
| N0 | 31 | 23 | 19 | 61.3 | 12 | 38.7 | |
| N1 | 51 | 37.8 | 27 | 52.9 | 24 | 47.1 | |
| N2 | 37 | 27.4 | 12 | 32.4 | 25 | 67.6 | |
| N3 | 16 | 11.8 | 4 | 25 | 12 | 75 | |
| State of metastasis | 0.381 † | ||||||
| M0 | 122 | 90.4 | 58 | 47.5 | 64 | 52.5 | |
| M1 | 13 | 9.6 | 4 | 30.8 | 9 | 69.2 | |
| Grade | 0.111 § | ||||||
| G1 | 3 | 2.2 | 2 | 66.7 | 1 | 33.3 | |
| G2 | 36 | 26.7 | 20 | 55.6 | 16 | 44.4 | |
| G3 | 96 | 71.1 | 40 | 41.7 | 56 | 58.3 | |
| Laurén | 0.020 ‡ | ||||||
| Intestinal | 74 | 54.8 | 42 | 56.8 | 32 | 43.2 | |
| Mixed | 8 | 5.9 | 3 | 37.5 | 5 | 62.5 | |
| Diffuse | 53 | 39.3 | 17 | 32.1 | 36 | 67.9 | |
| Lymph vessel invasion | 0.165 † | ||||||
| L0 | 73 | 54.1 | 38 | 52.1 | 35 | 47.9 | |
| L1 | 62 | 45.9 | 24 | 38.7 | 38 | 61.3 | |
| Blood vessel invasion | 0.483 † | ||||||
| V0 | 114 | 84.4 | 54 | 47.4 | 60 | 52.6 | |
| V1 | 21 | 15.6 | 8 | 38.1 | 13 | 61.9 | |
Fisher's exact test;
χ2‐test;
χ2‐test for trends. In the first row, overall distribution of the respective tumor parameters in the study population is listed.
Antibody specificity
For western blot and immunohistochemical analysis a monoclonal mouse antibody directed against human PLK1 (BD Transduction, San Diego, CA, USA) was used. To ascertain antibody specificity a Polyhistidine (His)‐tagged human PLK1 protein (amino acids 1–623 of the protein) was expressed in a baculovirus system and purified by nickel affinity chromatography. Preincubation experiments showed that immunostaining for PLK1 could be blocked by the PLK1‐protein (data not shown). In addition, western blot experiments ascertained that the PLK1 antibody reacted with the purified PLK1 protein (Fig. 1).
Figure 1.
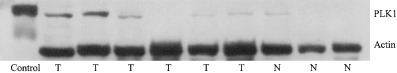
Expression of polo‐like kinase 1 (PLK1) in normal and neoplastic gastric tissue. Expression of PLK1 protein (68 kDa) in six cases of gastric carcinoma (T) and three cases of normal gastric mucosa (N) investigated by western blotting. Three carcinomas showed high PLK1 protein levels, whereas only one case of normal mucosa showed weak positivity for the protein. In the left lane, detection of control peptide by the antibody is depicted.
Immunoblotting
Tissue from gastric carcinomas and normal gastric mucosa was dissected by a senior pathologist in the operation room from surgical specimens sent for frozen section analysis and was frozen immediately in liquid nitrogen and stored at −80°C until analysis.
Protein samples (100 µg protein/sample) were loaded on a 10% polyacrylamide gel. Western blots were carried out as described previously( 25 ) using a mouse monoclonal anti‐PLK1 antibody (BD Transduction) diluted 1:500 and an anti‐β‐actin antibody (Chemicon, Temecula, CA, USA) diluted 1:3000.
Immunohistochemistry
Formalin‐fixed paraffin‐embedded tissue specimens were cut freshly (5 µm). The sections were mounted on superfrost slides (Menzel Gläser, Braunschweig, Germany), dewaxed with xylene and hydrated gradually. Antigen retrieval was achieved by pressure cooking in 0.01 M citrate buffer for 5 min. Slides were incubated with the primary PLK1 antibody diluted 1 : 500 in background reducing dilution buffer (Zymed, San Francisco, CA, USA) at room temperature for 1 h. Detection took place according to the manufacturer's instructions, using a streptavidin–biotin system (BioGenex, San Ramon, CA, USA) with alkaline phosphatase as the reporting enzyme. Fast‐Red (Sigma‐Aldrich, Munich, Germany) served as chromogen. Slides were then counterstained briefly with hematoxylin and mounted using Aquatex (Merck, Gernsheim, Germany).
Evaluation of tissue staining
All tissue slides were evaluated independently by two pathologists (WW and CD) who were blinded for patients’ characteristics and outcome. Whole‐tissue slides, each comprising a representative cross section of the tumor, were evaluated. To account for regional differences in staining a semiquantitative immunoreactivity scoring system (IRS) was applied. To obtain the IRS for each individual case, staining intensity (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining) as well as the percentage of cells stained (0 = no cells, 1 = <10% of cells, 2 = 11–50% of cells, 3 = 51–80% of cells, 4 = >80% of cells) were evaluated and the respective scores were multiplied resulting in an IRS range from 0 to 12. For statistical analysis, cases were grouped as either PLK1 negative (IRS 0–6) or PLK1 positive (IRS 7–12). Cases with discordant IRS scores were discussed at a multiheaded microscope until consensus was achieved.
Statistical analysis
Fisher's exact test and χ2‐test for trends were used to assess the statistical significance of associations between expression of PLK1 and clinicopathological parameters. To investigate correlations of PLK1 expression in primary tumors with PLK1 expression in lymph node metastases, Spearman's rank order correlation and Wilcoxon's test for non‐parametric paired samples were applied. Univariate survival analysis was carried out according to Kaplan–Meier, and differences in survival curves were assessed with the log rank test. Multivariate survival analysis was carried out using Cox's regression model. P‐values less than 0.05 were considered significant. For data compilation and statistical analysis the software package SPSS version 12.0 was used (SPSS Inc, Chicago, IL, USA).
Results
PLK1 expression in normal gastric mucosa
Moderate staining of PLK1 was observed in the cytoplasm of acid‐producing cells of normal gastric corpus mucosa (Fig. 2A). Foci of intestinal metaplasia of gastric mucosa, when present in the context of chronic gastritis, were PLK1‐positive as well (Fig. 2B). Ganglia of the autonomous neural system stained positive for PLK1 and served as an internal positive control.
Figure 2.
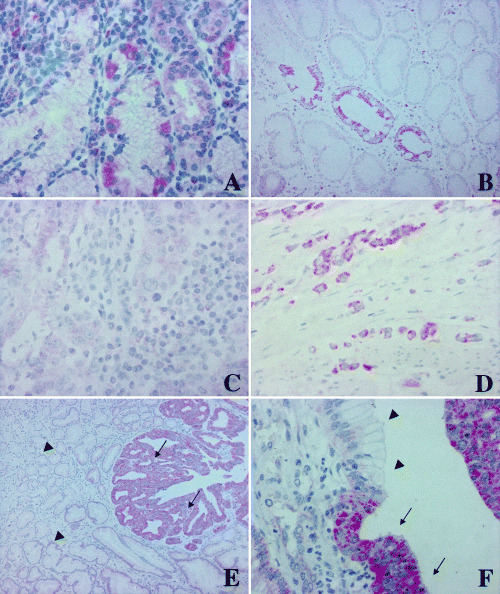
Expression of polo‐like kinase 1 (PLK1) in normal gastric mucosa and in gastric carcinoma. (A) Moderate PLK1 expression was observed in acid‐producing cells of the normal gastric mucosa. (B) Focus of intestinal metaplasia in the context of chronic gastritis. Note the strong PLK1 overexpression in the intestinal‐type cell lining in comparison to the adjacent gastric‐type cell lining. (C) Intestinal‐type gastric carcinoma negative for PLK1. (D) Diffuse‐type gastric carcinoma with overexpression of PLK1. (E,F) Gastric carcinomas adjacent to normal gastric mucosa. Note the strong overexpression in the neoplastic tissue (arrows) in comparison to the non‐transformed epithelial lining of the gastric glands (arrowheads).
In one out of three protein extracts from homogenized frozen normal gastric mucosa tissue very weak expression of PLK1 with a 68‐kDa band was detectable by western blot analysis (Fig. 1). This result is consistent with the small number of PLK1‐expressing cells observed in normal gastric mucosa.
PLK1 expression in gastric adenocarcinomas
In three out of six extracts from homogenized frozen gastric carcinoma tissue a strong 68‐kDa band representing PLK1 protein could be observed by western blot (Fig. 1). Two cases showed weak expression of PLK1, and one tumor had no detectable PLK1 band. In invasive gastric cancer we observed strong cytoplasmic PLK1 overexpression in 73 out of 135 cases (54.1%; Fig. 2C,D; Table 1) by immunohistochemistry.
Onset of overexpression in carcinoma tissue was usually abrupt, as noticed when comparing tumor tissue in the direct vicinity to normal non‐transformed mucosa (Fig. 2E,F). The staining observed was usually slightly granular and confined to the cytoplasm.
The IRS calculated comprised the whole range of possible IRS with a steady incline toward the high‐IRS end of the scale.
PLK1 expression in lymph node metastases
Thirty (65.2%) out of 46 cases of metastatic gastric carcinoma in regional lymph nodes were classified as PLK1 positive. Statistical analysis revealed no differences (Wilcoxon's test for paired samples, P = 0.895) of PLK1 IRS of the primary carcinomas (median: 8, quartiles: 4–12) when compared to the IRS of the lymph node metastases (median 8, quartiles: 5.5–9.75). Spearman's rank order correlation showed a high concordance (correlation coefficient r = 0.629, P < 0.001) of PLK1 positivity in primary tumors and metastases.
Correlation of PLK1 expression and clinicopathological parameters
PLK1 expression correlated positively with patient age (P = 0.016), tumor stage (P = 0.001) and nodal status (P = 0.003) (Table 1). A positive link was established between PLK1 expression and tumor growth pattern, classified according to Laurén. Non‐cohesively diffuse growing tumors with frequent signet ring cell formation were significantly more likely (P = 0.02) to express PLK1 than their conventional intestinal‐type gland‐forming counterparts.
Correlation of PLK1 expression with survival
In addition to the conventional prognostic factors, PLK1 expression had a highly significant impact on patient prognosis in univariate survival analysis (P = 0.003). Patients with PLK1 positivity in the primary tumor had a significantly shortened median survival time in comparison to patients whose tumors showed no PLK1 expression (11.3 months vs 22.3 months, respectively (Table 2, Fig. 3). In contrast, PLK1 expression in lymph node metastasis had no significant impact on prognosis (P = 0.14) in the study subgroup (n = 46) investigated for this parameter (data not shown).
Table 2.
Univariate survival analysis for the most important clinicopathological factors and for polo‐like kinase 1 (PLK1) expression
| Clinicopathological factor | No. cases | No. events | Median survival (months) | Standard error | Log‐rank test (P‐value) |
|---|---|---|---|---|---|
| PLK1 expression | 0.0026 | ||||
| Negative | 62 | 32 | 22.27 | 15.61 | |
| Positive | 73 | 55 | 11.30 | 2.68 | |
| Age at diagnosis | 0.0545 | ||||
| ≤65 years | 74 | 44 | 18.77 | 4.28 | |
| >65 years | 61 | 43 | 12.50 | 2.61 | |
| Tumor stage | 0.0001 | ||||
| T1 | 8 | 3 | Not reached | – | |
| T2 | 64 | 33 | 22.73 | 19.22 | |
| T3 | 49 | 39 | 11.30 | 1.71 | |
| T4 | 14 | 12 | 4.43 | 1.59 | |
| Nodal status | 0.0002 | ||||
| N0 | 31 | 16 | 36.07 | 17.25 | |
| N1 | 51 | 29 | 19.00 | 4.06 | |
| N2 | 37 | 29 | 11.77 | 2.47 | |
| N3 | 16 | 13 | 4.43 | 1.53 | |
| State of metastasis | 0.0868 | ||||
| M0 | 122 | 77 | 15.97 | 3.02 | |
| M1 | 13 | 10 | 6.73 | 5.63 | |
| Grade | 0.3043 | ||||
| G1 | 3 | 1 | Not reached | – | |
| G2 | 36 | 21 | 13.67 | 3.45 | |
| G3 | 96 | 65 | 15.47 | 1.81 |
Figure 3.
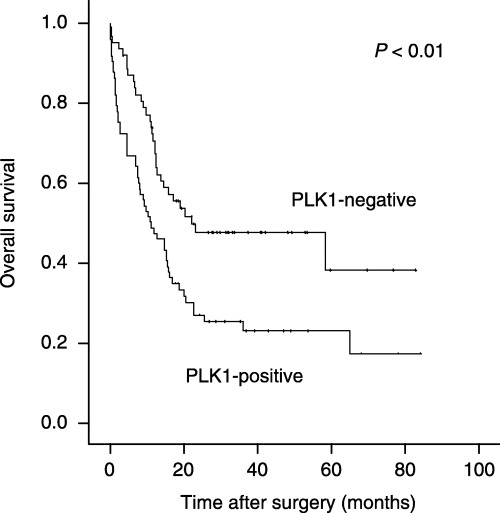
Kaplan–Meier survival curve of gastric carcinoma patients subgrouped for polo‐like kinase 1 (PLK1) expression. Overall survival by dependence of PLK1 expression in primary gastric carcinomas (n = 135). The Kaplan–Meier curve depicts significantly reduced survival for patients with PLK1‐positive tumors. The P‐value was calculated using the log rank test.
In multivariate survival analysis (Cox's regression model), under inclusion of patient age, lymph node status, tumor stage and PLK1 expression, only tumor stage (pT1/2 vs pT3/4, P = 0.007, Relative Risk (RR) = 1.88) and lymph node status (per positive node, P < 0.001, RR = 1.047) but not PLK1 expression (negative vs positive, P = 0.312) had independent prognostic significance. Comparable results were achieved when including the state of distant metastasis (M0 vs M1) in the analysis (data not shown). Therefore, in our study cohort, PLK1 expression status had no clear stage‐independent and metastasis‐independent prognostic value.
Discussion
Since the discovery of Polo over 25 years ago by Sunkel and Glover,( 4 ) a wealth of information has accumulated on the expression and function of human Polo homologs. PLK1, a central mitotic player, embodies the most relevant protein of this kinase family in humans. A plethora of functional studies confirmed a central role for PLK1 in mitotic entry,( 26 ) prophase,( 27 ) the metaphase/anaphase‐transition( 28 ) and cytokinesis.( 29 ) Based on these functional data it is not surprising that PLK1 mRNA downregulation in cell culture models results in mitotic arrest and subsequent strong induction of apoptosis,( 10 , 11 ) and that PLK1 inhibition in vivo leads to an inhibition of tumor growth.( 12 , 13 )
In the present study we found a strong overexpression of PLK1 in roughly half of gastric adenocarcinomas and we showed that PLK1 overexpression is especially prominent in locally advanced tumors with lymph node metastases and diffuse, non‐coherent growth patterns. Overexpression of PLK1 mRNA in gastric carcinomas has been reported in a preceding study by Tokumitsu and coworkers.( 30 ) In that study, a strong overexpression of PLK1 mRNA in gastric tumor tissue compared to adjacent gastric mucosa was evident in 73% of cases (n = 75). An interconnection between PLK1 mRNA overexpression and patient prognosis was not observed. In contrast, our results indicate that PLK1 protein expression levels had an impact on patient prognosis in univariate survival analysis. However, multivariate survival analysis revealed that PLK1 has no independent prognostic value in our study cohort, an observation that could most likely be attributed to the strong linkage of PLK1 expression and conventional independent prognostic factors such as tumor stage and lymph node status. Overexpression of PLK1 protein has been observed in a broad variety of malignant human tumors, amongst them tumors of the lung,( 14 ) breast,( 17 ) prostate,( 15 ) colon,( 18 ) head and neck,( 20 ) pancreas,( 19 ) thyroid,( 23 ) ovary,( 16 ) liver( 21 ) and skin.( 22 ) Whereas PLK1 overexpression was interlinked with reduced patient survival time in some tumor entities,( 14 , 16 , 20 ) in other entities such a statistical correlation could not be found.( 15 , 19 )
Another interesting observation in our study was the expression or overexpression of PLK1 in intestinal metaplasia of normal gastric mucosa. As intestinal metaplasia commonly precedes intestinal‐type gastric cancer,( 24 ) one may speculate that altered signal transduction pathways leading to an accumulation of PLK1 may be present in this precursor lesion, which in turn leads to an abnormality in growth behavior. Another possible explanation might be that increased proliferative capacity in intestinal metaplasia leads to an accumulation of PLK1 in enteric‐type cells. However, for both theories functional support is lacking at present.
The observed interlink between tumor growth pattern and PLK1 expression in our study is interesting given the fact that for intestinal‐type and diffuse‐type gastric cancer, certain differences in tumor behavior and genetic background have been proposed. Especially striking was the positivity of gastric carcinomas with non‐coherent, diffuse growth patterns. Loss of cell–cell adhesion in carcinomas overexpressing PLK1 may possibly be attributed to the proposed role of PLK isoenzymes in cytoskeletal reorganization.
In conclusion, we found the PLK1 was overexpressed in roughly half of all gastric carcinomas, with especially prominent overexpression in large, node‐positive, non‐cohesively growing tumors. In addition, PLK1 positivity had prognostic impact in univariate but not multivariate survival analysis in our study cohort. These data underscore the importance of PLK1 in gastric carcinogenesis and present a translational link for functional data into potential clinical use by defining PLK1 as an attractive protein for novel targeted chemotherapeutic approaches, especially for patients with advanced gastric carcinoma.
Acknowledgments
The excellent technical assistance of Ms Lisa Glanz is gratefully acknowledged. We thank Ms Martina Eickmann for editing and critical reading of the manuscript.
References
- 1. Jemal A, Murray T, Ward E et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55: 10–30. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi T, Kikuchi S, Lin Y et al. Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gastric Cancer 2004; 7: 233–9. [DOI] [PubMed] [Google Scholar]
- 3. Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 2003; 14: 31–6. [DOI] [PubMed] [Google Scholar]
- 4. Sunkel CE, Glover DM. Polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci 1988; 89: 25–38. [DOI] [PubMed] [Google Scholar]
- 5. Nigg EA. Polo‐like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol 1998; 10: 776–83. [DOI] [PubMed] [Google Scholar]
- 6. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 2002; 298: 1912–34. [DOI] [PubMed] [Google Scholar]
- 7. Barr FA, Sillje HH, Nigg EA. Polo‐like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 2004; 5: 429–40. [DOI] [PubMed] [Google Scholar]
- 8. Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo‐like kinases (Plks) and cancer. Oncogene 2005; 24: 287–91. [DOI] [PubMed] [Google Scholar]
- 9. Eckerdt F, Yuan J, Strebhardt K. Polo‐like kinases and oncogenesis. Oncogene 2005; 24: 267–76. [DOI] [PubMed] [Google Scholar]
- 10. Spankuch‐Schmitt B, Bereiter‐Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of polo‐like kinase‐1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst 2002; 94: 1863–77. [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Erikson RL. Polo‐like kinase (Plk) 1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA 2003; 100: 5789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan R, Tapang P, Leverson JD, Albert D, Giranda VL, Luo Y. Small interfering RNA‐mediated Polo‐like kinase 1 depletion preferentially reduces the survival of p53‐defective, oncogenic transformed cells and inhibits tumor growth in animals. Cancer Res 2005; 65: 2698–704. [DOI] [PubMed] [Google Scholar]
- 13. Nogawa M, Yuasa T, Kimura S et al. Intravesical administration of small interfering RNA targeting PLK‐1 successfully prevents the growth of bladder cancer. J Clin Invest 2005; 115: 978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolf G, Elez R, Doermer A et al. Prognostic significance of polo‐like kinase (PLK) expression in non‐small cell lung cancer. Oncogene 1997; 14: 543–9. [DOI] [PubMed] [Google Scholar]
- 15. Weichert W, Schmidt M, Gekeler V et al. Polo‐like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate 2004; 60: 240–5. [DOI] [PubMed] [Google Scholar]
- 16. Weichert W, Denkert C, Schmidt M et al. Polo like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer 2004; 90: 815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weichert W, Kristiansen G, Winzer KJ et al. Polo like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch 2005; 446: 442–50. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi T, Sano B, Nagata T et al. Polo‐like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci 2003; 94: 148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weichert W, Schmidt M, Jacob J et al. Overexpression of PLK1 is a common and early event in pancreatic cancer. Pancreatology 2005; 5: 259–65. [DOI] [PubMed] [Google Scholar]
- 20. Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo‐like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res 1999; 59: 2794–7. [PubMed] [Google Scholar]
- 21. Yamada S, Ohira M, Horie H et al. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor prognostic indicator of hepatoblastomas. Oncogene 2004; 23: 5901–11. [DOI] [PubMed] [Google Scholar]
- 22. Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo‐like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol 2002; 29: 354–8. [DOI] [PubMed] [Google Scholar]
- 23. Ito Y, Miyoshi E, Sasaki N et al. Polo‐like kinase 1 overexpression is an early event in the progression of papillary carcinoma. Br J Cancer 2004; 90: 414–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fenoglio‐Preiser C, Carneiro F, Correa P et al. Gastric carcinoma. In: Hamilton SR, Aaltonen A, eds. Tumours of the Digestive System, 1st edn. Lyon: IARC Press, 2000: 37–51. [Google Scholar]
- 25. Denkert C, Kobel M, Pest S et al. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am J Pathol 2002; 160: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toyoshima‐Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 2002; 3: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sumara I, Vorlaufer E, Stukenberg PT et al. The dissociation of cohesin from chromosomes in prophase is regulated by Polo‐like kinase. Mol Cell 2002; 9: 515–25. [DOI] [PubMed] [Google Scholar]
- 28. Golan A, Yudkovsky Y, Hershko A. The cyclin–ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1‐cyclin B and Plk. J Biol Chem 2002; 277: 15 552–7. [DOI] [PubMed] [Google Scholar]
- 29. Zhou T, Aumais JP, Liu X, Yu‐Lee LY, Erikson RL. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev Cell 2003; 5: 127–38. [DOI] [PubMed] [Google Scholar]
- 30. Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo‐like kinase expression in esophageal carcinoma. Int J Oncol 1999; 15: 687–92. [DOI] [PubMed] [Google Scholar]


