Abstract
The present study investigated the involvement of signaling of phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/protein kinase B (Akt) and transforming growth factor‐β (TGFβ) as well as receptor tyrosine kinases in the tumor promotion processes in a two‐stage hepatocarcinogenesis model using male F344 rats. The cellular localization of related molecules was examined in liver cell foci expressing glutathione S‐transferase placental form (GST‐P) at the early stage of tumor promotion by fenbendazole (FB), piperonyl butoxide, or thioacetamide. Distribution in the liver cell foci and neoplastic lesions positive for GST‐P was also examined at the later stage of FB promotion. In contrast to the initiation‐alone cases, subpopulations of GST‐P‐positive foci induced by promotion for 6 weeks, regardless of the promoting chemicals used, enhanced down‐regulation of PTEN and up‐regulation of phosphorylated (active) Akt2 and phosphorylated substrate(s) of Akt‐kinase activity. Also, up‐regulation of TGFβ receptor I and down‐regulation of epidermal growth factor receptor (EGFR) were enhanced in the subpopulation of GST‐P‐positive foci in all promoted cases. A similar pattern of cellular distribution of these molecules was also observed in the neoplastic lesions at the late stage. These results suggest a crosstalk between Akt2 and TGFβ signaling that involves a mechanism requiring EGFR down‐regulation during the entire tumor promotion process starting from the early stage. In particular, a shift in subcellular localization of phosphorylated substrate(s) of Akt from the cell membrane in liver cell foci to the cytoplasm in carcinomas was observed, suggesting an alteration of the function or activity of the corresponding molecule(s). (Cancer Sci 2009; 100: 813–820)
Abbreviations:
- Ab
antibody
- Akt
protein kinase B
- Bcl2
B‐cell CLL/lymphoma 2
- c‐erbB2
v‐erb‐b2 erythroblastic leukemia viral oncogene homolog 2
- DEN
N‐diethylnitrosamine
- EGFR
epidermal growth factor receptor
- FB
fenbendazole
- Grb10
growth factor receptor bound protein 10
- GST‐P
glutathione S‐transferase placental form
- IGF‐IRβ
insulin‐like growth factor‐I receptor β
- Mdm2
transformed mouse 3T3 cell double minute 2
- PB
phenobarbital
- PBO
piperonyl butoxide
- phospho‐Akt
phosphorylated (activated) form of Akt
- phospho‐Akt2
phosphorylated (active) Akt2
- phospho‐PTEN
phosphorylated form of PTEN
- PI3K
phosphatidylinositol 3‐kinase
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- Raf‐1
v‐raf‐leukemia viral oncogene 1
- RTK
receptor tyrosine kinase
- SDS‐PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TAA
thioacetamide
- TGF
transforming growth factor
- TGFβRI
transforming growth factor‐β receptor I
In a rat two‐stage hepatocarcinogenesis model, altered liver cell foci immunoreactive for glutathiuone S‐transferase placental form (GST‐P) have been shown to increase in number and area because of tumor promotion with hepatocarcinogens, in accordance with the hepatocarcinogenic potential; therefore, these foci have been confirmed as preneoplastic lesions of liver cells.( 1 , 2 ) However, only a few studies have examined the roles of cellular signaling and molecular linkage in the growth and development of the liver cell foci.( 3 , 4 , 5 , 6 )
Akt is a serine/threonine kinase that is a central regulator of widely divergent cellular processes, including proliferation, differentiation, migration, survival, and metabolism.( 7 ) Akt is activated by a variety of stimuli, through growth factor receptors, in a phosphatidylinositol 3‐kinase (PI3K)‐dependent manner, and it is also negatively regulated by the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN).( 8 ) A disruption of normal Akt/PTEN signaling frequently occurs in many human cancers, and thus these molecules play an important role in cancer development, progression, and therapeutic resistance.( 9 , 10 ) To date, three members of the Akt family have been identified in mammals: Akt1, Akt2, and Akt3.( 11 ) All three are ubiquitously expressed in all cell types and tissues, although Akt3 has a more restricted expression pattern. In rat hepatocarcinogenesis, there is a subpopulation of GST‐P‐positive liver cell foci exhibiting increased expression of PTEN and decreased expression of the phosphorylated (activated) form of Akt (phospho‐Akt), as well as high levels of ceramide species that are sensitive to sphingomyelin as a chemopreventive target.( 12 ) On the other hand, in a two‐stage hepatocarcinogenesis model, we recently obtained a contradictory result showing a lack of PTEN expression in a majority of GST‐P‐positive foci induced by promotion with phenob‐arbito (PB) or fenben‐dazole (FB), in contrast to the constitutive expression of PTEN in surrounding hepatocytes.( 13 ) This result suggests a decrease in the tumor suppressor function of PTEN by down‐regulation even during the early stage of hepatocarcinogenesis.
In the above‐mentioned study, we also found that transforming growth factor‐β receptor I (TGFβRI) was coexpressed with GST‐P in liver cell foci induced by promotion with PB or FB.( 13 ) Growth factor signaling through the activation of receptor tyrosine kinase (RTK) is crucial for regulation of PI3K/Akt signaling.( 14 ) Interestingly, TGFβ is able to activate PI3K/Akt in fetal hepatocytes by a mechanism dependent on activities of the erbB family RTK, epidermal growth factor receptor (EGFR), and c‐Src.( 15 )
Here, to clarify the involvement of Akt/PTEN signaling during the early stage of hepatocarcinogenesis, we further examined the cellular distribution of molecules related to PTEN/Akt signaling in preneoplastic lesions induced by 6 weeks of tumor promotion. For this purpose, three non‐genotoxic hepatocarcinogens, i.e. FB, piperonyl butoxide (PBO), and thioacetamide (TAA), were used as tumor‐promoting chemicals. We also examined immunolocalization of TGFβRI, as well as RTKs such as erbB family proteins, in these preneoplastic lesions. Moreover, immunolocalization of representative molecules were further examined in proliferative lesions developed after 57 weeks of promotion with FB to pursue involvement during the later stage of tumor promotion.
Materials and Methods
Chemicals. FB (CAS no. 43210‐67‐9) and N‐diethylnitrosamine (DEN) (CAS no. 55‐18‐5) were purchased from Sigma‐Aldrich Japan (Tokyo, Japan). PBO (CAS no. 51‐03‐6) was obtained from Nagase & Co. (Osaka, Japan), and TAA (CAS no. 62‐55‐5) was obtained from Wako Pure Chemicals Industries (Osaka, Japan).
Animal experiments. For analysis during the early stage of tumor promotion, 30 5‐week‐old male F344/NS1c rats were purchased from Japan SLC (Hamamatsu, Japan) and acclimatized on powdered basal diet (Oriental Yeast Co., Tokyo, Japan) and tap water ad libitum for 1 week. They were housed in stainless‐steel cages in a barrier‐maintained animal room on a 12‐h light–dark cycle and conditioned at 23 ± 2°C with a relative humidity of 55 ± 15%.
At 6 weeks of age, animals were subjected to two‐stage hepatocarcinogenesis using a medium‐term liver bioassay.( 1 , 2 ) All animals were initiated with a single intraperitoneal injection of DEN (200 mg/kg, dissolved in saline). Two weeks later, animals were divided into four groups and were fed basal diet (DEN‐alone) or diet containing either FB at 3600 p.p.m. (DEN + FB), PBO at 20 000 p.p.m. (DEN + PBO), or TAA at 400 p.p.m. (DEN + TAA). The animals were subjected to two‐thirds partial hepatectomy at week 3. At week 8, the animals were killed under deep ether anesthesia by exsanguinations from the abdominal aorta, and their livers were immediately removed and weighed. The liver slices were fixed in phosphate‐buffered 4% paraformaldehyde solution (pH 7.4) for 2 days and processed for histopathological examinations. Small portions of liver tissues, approximately 100 mg, were excised, quickly frozen in liquid nitrogen, and stored at –80°C. The selected doses of FB, PBO, and TAA have been reported to show tumor‐promoting activity or carcinogenic activity.( 16 , 17 , 18 )
To investigate the cellular distribution of molecules in neoplastic lesions during the late stage of tumor promotion, 35 F344/DuCrj rats (Charles River Japan, Yokohama, Japan) were promoted with FB at 3600 p.p.m. in the diet for 57 weeks.( 13 ) The livers of surviving animals were removed, and the liver slices containing gross neoplastic lesions were processed for histopathological examination as described previously.
The animal protocols were reviewed and approved by the Animal Care and Use Committee of the Tokyo University of Agriculture and Technology (short‐term study of 6‐week promotion) and the National Institute of Health Sciences, Japan (long‐term study of 57‐week promotion).
Immunohistochemistry. Paraffin‐embedded liver sections were subjected to immunohistochemistry using the horseradish peroxidase avidin–biotin complex method utilizing a VECTASTAIN Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) with 3,3′‐diaminobenzidine/H2O2 as the chromogen. Rabbit polyclonal Ab against GST‐P (1:1000; MBL, Nagoya, Japan) was used for all cases obtained at both 6 and 57 weeks of tumor promotion. In the livers after 6 weeks of promotion, serial sections were subjected to immunohistochemistry for EGFR (mouse monoclonal Ab, clone 6F1, 1:10; MBL), c‐erbB2 (mouse monoclonal Ab, clone SPM495, 1:50; Thermo Fisher Scientific, Fremont, CA, USA), TGFβRI (rabbit polyclonal Ab, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), insulin‐like growth factor‐I receptor β (IGF‐IRβ) (rabbit polyclonal Ab, 1:150; Cell Signaling Technology, Danvers, MA, USA), PTEN (rabbit monoclonal Ab, clone 138G6, 1:100; Cell Signaling Technology), phosphorylated form of PTEN (phospho‐PTEN; rabbit polyclonal Ab, 1:100; Cell Signaling Technology), Akt1 (mouse monoclonal Ab, clone 2H10, 1:50, Cell Signaling Technology), Akt2 (rabbit monoclonal Ab, clone 54G8, 1:150, Cell Signaling Technology), Akt3 (rabbit polyclonal Ab, 1:25, Cell Signaling Technology), phospho‐Akt at the position of Ser473 (rabbit monoclonal Ab, clone 193H12, 1:50; Cell Signaling Technology), phospho‐Akt2 (rabbit polyclonal Ab, 1:100; Abcam, Cambridge, UK), phospho‐(Ser/Thr) Akt substrate detecting phosphorylated Akt substrate peptides (rabbit polyclonal Ab, 1:250; Cell Signaling Technology), 14‐3‐3 (rabbit polyclonal Ab, 1:40; Abcam), growth factor receptor bound protein 10 (Grb10) (rabbit polyclonal Ab, A‐18, 1:20; Santa Cruz Biotechnology), Raf‐1 (rabbit polyclonal Ab, 1:50; Abcam) or Synip (rabbit monoclonal Ab, clone EP302Y, 1:250; Epitomics, Burlingame, CA, USA). Phospho‐PTEN is an inactive form of PTEN, and phospho‐Akt and phospho‐Akt2 are the active forms of Akt and Akt2, respectively. Because of the immunoreactivity of phospho‐Akt substrate(s) on the cell surface of GST‐P‐positive liver cell foci, 14‐3‐3, Grb10, Raf‐1, and Synip were selected as candidate substrates for Akt‐kinase activity that can localize to cellular membrane.( 19 , 20 , 21 , 22 , 23 , 24 , 25 )
For cases after 57 weeks of tumor promotion with FB, 10 slices that included tumor nodules were selected (one slice/animal), and serial sections were subjected to immunohistochemistry for Akt1, Akt2, Akt3, phospho‐Akt, phospho‐Akt2, phospho‐Akt substrate, 14‐3‐3, Grb10, TGFβRI, EGFR, c‐erbB2, and IGF‐IRβ.
For antigen retrieval, the sections were heated to 120°C in 10 mM citrate buffer (pH 6.0) by autoclaving for 10 min before incubation with each antibody.
Analysis of immunolocalization. The number and areas of GST‐P‐positive foci larger than 0.2 mm in diameter and the total areas of liver sections at week 6 of tumor promotion were measured using the WinROOF image analysis software package (version 5.7; Mitani Corp., Fukui, Japan), and then the number and areas of foci per unit area (cm2) of liver section were calculated. For evaluation of the expression of EGFR, TGFβRI, PTEN, phospho‐PTEN, phospho‐Akt2, 14‐3‐3, and Grb10, immunoreactivity was classified as increased, unchanged, or decreased in the GST‐P‐positive foci as compared with the expression levels of surrounding liver cells. For this evaluation, up to 50 GST‐P‐positive foci were randomly selected per animal. For the study of proliferative lesions generated after promotion with FB for 57 weeks, liver cell foci, adenomas and carcinomas in 10 animals showing immunoreactivity with GST‐P were examined by evaluation of immunoreactivity similarly to that of GST‐P‐positive foci at week 6 of promotion.
Expression analysis of polypeptide signals. Western blotting was performed with liver tissues at 6 weeks of tumor promotion (N = 2/group). With the same antibody used for immunohistochemistry, polypeptide signal levels of GST‐P, phospho‐Akt2, phospho‐Akt substrate, or TGFβRI were examined utilizing chemiluminescent reaction with ECL Plus Western Blotting Detection Reagents (GE Healthcare UK, Little Chalfont, UK). Tissue extraction and estimation of protein concentration were performed according to the methods described previously,( 26 ) and 10 µg of tissue extract was applied to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE). Resolved polypeptides were then transferred to a polyvinylidine difluoride membrane (Millipore, Billerica, MA, USA). Dilutions of applied Ab were 1:1000 for GST‐P, phospho‐Akt2, and phospho‐Akt substrate, and 1:200 for TGFβRI. Goat antirabbit IgG‐HRP (1:5000; Santa Cruz Biotechnology) was used as a secondary antibody. After signal detection of each molecule, membranes were reprobed with actin (1:200; Sigma, St. Louis, MO, USA) as a control for sample loading. Because GST‐P is known to be expressed, cell lysate from a rat hepatoma cell line, H‐4‐II‐E, was also included in the expression analysis.( 27 ) Signal level was estimated by measuring band intensity with the WinROOF image analysis software package.
Statistical analysis. Variance in data for the numbers and areas of GST‐P‐positive foci were checked for homogeneity using Bartlett's procedure. If the variance was homogenous, the data were assessed by one‐way anova. If not, the Kruskal–Wallis test was applied. When statistically significant differences were indicated, Dunnett's multiple comparison test was employed for comparison with the DEN‐alone group. Concordance ratios of the immunoreactive or immunonegative foci for molecules of interest with GST‐P‐positive foci were analyzed by χ2‐test for comparison between the DEN‐alone group and each treatment group.
Results
Immunolocalization during the early stage of tumor promotion. At week 6 of tumor promotion with FB or TAA, both the number and area of GST‐P‐positive liver cell foci were significantly increased compared with those in the DEN‐alone group (Table 1). Although statistically non‐significant, the number and area of GST‐P‐positive foci were also increased by promotion with PBO.
Table 1.
Quantitative data for GST‐P‐positive liver cell foci after promotion with FB, PBO, or TAA for 6 weeks
| Group | No. of animals examined | GST‐P‐positive foci | |
|---|---|---|---|
| Number (No./cm2) | Area (mm2/cm2) | ||
| DEN‐alone | 4 | 3.17 ± 1.05† | 0.38 ± 0.14 |
| DEN + FB 3600 p.p.m. | 8 | 28.35 ± 9.43* | 8.41 ± 4.46* |
| DEN + PBO 20 000 p.p.m. | 9 | 13.60 ± 7.11 | 2.30 ± 1.83 |
| DEN + TAA 400 p.p.m. | 9 | 58.34 ± 9.57** | 22.96 ± 5.39** |
Mean ± SD.
,
: P < 0.05, P < 0.01 versus DEN‐alone (Dunnett's test or Dunnett‐type rank‐sum test).
DEN, N‐diethylnitrosamine; FB, fenbendazole; GST‐P, glutathione S‐transferase placental form; PBO, piperonyl butoxide; TAA, thioacetamide.
Liver cells without GST‐P‐positive foci were diffusely immunoreactive with PTEN and its phosphorylated form in all groups, including the DEN‐alone group. With regard to immunolocalization in association with GST‐P‐positive foci, a small population of GST‐P‐positive foci showed decreased PTEN expression, forming PTEN‐negative foci, in the DEN‐alone group. On the other hand, GST‐P‐positive foci induced by promotion with FB, PBO, or TAA also formed PTEN‐negative foci with a significantly increased concordance ratio in GST‐P‐positive foci compared with the DEN‐alone group; FB promotion attained the highest ratio (Fig. 1a). Similarly, phosphorylated PTEN also showed significantly decreased expression in GST‐P‐positive foci in proportion with the magnitude of the promotion by each chemical compared with the DEN‐alone group (Fig. 1b).
Figure 1.
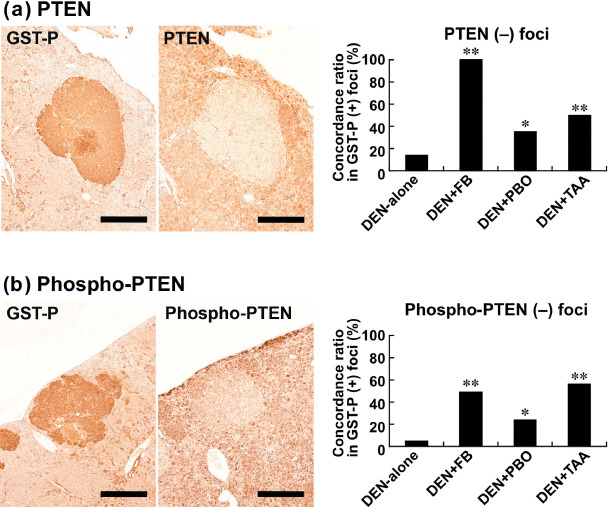
Immunohistochemical localization of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and phospho‐PTEN in association with glutathione S‐transferase placental form (GST‐P)‐positive liver cell foci after promotion for 6 weeks. (a) Lack of PTEN expression (right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 3600 p.p.m. fenbendazole (FB) after N‐diethylnitrosamine (DEN) initiation. Bar = 500 µm. The graph shows concordance ratios (%) of PTEN negativity with GST‐P‐positive foci generated after promotion with FB, piperonyl butoxide (PBO), or thioacetamide (TAA). *, **: P < 0.05, 0.01 versus DEN‐alone group (χ2‐test). (b) Lack of phospho‐PTEN expression (right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 3600 p.p.m. FB after DEN initiation. Bar = 500 µm. The graph shows concordance ratios (%) of phospho‐PTEN negativity with GST‐P‐positive foci generated after promotion with FB, PBO, or TAA. *, **: P < 0.05, 0.01 versus DEN‐alone group (χ2‐test).
Akt1, Akt2, and Akt3, as well as phospho‐Akt (Ser473), did not show specific immunolocalization in the liver in any group. On the other hand, as with weak and diffuse immunoreactivity in the liver cells not forming cellular foci, enhanced expression of phospho‐Akt2, forming phospho‐Akt2‐positive foci, was observed in a subpopulation of GST‐P‐positive foci in the livers of rats treated with DEN‐alone (Fig. 2a). Co‐expression with GST‐P‐positive foci was increased by promotion with FB, PBO, and TAA, with a statistically significant concordance ratio in GST‐P‐positive foci compared with the DEN‐alone group; FB promotion attained the highest ratio (Fig. 2a).
Figure 2.
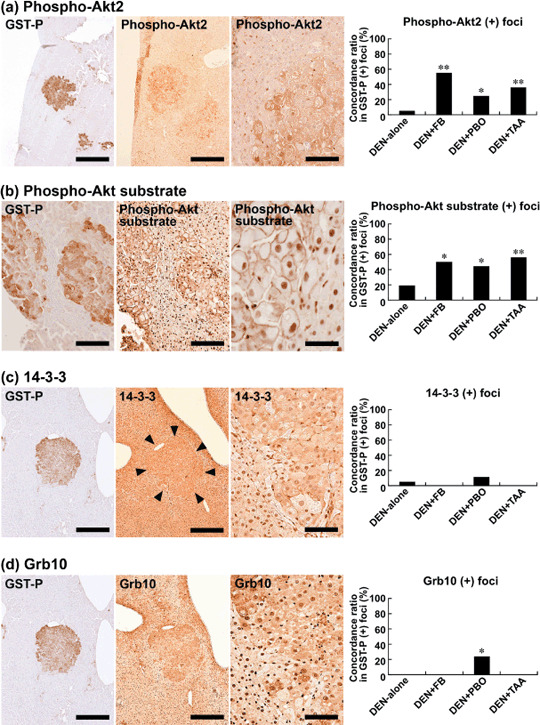
Immunohistochemical localization of phospho‐protein kinase B 2 (Akt2), phospho‐Akt substrate, 14‐3‐3, and Grb10 (growth factor receptor bound protein 10) in glutathione S‐transferase placental form (GST‐P)‐positive liver cell foci after promotion for 6 weeks. (a) Phospho‐Akt2 expression (middle and right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 3600 p.p.m. fenbendazole (FB) after N‐diethylnitrosamine (DEN) initiation. Note the mostly cytoplasmic expression with scattered nuclear immunoreactivity of phospho‐Akt2. Bar = 500 µm (left and middle), 100 µm (right). The graph shows concordance ratios (%) of phospho‐Akt2 immunoreactivity with GST‐P‐positive foci generated after promotion with FB, piperonyl butoxide (PBO), or thioacetamide (TAA). *, **: P < 0.05, 0.01 versus DEN‐alone group (χ2‐test). (b) Immunoreactivity against antiphospho‐Akt substrate antibody (middle and right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 400 p.p.m. TAA after DEN initiation. Note the predominant membrane immunoreactivity of phospho‐Akt substrate. Bar = 100 µm (left and middle), 50 µm (right). The graph shows concordance ratios (%) of phospho‐Akt substrate immunoreactivity with GST‐P‐positive foci generated after promotion with FB, PBO, or TAA. *, **: P < 0.05, 0.01 versus DEN‐alone group (χ2‐test). (c) 14‐3‐3 expression (middle and right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 20 000 p.p.m. PBO after DEN initiation. Margin of the focus expressing 14‐3‐3 is marked with arrowheads (middle). Note both of the nuclear and cytoplasmic immunoreactivities of 14‐3‐3. Bar = 500 µm (left and middle), 100 µm (right). The graph shows concordance ratios (%) of 14‐3‐3 immunoreactivity with GST‐P‐positive foci generated after promotion with FB, PBO, or TAA. (d) Grb10 expression (middle and right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 20 000 p.p.m. PBO after DEN initiation. The same focus expressing 14‐3‐3 as shown in panel (c) is presented. Note both of the nuclear and cytoplasmic immunoreactivities of Grb10. Bar = 500 µm (left and middle), 100 µm (right). The graph shows concordance ratios (%) of Grb10 immunoreactivity with GST‐P‐positive foci generated after promotion with FB, PBO, or TAA. *: P < 0.05 versus DEN‐alone group (χ2‐test).
Most foci positive for phospho‐Akt2 were negative for PTEN. Figure 3 shows concordance ratios of phospho‐Akt2‐immunoreactivity in PTEN‐negative foci in each group. All of the tumor‐promoted groups showed approximately 50% of concordance ratios. While DEN‐alone group showed slightly low concordance ratio (33%), there was no statistical significance between the DEN‐alone and tumor‐promoted groups.
Figure 3.
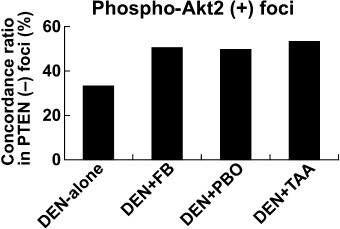
Concordance ratios of phospho‐protein kinase B 2 (Akt2) immunoreactivity with liver cell foci negative for phosphatase and tensin homolog deleted on chromosome 10 (PTEN) after promotion for 6 weeks with fenbendazole (FB), piperonyl butoxide (PBO), or thioacetamide (TAA).
Molecule(s) immunoreacted with antiphospho‐(Ser/Thr) Akt substrate antibody showed specific localization to the cellular membrane in accordance with GST‐P‐positive foci (Fig. 2b). The concordance ratio for GST‐P‐positive foci was significantly increased by promotion with FB, PBO, or TAA compared with that in the unpromoted cases (DEN‐alone), showing a similar magnitude among chemicals (Fig. 2b). Most foci positive for phospho‐Akt substrate were negative for PTEN. Nuclear immunoreactivity was also diffusely observed in liver cells without relation to GST‐P‐positive foci.
Among known Akt substrates, Raf1 and Synip did not show any specific immunolocalization in association with GST‐P‐positive foci. On the other hand, 14‐3‐3 was weakly positive in liver cells not forming altered foci, and enhanced expression was observed in the small population of GST‐P‐positive foci in the DEN‐alone and PBO‐promoted groups, showing a diffuse cytoplasmic expression pattern with scattered nuclear immunoreactivity. The concordance ratio with GST‐P‐positive foci between the two groups did not differ statistically (Fig. 2c). Grb10 also coexpressed in a small population of GST‐P‐positive foci only in the PBO‐promoted group, with a mainly cytoplasmic expression pattern and a statistically significant difference from the DEN‐alone group (Fig. 2d).
TGFβRI showed diffuse but weak immunoreactivity in the liver cells not forming cellular foci in all groups, including the DEN‐alone group. Populations of GST‐P‐positive foci in the livers of rats treated with DEN‐alone did not form TGFβRI‐positive foci (Fig. 4a). In contrast, coexpression of TGFβRI in GST‐P‐positive foci was apparent after promotion with FB, PBO, and TAA, with a statistically significant increase in the concordance ratio as compared with the DEN‐alone group. The highest concordance ratio was attained with FB promotion (Fig. 4a).
Figure 4.
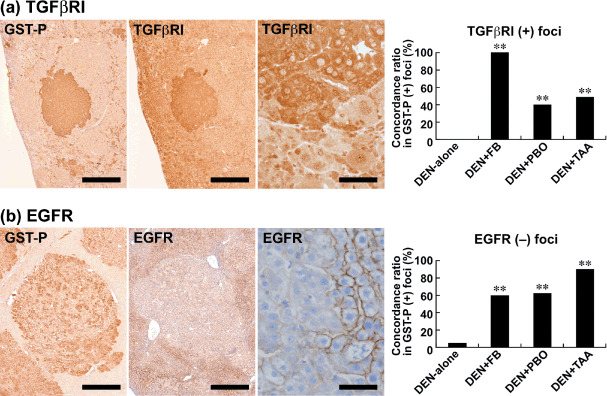
Immunohistochemical localization of transforming growth factor‐β receptor I (TGFβRI) and epidermal growth factor receptor (EGFR) in glutathione S‐transferase placental form (GST‐P)‐positive liver cell foci after promotion for 6 weeks. (a) TGFβRI expression (middle and right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 3600 p.p.m. fenbendazole (FB) after N‐diethylnitrosamine (DEN) initiation. Note the mostly cytoplasmic expression of TGFβRI. Bar = 500 µm (left and middle), 50 µm (right). The graph shows the concordance ratios (%) of TGFβRI immunoreactivity with GST‐P‐positive foci generated after promotion with FB, piperonyl butoxide (PBO), or thioacetamide (TAA). **: P < 0.01 versus DEN‐alone group (χ2‐test). (b) Lack of EGFR expression (middle and right) in a GST‐P‐positive focus (left) in the liver of a rat promoted with 3600 p.p.m. FB after DEN initiation. Bar = 500 µm (left and middle), 50 µm (right). The graph shows the concordance ratios (%) of EGFR negativity with GST‐P‐positive foci generated after promotion with FB, PBO, or TAA. **: P < 0.01 versus DEN‐alone group (χ2‐test).
Liver cells other than those consisting of GST‐P‐positive foci were diffusely immunoreactive with EGFR in all groups, including the DEN‐alone group. With regard to the immunoreactivity in GST‐P‐positive foci, a small population showed decreased EGFR expression, forming EGFR‐negative foci, in the DEN‐alone group. On the other hand, EGFR‐negative foci induced by promotion with FB, PBO, or TAA significantly increased the concordance ratio in GST‐P‐positive foci as compared with the DEN‐alone group; TAA promotion showed the highest concordance ratio (Fig. 4b). The receptor molecules c‐erbB2 and IGF‐IRβ did not show any specific expression pattern in association with GST‐P‐positive foci in any group.
Liver polypeptide signal levels during the early stage of tumor promotion. Although numbers of animals examined were two in each group, increased band intensity of polypeptide of GST‐P, phospho‐Akt2, and TGFβRI was observed in the liver after promotion with FB, PBO, or TAA, except for the reduction of TGFβRI signal with TAA‐promotion (Fig. 5). With regard to phospho‐Akt substrate, a single band migrating approximately 32 kDa could be detected in all groups including DEN‐alone group, and increase of this signal was evident in the liver by promotion with FB, PBO, or TAA.
Figure 5.
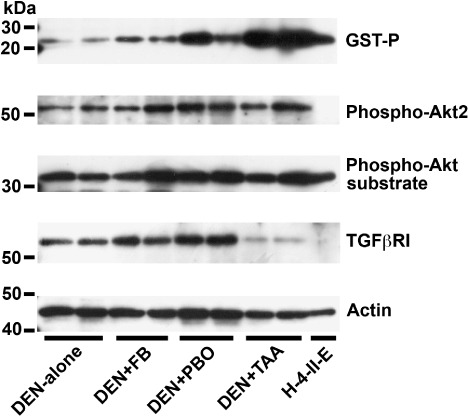
Polypeptide signal levels of glutathione S‐transferase placental form (GST‐P), phospho‐protein kinase B 2 (Akt2), phospho‐Akt substrate, and transforming growth factor‐β receptor I (TGFβRI) in the liver tissues after promotion for 6 weeks (N = 2/group). Blotted membranes were prepared for signal detection of each antigen and reprobed with actin as a control for sample loading. Cell lysate of a rat hepatoma cell line that is known to express GST‐P, H‐4‐II‐E, was also included in the analysis. As compared with N‐diethylnitrosamine (DEN)‐alone group, mean relative values of band intensity of GST‐P are 1.7 by promotion with fenbendazole (FB), 5.8 with piperonyl butoxide (PBO), and 13.8 with thioacetamide (TAA). With regard to phospho‐Akt2, relative values as compared with DEN‐alone group are 1.4 by promotion with FB, 2.0 with PBO, and 1.7 with TAA. With regard to phospho‐Akt substrate, a single band migrating approximately 32 kDa could be detected in all groups, and relative values to DEN‐alone group are 1.3 by promotion with FB, 1.5 with PBO, and 1.3 with TAA. Relative levels of TGFβRI signal as compared to DEN‐alone group are 1.5 by promotion with FB, 2.2 with PBO, and 0.2 with TAA. Relative levels of actin signal to the DEN‐alone group as represented by the samples examined for TGFβRI expression shown here are 0.8 by promotion with FB, 1.1 with PBO, and 0.7 with TAA.
Immunolocalization during the late stage of tumor promotion. Many adenomas and carcinomas were obtained after promotion by FB for 57 weeks.( 13 ) The adenomas often exhibited eosinophilic cytoplasm and solid growth, and the carcinomas frequently showed island and solid growth patterns. Foci observed at the late stage were mostly eosinophilic, but rarely, basophilic cell types were found.
All adenomas and carcinomas were positive for GST‐P, although a small subset of foci, such as the basophilic type, appeared negative. The numbers of liver cell foci, adenomas, and carcinomas showing GST‐P‐immunoreactivity and subjected to further immunohistochemical analyses were 61, 24, and 9, respectively.
Akt1, Akt2, and Akt3, as well as phospho‐Akt (Ser473), did not show specific immunolocalization in association with GST‐P‐positive lesions. On the other hand, localized expression of phospho‐Akt2 was observed in the liver cell foci and neoplastic lesions, and the concordance ratio of this immunolocalization with GST‐P immunoreactivity was increased in association with lesion development from foci to carcinomas (6, 7). All of the nine carcinomas examined showed positive immunolocalization of phospho‐Akt2.
Figure 6.
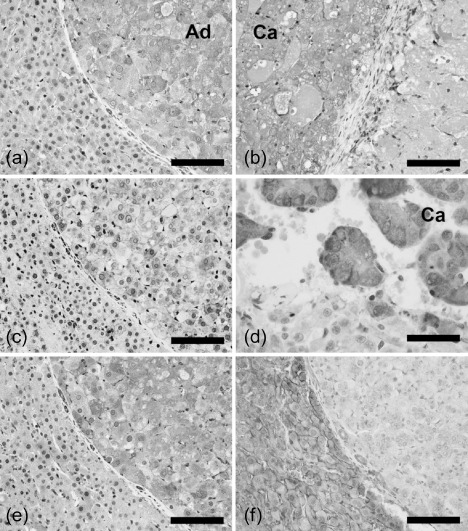
Immunolocalization of phospho‐protein kinase B 2 (Akt2), phospho‐Akt substrate, transforming growth factor‐β receptor I (TGFβRI), and epidermal growth factor receptor (EGFR) in the glutathione S‐transferase placental form‐positive proliferative lesions generated after promotion by fenbendazole for 57 weeks. (a,b) Selective expression of phospho‐Akt2 in an adenoma (a) and in a carcinoma (b) in contrast to surrounding non‐tumor tissue. (c,d) An adenoma showing both cytoplasmic and membrane immunoreactivities (c), and a carcinoma showing predominantly cytoplasmic immunoreactivity (d) with the antibody against phospho‐Akt substrate. (e,f) Positive immunoreactivity of TGFβRI (e) and negative reactivity of EGFR (f) in an adenoma. Immunoreactivity in the same portion of the identical adenoma is presented in panels (a), (c), (e), and (f). (a), (b), (c), (e), (f) Bar = 100 µm (d) Bar = 50 µm. Ad, adenoma; Ca, carcinoma.
Figure 7.
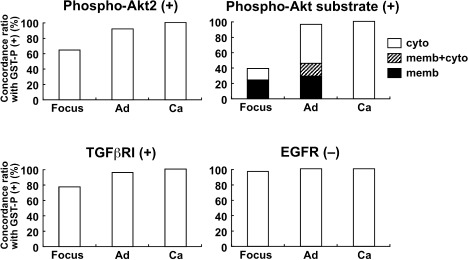
The concordance ratios (%) of the immunoreactive proliferative lesions for phospho‐protein kinase B 2 (Akt2), phospho‐Akt substrate, and transforming growth factor‐β receptor I (TGFβRI), and negative foci for epidermal growth factor receptor (EGFR) in the glutathione S‐transferase placental form (GST‐P)‐positive proliferative lesions produced after promotion by fenbendazole for 57 weeks. Ad, adenoma; Ca, carcinoma; cyto, cytoplasm; memb, membrane.
With regard to the distribution of molecule(s) immunoreacted with antiphospho‐(Ser/Thr) Akt substrate antibody, lesion‐specific immunoreactivity was increased in association with lesion development from foci to carcinomas, reflecting the concordance ratio with GST‐P immunoreactivity (6, 7). Regarding subcellular localization, liver cell foci showed immunolocalization either at the cell surface membrane or in the cytoplasm. Adenomas showed three types of localization, i.e. cell membrane alone, cytoplasm alone, and both membrane and cytoplasm. The ratio of adenomas showing only cytoplasmic expression was increased compared with hepatocellular foci. All carcinomas examined showed a cytoplasmic immunolocalization pattern. Weak nuclear immunoreactivity was also observed in the liver cells without showing specific immunolocalization to foci or neoplastic lesions.
With regard to TGFβRI immunoexpression, the concordance ratio with GST‐P immunoreactivity was rather high in all types of lesions (6, 7). Decreased EGFR expression was observed in almost all of the GST‐P‐positive proliferative lesions from foci to carcinomas (6, 7). Other RTKs, c‐erbB2, and IGF‐IRβ, as well as 14‐3‐3 and Grb10, did not show any specific expression pattern in association with proliferative lesions.
Discussion
Our previous study demonstrated down‐regulation of PTEN expression, including its inactive phosphorylated form, in a majority of altered liver cell foci generated by tumor promotion in the early stage.( 13 ) Here, we investigated the cellular localization of molecules involved in Akt signaling that are negatively regulated by PTEN in GST‐P‐positive preneoplastic lesions at the early stage of promotion. As a result, we found an increase in the ratios of GST‐P‐positive foci coexpressing phospho‐Akt2 and the undetermined downstream substrate molecule(s) of Akt‐kinase activity in the promoted cases. Further, most of phospho‐Akt2‐positive foci and phospho‐Akt substrate‐positive foci were found in subpopulations of PTEN‐negative foci. On the other hand, total phosphorylated and non‐phosphorylated forms of Akt1, Akt2, and Akt3, as well as phospho‐Akt did not show any specific localization in association with liver cell foci. These results suggest an enhanced activation of Akt2 in response to promotion stimuli in subpopulations of GST‐P‐positive foci to show lack of PTEN‐expression. GST‐P‐positive foci also showed up‐regulation of TGFβRI and down‐regulation of EGFR in response to tumor promotion with hepatocarcinogens. Although the possibility of chemical‐dependent response remains to be addressed, these results suggest a crosstalk between the TGFβ and PTEN/Akt2 signaling cascades, involving a mechanism to cause EGFR down‐regulation by promotion at the early stage. A similar pattern of cellular distribution of these molecules was observed in the neoplastic lesions at the late stage, suggesting these are essential molecular events in the entire process of tumor promotion.
The AKT2 gene product is strongly correlated with the regulation of glucose homoeostasis and is the predominant Akt isoform expressed in insulin‐responsive tissues.( 28 ) Akt2 has also been implicated in human malignancies, and recently, Akt2 has been shown to regulate cancer cell survival, migration, and invasion, acting as a transcriptional regulatory target of the basic helix‐loop‐helix transcriptional factor Twist.( 29 ) In the present study, among Akt isoforms, we could only detect increased coexpression of the activated form, phospho‐Akt2, in subpopulations of GST‐P‐positive foci induced by tumor promotion. Increase in the polypeptide level of phospho‐Akt2 was also observed in the liver of tumor‐promoted cases. The following phenomena may underlie the mechanisms for clonal expansion of GST‐P‐positive cells: lack of a p53 response to DNA damage because of transactivation of Mdm2 oncogene,( 4 , 5 ) down‐regulation of apoptosis,( 6 , 30 ) and up‐regulation of cell proliferation.( 31 ) Although the direct evidence for the functional relevance of active Akt2 in GST‐P‐positive foci needs to be clarified, Akt, once phosphorylated and activated, is known to relocalize to several subcellular locations where it phosphorylates proteins, such as Forkhead transcription factors, glycogen synthesis kinase‐3, Bcl2‐antagonist of cell death, and Mdm2 – some of which contribute to antiapoptotic signaling.( 32 )
In this study, we also found membrane localization of molecule(s) immunoreactive with antiphospho‐(Ser/Thr) Akt substrate antibody in the GST‐P‐positive preneoplastic lesions at 6 weeks of tumor promotion. To identify the corresponding molecule, we first examined the cellular localization of four candidates of Akt substrates that were selected because of reported cell surface membrane localization.( 19 , 20 , 21 , 22 , 23 , 24 , 25 ) As a result, 14‐3‐3 and Grb10 showed increased cytoplasmic expression in a subpopulation of GST‐P‐positive foci induced only by promotion with PBO. We next examined polypeptide signal levels immunoreacted with the antiphospho‐(Ser/Thr) Akt substrate antibody in the liver of tumor promoted cases. As a result, a single polypeptide signal, migrating approximately at 32 kDa, was detected in all groups, and enhanced expression was observed after tumor promotion. Interestingly, the pattern of immunolocalization changed from the membrane to the cytoplasm in relation with lesion development. All carcinomas showed cytoplasmic localization, suggesting an alteration of the function or activity of the corresponding molecule(s).
ΤGFβ exerts a growth‐inhibitory effect on epithelial cells, which explains its role as a tumor suppressor that cancer cells must elude for malignant evolution.( 33 ) Yet, paradoxically, TGFβ also modulates processes such as cell invasion, immune regulation, and microenvironment modification that cancer cells may exploit to their advantage.( 34 ) In our previous study, we found that TGFβRI was selectively expressed in proliferative lesions from early preneoplastic liver cell foci induced by promotion with PB or FB, whereas TGFβRII showed less selective expression in relation with proliferative lesions.( 13 ) In the present study, we also found TGFβRI expression in subpopulations of GST‐P‐positive foci generated after promotion with PBO or TAA as well as FB, whereas no apparent immunoreactivity was found with GST‐P‐positive foci induced without any promotion. In parallel with the immunohistochemical results, polypeptide signal of TGFβRI was increased in the liver after promotion with FB or PBO. TAA‐promoted cases, on the other hand, decreased the polypeptide signal, in contrast to the increased expression in the preneoplastic liver cell foci. In the immunohistochemical analysis of focal strong expression as in the present study, weak constitutive expression is usually excluded from the evaluation of immunohistochemical results. However, constitutive expression could be detected by immunoblot analysis. Therefore, although the reason for the discrepancy in the result between the immunohistochemistry and immunoblotting was not clear, difference in the constitutively expressed level of TGFβRI in liver cells not forming preneoplastic foci between groups including DEN‐alone group might be involved.
Considering the similarity of the immunolocalization pattern of TGFβRI with the above‐examined PTEN/Akt signaling molecules, some signal crosstalk may occur between TGFβ and PTEN/Akt signaling for formation of preneoplastic lesions under tumor‐promoting stimuli. TGFβ has been shown to induce transcriptional down‐regulation of PTEN in neoplastic cells, suggesting a role for TGFβ signaling in their growth promotion.( 35 ) It has also been reported that activation of Akt is required for TGFβ‐mediated cell survival, epithelial‐to‐mesenchymal transition, and cell migration.( 36 , 37 ) This activation can result from TGFβ‐induced TGF‐α expression and consequent EGF‐receptor activation.( 37 ) According to our previous study results, populations of PTEN‐negative proliferative lesion were slightly but consistently larger than TGFβRI‐positive ones at both early and late stages of tumor promotion by FB and PB,( 13 ) suggesting an acquisition of TGFβ signaling in a subpopulation of PTEN‐negative lesions. On the other hand, in the analysis of the present study, GST‐P‐positive neoplastic lesions at the late stage of tumor promotion by FB showed higher concordance ratios with Akt2, phospho‐Akt substrate, and TGFβRI, than GST‐P‐positive liver cell foci. Most of these neoplastic lesions expressed above all molecules. These results suggest that crosstalk between TGFβ and PTEN/Akt signaling plays a role for the development of neoplastic lesions as an outcome of tumor promotion.
EGFR is a c‐erbB family protein that can heterodimerize for ligand‐dependent activation of downstream signaling. Interestingly, selective down‐regulation of EGFR in a majority of GST‐P‐positive foci was observed at 6 weeks of promotion in the present study, while c‐erbB2 did not show any specific expression pattern. Moreover, most of the GST‐P‐positive proliferative lesions, including carcinomas presented here, also down‐regulated EGFR at the later stage. It is well known that EGFR can be down‐regulated in a ligand‐dependent manner because of internalization and the following efficient degradation, resulting in a dramatic decrease in the half‐life of the EGFR protein,( 38 ) and long‐term down‐regulation of receptor activity can occur through this mechanism.( 39 ) This type of EGFR down‐regulation can occur via clathrin‐dependent or independent mechanisms; activity of PI3K as well as that of EGFR kinase and dynamin would be required for the latter.( 40 ) Another possibility for the mechanism underlying selective down‐regulation of EGFR is transcriptional gene silencing by CpG island hypermethylation.( 41 ) Although further studies are needed to examine whether EGFR is activated or inactivated in the GST‐P‐positive foci and neoplastic cells, TGFβ activates survival signals such as Akt, and EGFR is required for its activation.( 42 ) A subpopulation of GST‐P‐positive liver cell foci expresses TGFα, acting as a c‐erbB ligand.( 43 )
In summary, we found an enhanced activation of PTEN/Akt2 signaling in preneoplastic and neoplastic lesions in response to tumor promotion from the early stages of hepatocarcinogenesis. Considering the up‐regulation of TGFβRI and down‐regulation of EGFR in relation with PTEN/Akt2 in these lesions, there is likely crosstalk between Akt2 and TGFβ signaling, involving a mechanism requiring EGFR down‐regulation during the entire process of tumor promotion starting from the early stage.
Acknowledgments
We thank Mrs Sumiko Hayashi for her technical assistance in analysis of immunoblotting. This work was supported by Health and Labour Sciences Research Grants (Research on Food Safety) from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. Shirai T. A medium‐term rat liver bioassay as a rapid in vivo test for carcinogenic potential, a historical review of model development and summary of results from 291 tests. Toxicol Pathol 1997; 25: 453–60. [DOI] [PubMed] [Google Scholar]
- 2. Ito N, Imaida K, Asamoto M, Shirai T. Early detection of carcinogenic substances and modifiers in rats. Mutat Res 2000; 462: 209–17. [DOI] [PubMed] [Google Scholar]
- 3. Miller RT, Cattley RC, Marsman DS, Lyght O, Popp JA. TGFα differentially expressed in liver foci induced by diethylnitrosamine initiation and peroxisome proliferator promotion. Carcinogenesis 1995; 16: 77–82. [DOI] [PubMed] [Google Scholar]
- 4. Van Gijssel HE, Ohlson LC, Torndal UB et al . Loss of nuclear p53 protein in preneoplastic rat hepatocytes is accompanied by Mdm2 and Bcl‐2 overexpression and by defective response to DNA damage in vivo . Hepatology 2000; 32: 701–10. [DOI] [PubMed] [Google Scholar]
- 5. Finnberg N, Silins I, Stenius U, Högberg J. Characterizing the role of MDM2 in diethylnitrosamine induced acute liver damage and development of pre‐neoplastic lesions. Carcinogenesis 2004; 25: 113–22. [DOI] [PubMed] [Google Scholar]
- 6. Ogawa K, Asamoto M, Suzuki S, Tsujimura K, Shirai T. Downregulation of apoptosis revealed by laser microdissection and cDNA microarray analysis of related genes in rat liver preneoplastic lesions. Med Mol Morph 2005; 38: 23–9. [DOI] [PubMed] [Google Scholar]
- 7. Vanhaesebroeck B, Alessi DR. The PI3K‐PDK1 connection: more than just a road to PKB. Biochem J 2000; 346: 561–76. [PMC free article] [PubMed] [Google Scholar]
- 8. Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem 2001; 70: 247–79. [DOI] [PubMed] [Google Scholar]
- 9. West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 2002; 5: 234–48. [DOI] [PubMed] [Google Scholar]
- 10. Brader S, Eccles SA. Phosphoinositide 3‐kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori 2004; 90: 2–8. [DOI] [PubMed] [Google Scholar]
- 11. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 1999; 13: 2905–27. [DOI] [PubMed] [Google Scholar]
- 12. Silins I, Högberg J, Stenius U. Dietary sphingolipids suppress a subset of preneoplastic rat liver lesions exhibiting high PTEN, low phospho‐Akt and high levels of ceramide species. Food Chem Toxicol 2006; 44: 1552–61. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi M, Shibutani M, Woo GH et al . Cellular distributions of molecules with altered expression specific to the tumor promotion process from the early stage in a rat two‐stage hepatocarcinogenesis model. Carcinogenesis 2008; 29: 2218–26. [DOI] [PubMed] [Google Scholar]
- 14. Zhang H, Bajraszewski N, Wu E et al . PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest 2007; 117: 730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murillo MM, del Castillo G, Sánchez A, Fernández M, Fabregat I. Involvement of EGF receptor and c‐Src in the survival signals induced by TGF‐beta1 in hepatocytes. Oncogene 2005; 24: 4580–7. [DOI] [PubMed] [Google Scholar]
- 16. Shoda T, Onodera H, Takeda M et al . Liver tumor promoting effects of fenbendazole in rats. Toxicol Pathol 1999; 27: 553–62. [DOI] [PubMed] [Google Scholar]
- 17. Muguruma M, Unami A, Kanki M et al . Possible involvement of oxidative stress in piperonyl butoxide induced hepatocarcinogenesis in rats. Toxicology 2007; 236: 61–75. [DOI] [PubMed] [Google Scholar]
- 18. Kuroda K, Terao K, Akao M. Inhibitory effect of fumaric acid on hepatocarcinogenesis by thioacetamide in rats. J Natl Cancer Inst 1987; 79: 1047–51. [PubMed] [Google Scholar]
- 19. Shikano S, Coblitz B, Wu M, Li M. 14‐3‐3 proteins: regulation of endoplasmic reticulum localization and surface expression of membrane proteins. Trends Cell Biol 2006; 16: 370–5. [DOI] [PubMed] [Google Scholar]
- 20. Powell DW, Rane MJ, Chen Q, Singh S, McLeish KR. Identification of 14‐3‐3ζ as a protein kinase B/Akt substrate. J Biol Chem 2002; 277: 21639–42. [DOI] [PubMed] [Google Scholar]
- 21. Kebache S, Ash J, Annis MG et al . Grb10 and active Raf‐1 kinase promote Bad‐dependent cell survival. J Biol Chem 2007; 282: 21873–83. [DOI] [PubMed] [Google Scholar]
- 22. Jahn T, Seipel P, Urschel S, Peschel C, Duyster J. Role for the adaptor protein Grb10 in the activation of Akt. Mol Cell Biol 2002; 22: 979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goetz CA, O’Neil JJ, Farrar MA. Membrane localization, oligomerization, and phosphorylation are required for optimal raf activation. J Biol Chem 2003; 278: 51184–9. [DOI] [PubMed] [Google Scholar]
- 24. Kao G, Tuck S, Baillie D, Sundaram MVC. elegans SUR‐6/PR55 cooperates with LET‐92/protein phosphatase 2A and promotes Raf activity independently of inhibitory Akt phosphorylation sites. Development 2004; 131: 755–65. [DOI] [PubMed] [Google Scholar]
- 25. Yamada E, Okada S, Saito T et al . Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4‐containing vesicles. J Cell Biol 2005; 168: 921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee KY, Shibutani M, Inoue K et al . Methacarn fixation – effects of tissue processing and storage conditions on detection of mRNAs and proteins in paraffin‐embedded tissues. Anal Biochem 2006; 351: 36–43. [DOI] [PubMed] [Google Scholar]
- 27. Ikeda H, Nishi S, Sakai M. Transcription factor Nrf2/MafK regulates rat placental glutathione S‐transferase gene during hepatocarcinogenesis. Biochem J 2004; 380: 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans 2007; 35: 231–5. [DOI] [PubMed] [Google Scholar]
- 29. Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res 2008; 68: 957–60. [DOI] [PubMed] [Google Scholar]
- 30. Luebeck EG, Buchmann A, Stinchcombe S, Moolgavkar SH, Schwarz M. Effects of 2,3,7,8‐tetrachlorodibenzo‐P‐dioxin on initiation and promotion of GST‐P‐positive foci in rat liver: A quantitative analysis of experimental data using a stochastic model. Toxicol Appl Pharmacol 2000; 167: 63–73. [DOI] [PubMed] [Google Scholar]
- 31. Kinoshita A, Wanibuchi H, Morimura K et al . Phenobarbital at low dose exerts hormesis in rat hepatocarcinogenesis by reducing oxidative DNA damage, altering cell proliferation, apoptosis and gene expression. Carcinogenesis 2003; 24: 1389–99. [DOI] [PubMed] [Google Scholar]
- 32. Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol 2005; 17: 150–7. [DOI] [PubMed] [Google Scholar]
- 33. Derynck R, Zhang YE. Smad‐dependent and Smad‐independent pathways in TGF‐β family signalling. Nature 2003; 425: 577–84. [DOI] [PubMed] [Google Scholar]
- 34. Massagué J. TGFβ in cancer. Cell 2008; 134: 215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chow JY, Quach KT, Cabrera BL, Cabral JA, Beck SE, Carethers JM. RAS/ERK modulates TGFβ‐regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinogenesis 2007; 28: 2321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3‐kinase function is required for transforming growth factor β‐mediated epithelial to mesenchymal transition and cell migration. J Biol Chem 2000; 275: 36803–10. [DOI] [PubMed] [Google Scholar]
- 37. Vinals F, Pouysségur J. Transforming growth factor β1 (TGF‐β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF‐α signaling. Mol Cell Biol 2001; 21: 7218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 2008; 314: 3093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays 1993; 15: 375–82. [DOI] [PubMed] [Google Scholar]
- 40. Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res 2006; 66: 3603–10. [DOI] [PubMed] [Google Scholar]
- 41. Montero AJ, Díaz‐Montero CM, Mao L et al . Epigenetic inactivation of EGFR by CpG island hypermethylation in cancer. Cancer Biol Ther 2006; 5: 1494–501. [DOI] [PubMed] [Google Scholar]
- 42. Caja L, Ortiz C, Bertran E et al . Differential intracellular signaling induced by TGF‐β in rat adult hepatocytes and hepatoma cells: Implications in liver carcinogenesis. Cell Signal 2007; 19: 683–94. [DOI] [PubMed] [Google Scholar]
- 43. Kitano M, Ichihara T, Matsuda T et al . Presence of a threshold for promoting effects of phenobarbital on diethylnitrosamine‐induced hepatic foci in the rat. Carcinogenesis 1998; 19: 1475–80. [DOI] [PubMed] [Google Scholar]


