Abstract
A conventional DC‐based immunotherapy has been tested clinically for treatment of patients with advanced cancer but requires modification to further improve the clinical results. In this study, we evaluated the in vivo antitumor effects of DC therapy, non‐viral‐mediated IL‐12 gene therapy, and a combination of the two in a murine bilateral subcutaneous tumor model. DC therapy alone and IL‐12 gene therapy alone suppressed tumor growth at the injected sites. However, the antitumor effect on the distant contralateral tumor was insufficient. When DC therapy and IL‐12 gene therapy were carried out simultaneously, tumor growth was significantly suppressed bilaterally (P < 0.001). Cytolytic activity was augmented significantly in mice given the combination treatment compared to in mice treated with either DC or IL‐12 gene therapy alone (P < 0.05). Microvessel density of both tumors was significantly lower in mice subjected to the combination therapy than in mice treated otherwise (P < 0.05). Furthermore, no side‐effects were observed in the treated mice. DC therapy combined with non‐viral‐mediated intratumoral IL‐12 gene delivery has a synergistic antitumor effect not only on targeted tumors but also on contralateral distant tumors and may be of great potential as a therapeutic treatment for patients with advanced cancer. (Cancer Sci 2005; 96: 303 –308)
Abbreviations:
- APC
antigen presenting cells
- BM
bone marrow
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- IFN
interferon
- IL
interleukin
- IP‐10
IFN‐inducible protein‐10
- LDH
lactate dehydrogenase
- MIG
monokine induced by IFN‐γ
- NK
natural killer
- PBS
phosphate‐buffered saline
- PDBA
poly(d,l‐2,4‐diaminobutyric acid).
Dendritic cells are the most potent APC and are clearly central to the regulation, maturation and maintenance of the cellular immune response to cancer. (1) DC‐based immunotherapy is promising as a strategy against cancer, and it is currently being tested in clinical trials. 2 , 3 , 4 , 5 , 6 , 7 Although several clinical trials, including ours, (3) showed the safety and feasibility of DC therapy, objective clinical responses did not persist. Thus, new strategies for DC‐based immunotherapy are anticipated to improve the clinical efficacy of this treatment.
We recently developed a novel non‐viral cytokine gene delivery system using PDBA. (8) PDBA differs from polylysine in the length of the side chain, resulting in high transfection efficiency. IL‐12 gene transfer to established tumors with this system successfully induced high tumor levels of IL‐12 protein, whereas serum levels of IL‐12 in treated mice were below the limits of detection. Moreover, IL‐12 gene delivery augmented both NK cell and CTL activity, resulting in growth inhibition of transfected tumors. (8)
We hypothesized that DC therapy could be more effective when used in combination with PDBA‐mediated IL‐12 gene delivery. In mice, we investigated whether intratumoral IL‐12 gene delivery enhanced the in vivo antitumor effects of DC‐based immunotherapy. Our results showed that this strategy produced potent antitumor effects not only at the injected tumor sites but also in distant non‐injected tumors in the contralateral flank. In the present study, the mechanism of the synergetic antitumor effect of this combination therapy was explored from the standpoint of cytolytic activity and angiogenesis.
Materials and Methods
Tumor cell lines and animals. B16F10 mouse melanoma cells and YAC‐1 cells (Cell Resource Center for Biomedical Research, Tohoku University, Sendai, Japan) were maintained in vitro in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, 100 µg/mL streptomycin and 2 mM l‐glutamine (all from Life Technologies, Grand Island, NY, USA). Seven‐week‐old female B57BL/6 mice were purchased from Seac Yoshitomi (Fukuoka, Japan) and housed in the Laboratory Animal Research Center of Oita University. Mice were maintained on rodent feed and water ad libitum in an atmosphere of 23°C, 50% humidity. All mice were acclimated for at least 1 week before tumor implantation. All studies were carried out in accordance with the guidelines established by the Oita University Institutional Animal Care and Use Committee.
Preparation of tumor lysate. Tumor cells were collected and lysed in three freeze‐thaw cycles. Lysis was monitored by light microscopy. After centrifugation (10 min, 900 g ), supernatant was collected and passed through a 0.2‐µm pore filter. The protein concentration of the lysate was determined by commercial assay (BCA Protein Assay; Pierce, Rockford, IL, USA).
Mouse IL‐12 plasmid and PDBA/plasmid DNA complex preparations. Murine IL‐12 expression plasmid (pmIL‐12), designated pCAGGS‐mIL‐12, was obtained as a gift from Dr Jun‐ichi Miyazaki of Osaka University Medical School, Japan. Plasmid DNA was amplified in Escherichia coli strain DH5α, isolated and purified with a Qiagen Plasmid Maxi Kit (Qiagen, Hilden, Germany). The concentration of pmIL‐12 was determined using UV absorption at 260 nm. PDBA (PDBA8000, a gift from Hisamitsu Pharmaceutical Company, Tokyo, Japan) and pmIL‐12 were diluted in PBS to a concentration of 50 µg/mL or 150 µg/mL, respectively. The PDBA/pmIL‐12 complex was then prepared by gentle vortexing and used for gene transfection within 60 min.
Dendritic cell culture. Bone marrow‐derived DC were prepared as described previously (9) with some modifications. Briefly, three mice were killed, and BM cells were harvested from the femur and tibia (day 0). Contaminated erythrocytes were lysed with NH4Cl buffer, and lymphocytes were depleted with a mixture of anti‐B220 (RA3‐6B2), anti‐CD4 (GK1.5) and anti‐CD8a monoclonal antibodies (Ly‐2; PharMingen, San Diego, CA, USA), and rabbit complement (Cedarlane Laboratories, Ontario, Canada). These cells were cultured overnight in six‐well plates (106 cells/mL, 4 mL/well) in RPMI 1640 supplemented with 10% heat‐inactivated fetal bovine serum, antibiotics and antimycotics. Non‐adherent cells were then placed in fresh culture medium containing recombinant murine granulocyte/macrophage colony‐stimulating factor (1000 IU/mL) and recombinant murine IL‐4 (1000 IU/mL) (both from PeproTech EC, London, UK) on day 1. On day 6 of culture, non‐adherent cells were collected, placed in six‐well plates (5 × 105 cells/mL; 4 mL/well) and pulsed with prepared tumor lysate (100 µg/mL) overnight. On day 7 of culture, lipopolysaccharide (50 ng/mL; Calbiochem, Bad Soden, Germany) and IFN‐γ (50 ng/mL; PeproTech EC) were added to the culture to induce sufficient maturation of these cells. On day 9, fully matured DC were collected and used for the immunization of mice. Phenotypic analysis by flow cytometry was carried out on all preparations used in this study to ensure the quality of the cell preparations. DC were stained with fluorescein‐isothiocyanate‐ or phycoerythrin‐conjugated monoclonal antibodies against murine cell surface molecules (CD11c, CD80, CD86) and with appropriate isotype control antibodies.
Tumor implantation and treatment. To generate tumors, mice were challenged subcutaneously in both flanks with 0.1 mL of a single‐cell suspension containing 2.0 × 105 B16F10 cells. Treatment of the right lateral flank tumor began after 7 days, when the tumor reached approximately 100–125 mm3. Mice underwent intratumoral injection of 250 µL PDBA/pmIL‐12 complex. At 3–4 h later, 106 DC were injected around the tumor. Treatment was carried out once on day 7 and once on day 8. Control groups of mice received DC therapy, IL‐12 gene therapy or PBS. Each group consisted of eight animals. The tumor was measured every 2 or 3 days for a period of 21 days after tumor inoculation, and tumor volume was calculated according to the formula V = A × B2/2 (mm3), where A is the largest diameter (mm) and B is the smallest diameter (mm).
Cytotoxicity assay. Lymphoid cells were obtained from the groin lymph nodes of the mice. To obtain CTL from these cells, 2 × 106 lymphoid cells were restimulated in vitro with 2 × 105 mitomycin C‐treated B16F10 cells in the presence of recombinant murine IL‐2 (25 IU/mL; Sigma, St Louis, MO, USA) for 5 days. Lymphoid cells were harvested and washed three times in serum‐free medium and applied as effectors at various effector/target ratios. B16F10 cells or YAC‐1 cells (NK‐sensitive cells) were used as targets. To measure LDH released from targets, 100 µL supernatant was transferred to a flat‐bottomed enzyme assay plate 4 h after incubation in 96‐well round‐bottomed plates. Cytotoxic activity was examined with the LDH Cytotoxicity Detection Kit (Takara Bio, Tokyo, Japan) as described previously. 10 , 11 , 12 Briefly, 100 µL substrate of LDH was added to the enzymic assay plates and incubated for 30 min at room temperature. The optical absorbances of red formazan at a measurement wavelength of 490 nm and a reference wavelength of 620 nm were then recorded. The percentage of specific toxicity was calculated according to the following formula:
| %cytotoxicity = 100 × ([experimental − effecter spontaneous − target spontaneous]OD/[target maximum − target spontaneous]OD). |
Morphological analysis and staining and counting of microvessels. On day 14 after tumor inoculation (6 days after the final treatment), tumors (bilateral) in each group were resected, fixed in 10% buffered formalin, cut into slices, embedded in paraffin, prepared as 3‐µm sections and stained routinely with hematoxylin and eosin. All blood vessels were highlighted by staining endothelial cells for CD34 (PharMingen). Primary antibody was detected with an LSAB + Kit (Japan DakoCytomation, Kyoto, Japan) according to the manufacturer's instructions. The number of CD34‐positive microvessels was determined in three 0.25‐mm2 areas within a specimen under a magnification of ×200; three mice were examined in each group. The average of all fields examined within the area was recorded and expressed as counts/mm2.
Statistical analysis. Differences in tumor growth were evaluated statistically by repeated measures ANOVA. Statistical significance of differences between groups was determined using the non‐parametric Mann–Whitney U‐test. P < 0.05 was considered statistically significant.
Results
Enhanced antitumor effect of DC therapy in combination with IL‐12 gene therapy. The growth of injected tumors in the right flank was inhibited significantly with IL‐12 gene therapy alone, DC therapy alone and DC therapy plus IL‐12 gene therapy in comparison to injection of PBS (P < 0.001; Fig. 1a). Growth of the non‐injected tumors on the contralateral side was inhibited significantly with IL‐12 gene therapy alone or DC therapy alone in comparison to injection of PBS (P < 0.05). When DC therapy plus IL‐12 gene therapy was used, the growth inhibition was significantly greater than with IL‐12 gene therapy alone or DC therapy alone (P < 0.05; Fig. 1b). Adverse effects such as death or diarrhea were not observed with any treatment.
Figure 1.
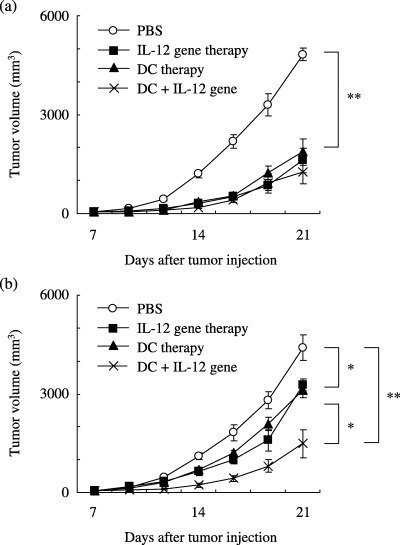
Effect of combination treatment with DC therapy and intratumoral IL‐12 gene delivery against a B16F10 subcutaneous tumor. (a) Growth of injected tumors in the right flank was inhibited significantly with IL‐12 gene therapy alone, DC therapy alone and DC therapy plus IL‐12 gene therapy in comparison to injection of PBS (P < 0.001). (b) Growth of the non‐injected tumors on the contralateral side was inhibited significantly with IL‐12 gene therapy alone and DC therapy alone in comparison to that with PBS (P < 0.05). The growth was inhibited significantly with DC therapy plus IL‐12 gene therapy in comparison to that with IL‐12 gene therapy alone and DC therapy alone (P < 0.05). The growth inhibition was greater in the mice given DC therapy plus IL‐12 gene therapy than in those injected with PBS (P < 0.001). Each group comprised eight mice. Data are presented as mean ± SE. *P < 0.05, **P < 0.001.
Augmentation of cellular immune response with DC plus IL‐12 gene therapy. To analyze the mechanism underlying the antitumor effect, draining lymph nodes in the right lower flank were resected after treatment. Lymphadenopathy was observed after treatment (Fig. 2a). Mean weight of the lymph nodes was 13.8 ± 1.0 mg in the mice who received IL‐12 gene therapy, 11.5 ± 0.6 mg in those who received DC therapy, 17.7 ± 0.6 mg in those who received combination therapy, and 5.9 ± 2.5 mg in those given PBS (Fig. 2b). Lymph nodes were markedly enlarged in the group treated with DC plus IL‐12 gene therapy in comparison to those of mice treated under the other protocols. Cytolytic activity against B16F10 cells was augmented significantly in the combination treatment group compared to that in the other groups, as shown in Fig. 2c (P < 0.05). Cytolytic activity against YAC‐1 cells was also augmented significantly in the combination treatment group compared to that in the other groups, as shown in Fig. 2d (P < 0.05). These results confirmed that enhancement of the antitumor effect in mice with combination therapy corresponded to augmentation of the cytolytic activity.
Figure 2.
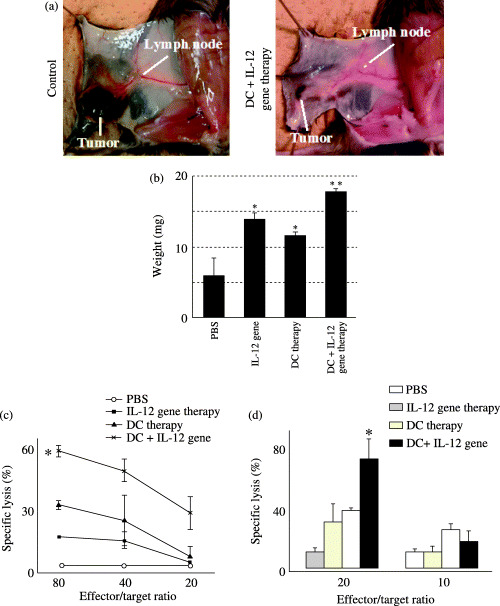
Changes in draining lymph nodes after treatment. (a) Lymphadenopathy was observed after treatment. (b) Mean weight of the lymph nodes in each group is shown. *P < 0.05 versus PBS; **P < 0.05 versus other protocols. (c) To obtain effector cells, 2 × 106 cells derived from regional lymph nodes were restimulated in vitro with 2 × 105 mitomycin C‐treated B16F10 cells in the presence of recombinant murine IL‐2 (25 IU/mL) for 5 days. Lymphoid cells were harvested and washed three times in serum‐free medium and applied as effectors. Cytolytic activity was assessed against B16F10 cells at various effector/target ratios from mice treated with DC alone, IL‐12 gene therapy alone, or DC and IL‐12 gene therapy in combination. Data are presented as mean ± SD cytotoxicity of effector cells obtained from draining lymph nodes. *P < 0.05 versus other protocols. (d) Cytolytic activity was assessed against YAC‐1 cells at effector/target ratios of 10 and 20 from mice treated with DC alone, IL‐12 gene therapy alone or DC and IL‐12 gene therapy in combination. Data are presented as mean ± SD cytotoxicity of effector cells obtained from draining lymph nodes. *P < 0.05 versus other protocols.
Morphology and microvessel counts. An extensive necrotic area, massive lymphatic infiltration and lack of neovascularization were observed in the mice that underwent combination therapy. In terms of tumor‐related angiogenesis, we quantified the distribution of vessels in tumors by immunocytochemical study with CD34 specific to endothelial cells. Representative immunohistochemical findings for CD34 staining are shown in Fig. 3. Mean microvessel counts of tumors are summarized in Table 1. The combination therapy significantly reduced distribution of vessels in tumors on both sides (P < 0.05).
Figure 3.
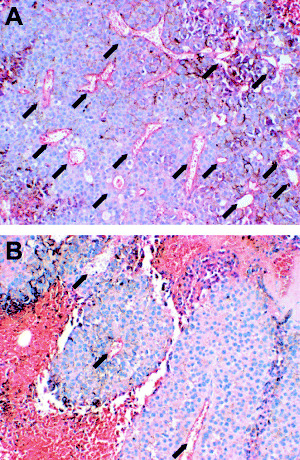
Microvessels (arrows) in the B16F10 tumors stained with antimouse CD34 monoclonal antibody. (a) Neovasclarization of untreated tumor (PBS control). (b) Anti‐angiogenic effect observed in the tumor treated by DC therapy plus intratumoral IL‐12 gene delivery.
Table 1.
Microvessel density (count/mm2) in the mice tumors †
| Therapy | Injected tumor | Noninjected distant tumor |
|---|---|---|
| PBS (control) | 102.7 ± 22 | 82.2 ± 26 |
| IL‐12 gene | 40.0 ± 17* | 55.6 ± 12* |
| DC | 70.2 ± 17* | 66.2 ± 33 |
| Combination | 21.3 ± 11** | 42.2 ± 15** |
Resected 6 days after treatment;
significant (P < 0.05) versus PBS;
significant (P < 0.05) versus all other groups.
Discussion
Dendritic cells are used extensively for antigen‐specific immunotherapy for cancer because only APC are capable of initiating an immune response. (13) DC therapy has been used to treat advanced cancer in particular. 3 , 14 , 15 Although antitumor cellular immune responses are induced by DC vaccination, the objective clinical response observed is limited. Thus, an additional therapy to be used in combination with DC immunotherapy is anticipated to render a synergistic antitumor effect and enhance the clinical effectiveness of DC therapy. IL‐12 was initially identified and isolated as an NK cell stimulatory factor. (16) In addition to this stimulatory effect on NK cells, bioactive IL‐12 can activate CTL or NKT cells. 17 , 18 , 19 Induction of cytokines, such as IFN‐γ, IP‐10 and MIG, has also been implicated as a mechanism of antitumor activity of IL‐12. 20 , 21
We reported recently that PDBA‐mediated intratumoral IL‐12 gene delivery enhances local IL‐12 expression in a murine subcutaneous tumor model, resulting in a significant antitumor response without toxicity. (8) In the present study, we first evaluated the antitumor effects of the combination DC plus IL‐12 gene therapy in comparison to that of DC therapy or IL‐12 gene therapy alone. In the group given combination therapy, a significant antitumor effect was observed not only in the injected tumors but also in the non‐injected tumors on the contralateral side. Consistent with results of our previous study, (8) both IL‐12 gene therapy alone and DC therapy alone showed enhanced CTL activity in vivo. In the present study, we observed significant augmentation of cytolytic activity against both B16F10 and YAC‐1 cells in the combination treatment group. These results suggest that DC therapy in combination with PDBA‐mediated IL‐12 gene therapy cooperatively enhances cytolytic activity and successfully induces systemic antitumor immunity.
We further investigated morphological change in the treated tumors. An extensive necrotic area, massive lymphatic infiltration and lack of neovascularization in the treated tumors were observed. These findings were significant in the tumors treated by DC therapy in combination with PDBA‐mediated IL‐12 gene therapy. IL‐12 has the ability to induce chemokines such as IP‐10 and MIG. (20) IP‐10 and MIG have been shown to function as chemoattractants for activated T lymphocytes. Therefore, our findings indicate that DC therapy and IL‐12 gene therapy act synergistically through activation and attraction of T lymphocytes, as several studies have shown. 22 , 23 IL‐12 also produces antiangiogenic effects via induction of IFN‐γ. (24) IL‐12 is produced mainly by APC such as macrophages or DC. DC express IL‐12 receptor on their cell surface, and the production of IL‐12 by DC is promoted by IL‐12 itself. (25) Therefore, antiangiogenic effects observed in the treated tumors might reflect local elevation of IL‐12 protein after the treatment. In addition, the antitumor effect of the combination therapy in this B16F10 mice model was considered to depend partially on the antiangiogenic effect.
Leonard et al. warned that IFN‐γ elevation induced by systemic administration of IL‐12 might lead to severe adverse effects including death. (26) Thus, local but not systemic elevation of IL‐12 is ideal for the treatment of cancer. As in our previous study, IL‐12 and IFN‐γ protein in sera were under detectable levels in the present study (data not shown). Moreover, the combination therapy yielded a potent antitumor effect without severe adverse effects. Thus, the combination of DC plus PDBA‐mediated IL‐12 gene therapy appears to be a safe and potent treatment for advanced cancer. Intratumoral delivery of adenoviruses 27 , 28 or retroviruses (29) containing the IL‐12 gene can cause regression of some established tumors in mice. However, they can exhibit some harmful antigenicity, and serious concerns have been voiced about the use of viral vectors, especially when clinical trials are involved. As far as we know, this is the first report of an enhanced antitumor effect of DC therapy with non‐viral‐mediated IL‐12 gene therapy. Because DC plus IL‐12 is a well‐established strategy for cancer immunotherapy, new technologies for safe and effective IL‐12 gene delivery should be developed. Recently, non‐viral gene delivery systems with polyvinylpyrrolidone, (30) poly(α‐[4‐aminobutyl]‐l‐glycolic acid) 31 , 32 and water‐soluble lipopolymers, (33) and rather unfamiliar non‐viral gene transfer methods such as gene gun (34) or electroporation, (35) were developed and reported. These methods could also be options for enhancement of the antitumor effect of DC therapy.
In the present study, DC therapy with PDBA‐mediated IL‐12 gene therapy cooperatively enhanced antitumor effects through induction of cytolytic activity, antiangiogenic effects and lymphatic infiltration even to non‐injected tumor sites. Thus, DC‐based immunotherapy in combination with intratumoral IL‐12 gene delivery may be useful for suppressing tumor growth in advanced cancer patients who have systemic multiple metastases. In conclusion, the synergistic antitumor effect of DC immunotherapy and IL‐12 gene therapy is attractive as a strategy for cancer treatment, although further studies are necessary before it can be applied in clinical settings.
Acknowledgments
This work was supported by the JSPS Fujita Memorial Fund for Medical Research and in part by a Grant‐in‐Aid for Scientific Research (C, 16591329) from the Japan Society for the Promotion of Science. We thank Ms Michiyo Hisaka and Ms Fusayo Kawamura for their excellent technical support in the histopathological study. We also thank Dr Fumiaki Tanaka (Kyushu University, Japan) for his helpful suggestions.
References
- 1. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991; 9: 271–96. [DOI] [PubMed] [Google Scholar]
- 2. Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide‐ or tumor lysate‐pulsed dendritic cells. Nat Med 1998; 4: 328–32. [DOI] [PubMed] [Google Scholar]
- 3. Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell‐based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother 2003; 52: 155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao X, Wei YQ, Peng ZL. Induction of T cell responses against autologous ovarian tumors with whole tumor cell lysate‐pulsed dendritic cells. Immunol Invest 2001; 30: 33–45. [DOI] [PubMed] [Google Scholar]
- 5. Geiger J, Hutchinson R, Hohenkirk L, McKenna E, Chang A, Mule J. Treatment of solid tumours in children with tumour‐lysate‐pulsed dendritic cells. Lancet 2000; 356: 1163–5. [DOI] [PubMed] [Google Scholar]
- 6. Holtl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol 1999; 161: 777–82. [PubMed] [Google Scholar]
- 7. Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K, Akiyoshi T, Mori M. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res 2001; 7: 2277–84. [PubMed] [Google Scholar]
- 8. Iwashita Y, Ogawa T, Goto S, Nakanishi M, Goto T, Kitano S. Effective transfer of interleukin‐12 gene to solid tumors using a novel gene delivery system, poly [d,l‐2,4‐diaminobutyric acid]. Cancer Gene Ther 2004; 11: 103–8. [DOI] [PubMed] [Google Scholar]
- 9. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony‐stimulating factor. J Exp Med 1992; 176: 1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao LZ, Lin ZB. Regulatory effect of Ganoderma lucidum polysaccharides on cytotoxic T‐lymphocytes induced by dendritic cells in vitro . Acta Pharmacol Sin 2003; 24: 321–6. [PubMed] [Google Scholar]
- 11. Hao X, Shao Y, Ren X, Liu H, Xu Q, Li H, Zhang P, An X, Ren B. Induction of specific CTL by MAGE‐3/CEA peptide‐pulsed dendritic cells from HLA‐A2/A24(+) gastrointestinal cancer patients. J Cancer Res Clin Oncol 2002; 128: 507–15. [DOI] [PubMed] [Google Scholar]
- 12. Yang YW, Wu CA, Morrow WJ. Cell death induced by vaccine adjuvants containing surfactants. Vaccine 2004; 22: 1524–36. [DOI] [PubMed] [Google Scholar]
- 13. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811. [DOI] [PubMed] [Google Scholar]
- 14. Chang AE, Redman BG, Whitfield JR, Nickoloff BJ, Braun TM, Lee PP, Geiger JD, Mule JJ. A phase I trial of tumor lysate‐pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res 2002; 8: 1021–32. [PubMed] [Google Scholar]
- 15. Hernando JJ, Park TW, Kubler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen‐pulsed dendritic cells in advanced gynaecological malignancies. Clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother 2002; 51: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med 1989; 170: 827–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hendrzak JA, Brunda MJ. Interleukin‐12. Biologic activity, therapeutic utility, and role in disease. Laboratory Invest 1995; 72: 619–37. [PubMed] [Google Scholar]
- 18. Trinchieri G. Interleukin‐12: a cytokine produced by antigen‐presenting cells with immunoregulatory functions in the generation of T‐helper cells type 1 and cytotoxic lymphocytes. Blood 1994; 84: 4008–27. [PubMed] [Google Scholar]
- 19. Gately MK, Wolitzky AG, Quinn PM, Chizzonite R. Regulation of human cytolytic lymphocyte responses by interleukin‐12. Cell Immunol 1992; 143: 127–42. [DOI] [PubMed] [Google Scholar]
- 20. Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP‐10 and Mig are necessary for IL‐12‐mediated regression of the mouse RENCA tumor. J Immunol 1998; 161: 927–32. [PubMed] [Google Scholar]
- 21. Brunda MJ, Luistro L, Hendrzak JA, Fountoulakis M, Garotta G, Gately MK. Role of interferon‐gamma in mediating the antitumor efficacy of interleukin‐12. J Immunother Emphasis Tumor Immunol 1995; 17: 71–7. [DOI] [PubMed] [Google Scholar]
- 22. Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Improved efficacy of dendritic cell vaccines and successful immunization with tumor antigen peptide‐pulsed peripheral blood mononuclear cells by coadministration of recombinant murine interleukin‐12. Int J Cancer 1999; 80: 324–33. [DOI] [PubMed] [Google Scholar]
- 23. Tatsumi T, Takehara T, Kanto T, Miyagi T, Kuzushita N, Sugimoto Y, Jinushi M, Kasahara A, Sasaki Y, Hori M, Hayashi N. Administration of interleukin‐12 enhances the therapeutic efficacy of dendritic cell‐based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res 2001; 61: 7563–7. [PubMed] [Google Scholar]
- 24. Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst 1995; 87: 581–6. [DOI] [PubMed] [Google Scholar]
- 25. Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL‐12 acts directly on DC to promote nuclear localization of NF‐kappaB and primes DC for IL‐12 production. Immunity 1998; 9: 315–23. [DOI] [PubMed] [Google Scholar]
- 26. Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single‐dose interleukin‐12 exposure on interleukin‐12‐associated toxicity and interferon‐gamma production. Blood 1997; 90: 2541–8. [PubMed] [Google Scholar]
- 27. Caruso M, Pham‐Nguyen K, Kwong YL, Xu B, Kosai KI, Finegold M, Woo SL, Chen SH. Adenovirus‐mediated interleukin‐12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci USA 1996; 93: 11 302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin‐12 induces regression and long‐lasting immunity that is associated with highly localized expression of interleukin‐12. Hum Gene Ther 1996; 7: 1995–2002. [DOI] [PubMed] [Google Scholar]
- 29. Tahara H, Zitvogel L, Storkus WJ, Zeh HJ 3rd, McKinney TG, Schreiber RD, Gubler U, Robbins PD, Lotze MT. Effective eradication of established murine tumors with IL‐12 gene therapy using a polycistronic retroviral vector. J Immunol 1995; 154: 6466–74. [PubMed] [Google Scholar]
- 30. Mendiratta SK, Quezada A, Matar M, Wang J, Hebel HL, Long S, Nordstrom JL, Pericle F. Intratumoral delivery of IL‐12 gene by polyvinyl polymeric vector system to murine renal and colon carcinoma results in potent antitumor immunity. Gene Ther 1999; 6: 833–9. [DOI] [PubMed] [Google Scholar]
- 31. Maheshwari A, Mahato RI, McGregor J, Han S, Samlowski WE, Park JS, Kim SW. Soluble biodegradable polymer‐based cytokine gene delivery for cancer treatment. Mol Ther 2000; 2: 121–30. [DOI] [PubMed] [Google Scholar]
- 32. Maheshwari A, Han S, Mahato RI, Kim SW. Biodegradable polymer‐based interleukin‐12 gene delivery: role of induced cytokines, tumor infiltrating cells and nitric oxide in anti‐tumor activity. Gene Ther 2002; 9: 1075–84. [DOI] [PubMed] [Google Scholar]
- 33. Mahato RI, Lee M, Han S, Maheshwari A, Kim SW. Intratumoral delivery of p2CMVmIL‐12 using water‐soluble lipopolymers. Mol Ther 2001; 4: 130–8. [DOI] [PubMed] [Google Scholar]
- 34. Rakhmilevich AL, Timmins JG, Janssen K, Pohlmann EL, Sheehy MJ, Yang NS. Gene gun‐mediated IL‐12 gene therapy induces antitumor effects in the absence of toxicity: a direct comparison with systemic IL‐12 protein therapy. J Immunother 1999; 22: 135–44. [DOI] [PubMed] [Google Scholar]
- 35. Yamashita YI, Shimada M, Hasegawa H, Minagawa R, Rikimaru T, Hamatsu T, Tanaka S, Shirabe K, Miyazaki JI, Sugimachi K. Electroporation‐mediated interleukin‐12 gene therapy for hepatocellular carcinoma in the mice model. Cancer Res 2001; 61: 1005–12. [PubMed] [Google Scholar]


