Abstract
The effects of leuprorelin acetate, a luteinizing hormone‐releasing hormone agonist (LHRH‐A), on prostate carcinogenesis in probasin/SV40 Tag transgenic rat was investigated. Fifteen weeks after administration of 0.28 and 2.8 mg/kg leuprorelin, prostate weights and serum testosterone levels were significantly decreased compared to values for transgenic controls. Histopathological findings revealed that the incidence of prostatic adenocarcinomas was significantly reduced in ventral, dorsal and lateral lobes of the prostate, correlating with decreased expression of SV40 Tag oncoprotein as well as inhibition of DNA synthesis and proliferation of epithelial cells in neoplastic lesions of the ventral prostate. Microarray analysis further showed leuprorelin acetate to significantly inhibit testicular steroidogenesis, suppressing the expression of SV40 Tag oncoprotein and altering the expression of a large number of genes which might be involved in the inhibition of prostate cancer progression in this rat model. (Cancer Sci 2006; 97: 459–467)
Prostate cancer has become the most commonly diagnosed malignancy in men, and the second commonest cause of cancer death after lung neoplasms in the USA.( 1 ) Initial treatment of prostate cancer is usually androgen‐ablative therapy, radiotherapy or radical prostatectomy, and patients with early stage disease respond well. However, in many patients the therapy eventually fails and death occurs from recurrent androgen‐independent prostate cancer and metastasis.
Luteinizing hormone‐releasing hormone (LHRH), which is synthesized in hypothalamic neurons and secreted directly into the hypophyseal‐portal blood circulation in a pulsatile manner, binds to high‐affinity receptors (LHRH‐R) on the gonadotrophic cells in the pituitary, stimulating the synthesis and release of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH), which in turn stimulate the synthesis of sex steroids by the testes. Based on the hypothalamus‐pituitary‐gonad hormonal relationship, LHRH analogs have been developed and used to treat prostate cancer through suppression of the pituitary release of gonadotropins to achieve a chemical castration effect. The mechanism of action is presumed to result from desensitization or downregulation of LHRH receptors in the pituitary gonadotrophs after chronic exposure to LHRH agonists and a consequent decline of gonadotropin secretion and subsequent gonadal atrophy.( 2 , 3 ) However, the molecular mechanisms involved in the inhibition of prostatic carcinomas by LHRH agonists are poorly defined.
Various studies have demonstrated that LHRH analogs inhibit the proliferation of human prostate cancer cell lines and prostate cancer xenografts, and also reduce the growth of androgen‐dependent and independent rat Dunning tumors, suggesting that their effects are mediated by specific LHRH receptors. This has been confirmed by detection of LHRH receptor mRNA expression in human prostate cancer cell lines and prostate cancer tissues. Therefore, activation of LHRH receptors at the prostate tumor level may represent an additional and more direct mechanism of action for antitumoral LHRH agonists.( 4 , 5 )
Leuprorelin acetate is a highly superactive agonistic analog of LHRH which is reported to inhibit pituitary gonadotropin secretion and suppress testicular steroidogenesis when administered chronically in therapeutic doses.( 6 , 7 ) Leuprorelin treatment is an established effective palliative measure in men with previously untreated advanced prostatic cancer, and is therefore a reasonable non‐surgical alternative in patients with prostatic disorders associated with aging.( 8 )
A rat transgenic model producing well‐differentiated prostate adenocarcinomas in all prostatic lobes and in a short period (15 weeks of age) using the Simian virus 40 T antigen under control of the probasin gene promoter (PB/SV40 Tag) has been established in our laboratory.( 9 ) This rat model of prostate carcinogenesis, which is completely androgen‐dependent, provides a good tool to evaluate strategies for prevention and treatment of prostate cancer in a relatively short‐term.
The present study was undertaken to investigate the effects of leuprorelin acetate on prostate carcinogenesis using our PB/SV40 Tag transgenic rats, with special attention to molecular changes in response to leuprorelin treatment, assessed by microarray analysis.
Materials and Methods
Animals
Heterozygous probasin‐SV40 large T antigen (PB/SV40 Tag) transgenic male rats for this study were obtained by mating heterologous transgenic males and wild‐type Sprague Dawley female rats (Clea, Tokyo, Japan). Rats weighing 110–150 g and aged 5 weeks at the commencement were used. They were ear‐tagged and housed three rats per plastic cage on wood‐chip bedding in an air‐conditioned specific pathogen‐free (SPF) animal room at 22 ± 2°C and 55 ± 5% humidity with a 12 h light/dark cycle. The animals had free access to food (Oriental MF, Oriental Yeast, Tokyo, Japan) and water. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University School of Medicine.
Screening of transgenic rats
DNA samples were obtained from rat tails by the proteinase K/phenol/chloroform method. Polymerase chain reaction (PCR) was performed using Taq polymerase (TaKaRa, Japan) to amplify a 300 bp fragment of SV40 Tag. Primers used were 5′‐AGCCCTGTCCTCCTGCAGGAT‐3′ (upper primer) and 5′‐GGCCAGCCTCACGGGGTTCA‐3′ (lower primer) (Hokkaido System Science, Japan).
Chemicals
Leuprorelin acetate (Leuplin) was kindly donated by Takeda Chemical Industries (Osaka, Japan) in a white powder form as a microcapsule sustained‐release preparation (microspheres of 20 µm diameter). The molecular weight is 1269.47 and the chemical comprises nine amino acids and has the empiric formula of C59H84N16O12.C2H4O2.( 10 )
Stock leuprorelin solution (1.875 mg/mL) was freshly prepared by suspending a vial of leuprorelin (3.75 mg leuprorelin acetate, 33.1 mg DL‐lactic and glycolic acids copolymer [3:1], 0.65 mg purified gelatin and 6.6 mg D‐mannitol) into 2 mL of diluent (100 mg D‐mannitol, 10 mg sodium carboxymethyl cellulose and 2 mg polysorbate‐80) from which two doses (high‐dose; 2.8 mg/kg and low‐dose; 0.28 mg/kg) were prepared and subcutaneously administered to the back of rats once every 4 weeks for a total of four injections.( 11 )
Study design
A total of 36 heterozygous transgenic male rats were allocated into four equally sized groups so that there were no significant differences in mean bodyweights. Group I (controls) comprised transgenic rats with the probasin/SV40 Tag serving as the reference group. Group II (vehicle) comprised PB/SV40 Tag transgenic animals which received a subcutaneous injection of the diluent (1.5 mL/kg) once every 4 weeks for a total of four injections. Group III (low‐dose) comprised transgenic animals that received a subcutaneous injection of leuprorelin at 0.28 mg/kg once every 4 weeks for a total of four injections. Group IV (high‐dose) comprised transgenic animals which received a subcutaneous injection of leuprorelin at 2.8 mg/kg once every 4 weeks for a total of four injections. Animal weights were recorded weekly throughout the experimental period (15 weeks).
Blood collection and tissue sampling
At the end of the treatment period (15 weeks), animals in all groups were intraperitoneally injected 1 h before being killed with 2% 5‐bromo‐2′‐deoxyuridine solution (BrdU) at a dose of 100 mg/kg bodyweight.( 12 ) Blood was collected from the abdominal aorta under ether anesthesia into 10 mL plastic vacuum tubes, kept on ice to clot and centrifuged. Serum samples were then analyzed for total testosterone with a direct radioimmunoassay (RIA) kit (Diagnostic Products Corporation, USA), for LH and FSH with double antibody RIA research kits (Amersham Biosciences, UK), and for urea nitrogen and creatinine levels using commercial kits (Alfresa Pharma, Japan). The urinogenital organs, comprising the prostate gland, seminal vesicles and urinary bladder were excised, weighed and photographed. Both ventral prostate lobes were separated and weighed. One lobe together with the pituitary gland was immediately frozen in liquid nitrogen then stored at −80°C until RNA extraction, while the other lobe together with the remaining prostates and tongues was fixed in 10% phosphate‐buffered formalin for 48 h, routinely processed to hematoxylin and eosin (HE) stained sections and histopathologically examined. Livers, kidneys and testes were excised at necropsy and weighed.
Immunohistochemistry
Immunohistochemical analyses of androgen receptor (AR) and SV40 Tag expression were performed with a Discovery instrument using DAB Map kits (Ventana Medical Systems, USA) with polyclonal rabbit antiandrogen receptor (PA1‐110, Affinity BioReagents, USA) and monoclonal mouse anti‐SV40 large T antigen (554149, BD PharMingen, USA) antibodies. Binding was visualized with a Vectastain Elite ABC kit (Vector Laboratories, USA) and light hematoxylin counterstaining was conducted to facilitate microscopic examination. Furthermore, the effects of administration of leuprorelin acetate on DNA synthesis in the epithelial cells of the ventral prostates of PB/SV40 Tag transgenic rats was investigated using BrdU immunostaining using a monoclonal mouse antibromodeoxyuridine antibody (Dako, Denmark). The numbers of BrdU positive cells were counted in 1000 cells/slide and BrdU labeling indices was determined with the following equation: (number of labeled cells/number of total cells) × 100.
Extraction of RNA
Extraction of total RNA from rat ventral prostate lobes as well as pituitary glands for reverse transcription (RT)‐PCR and microarray analyses was performed according to an ISOGEN protocol (Nippon Gene, Japan) with DNase treatment using a RQ1 RNase‐Free DNase kit (Promega Corporation, USA). Concentration and purity of total RNAs were assessed by measuring absorbance at 260 and 280 nm with a spectrophotometer (Ultrospec 3300 pro, Amersham Pharmacia Biotech, USA) and quality was assessed with an Agilent 2100 Bioanalyzer using a RNA 6000 Nano LabChip Kit (Agilent Technologies, USA). For microarray assays, the concentration, purity and quality of RNA should be >2, with an A260:A280 between 1.8 and 2.1, and the 28S:18S ratio should approach 2. Extracted RNA samples were stored at −80°C.
Quantitative RT‐PCR analyses of mRNA expression of SV40 Tag, androgen receptor and LHRH‐receptors
One microgram of RNA was converted to cDNA with avian myeloblastosis virus (AMV) reverse transcriptase (TaKaRa, Japan) in a 20 µg reaction mixture. Aliquots of 2 µg of cDNA samples were subjected to quantitative RT‐PCR using SYBR Premix ExTaq (TaKaRa) in a light cycler apparatus (Roche Diagnostic, Mannheim, Germany). Primers used for SV40 Tag were 5′‐GTCAGCAGTAGCCTCATCAT‐3′ and 5′‐GGTTGATTGCTACTGCTTCG‐3′; primers for AR were 5′‐GACTATTACTTCCCACCCCAG‐3′ and 5′‐ACATTTCCGGAGACGACACGA‐3′; primers for LHRH‐R were 5′‐CTTGAAGCCCGTCCTTGGAGAAAT‐3′ and 5′‐GCGATCCAGGCTAATCACCACCAT‐3′; and primers for rat cyclophilin (housekeeping gene) were 5′‐TGCTGGACCAAACACAAATG‐3′ and 5′‐GAAGGGGAATGAGGAAAATA‐3′. The LightCycler amplification protocol consisted of four programs: program 1, preincubation and denaturation of the template DNA (one cycle; 95°C for 30 s); program 2, amplification of the target DNA (30–40 cycles of denaturation at 95°C for 5 s, primer annealing at 45°C for SV40 Tag, 52°C for AR, 55°C for cyclophilin and 60°C for LHRH‐R for 15 s and elongation at 72°C for 30 s); program 3, melting curve analysis for product identification (95°C for 0 s, 65°C for 15 s and 95°C for 0 s); and program 4, cooling of the rotor and thermal chamber (one cycle; 40°C for 30 s). Cyclophilin mRNA levels were used to normalize sample cDNA contents.
Microarray analysis of gene expression profiles
Gene expression profiling was conducted using the CodeLink Expression Bioarray System (Amersham Biosciences, USA). The CodeLink Rat Whole Genome Bioarray targets ∼34 000 transcripts and Expressed Sequence Tags (ESTs) including over 29 000 well substantiated rat genes along with probes for housekeeping genes for normalization as well as positive and negative bacterial controls.
Because both low and high doses of leuprorelin showed parallel inhibition of prostatic adenocarcinoma development, the low dose was chosen for gene expression profiling analysis compared to the controls.
One RNA sample with a final concentration of 2 µg (pooled from three animals/group) was prepared and used to probe a single microarray chip. Hybridizations were performed as directed by CodeLink instructions. Briefly, mRNA was hybridized with an oligo‐dT primer that contained additional sequences corresponding to one strand of T7 RNA polymerase promoter. The oligo‐dT‐primed mRNA was converted to single‐stranded cDNA with reverse transcriptase then into double‐stranded cDNA with DNA polymerase. Double‐stranded cDNA was captured using QIAquick columns (QIAGEN, Germany) and served as a template for in vitro transcription (IVT) by T7 RNA polymerase in the presence of biotin‐UTP (PerkinElmer Life Sciences, USA) to produce biotin‐labeled target cRNA transcripts that were collected on RNeasy columns (QIAGEN). Target cRNA was fragmented followed by overnight hybridization with the bioarray chip in a temperature‐controlled shaking incubator. Spots were visualized using Cy5‐streptavidin dye conjugate and bioarrays were scanned and hybridization intensities were analyzed with CodeLink Expression Analysis software (Amersham Biosciences). The expression ratio for each gene was calculated between leuprorelin‐treated transgenic animals and controls. More than a two‐fold increase or decrease was regarded as a significant change (>2 as upregulated and <0.5 as downregulated). Overexpressed and downregulated genes were then annotated and grouped by function using the public database SOURCE.
Statistical analysis
The statistical significance of the incidence of neoplastic lesions in the prostates was assessed by Scheffe's analysis. Statistical analysis of differences between means was carried out using analysis of variance (ANOVA). When significant differences were obtained between means, the post‐hoc Bonferroni's test for multiple comparisons was used to evaluate the statistical significance between treatment groups at the P < 0.05 level of significance.
Results
Effects of leuprorelin treatment on body and organ weights in PB/SV40 Tag transgenic rats
Non‐significant changes in total bodyweights were recorded in vehicle or leuprorelin treated animals throughout the experiment compared to controls, demonstrating subchronic administration (15 weeks) of leuprorelin to transgenic rats to be non‐toxic.
Effects of leuprorelin treatment on the gross appearance of prostate and seminal vesicles
Macroscopically, prostates of the control and vehicle treated rats showed irregular surfaces with no apparent nodule or mass formation. Treatment of rats with low and high doses of leuprorelin markedly reduced the gross weights of the prostate and seminal vesicles in comparison to control and vehicle treated animals, without any apparent difference between the two dose groups (Fig. 1, Table 1).
Figure 1.
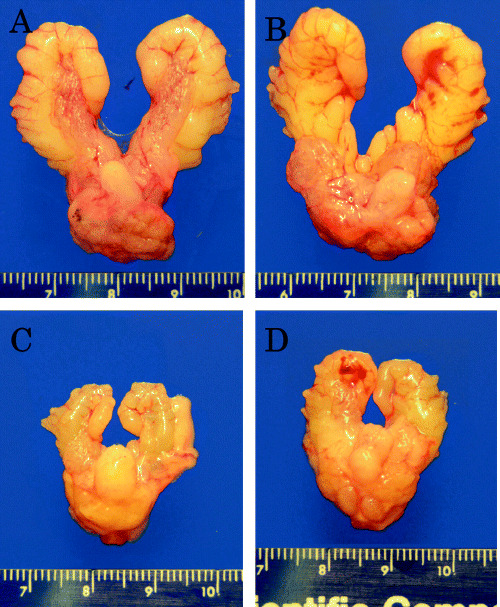
Photomicrographs showing the macroscopic appearance of urogenital organs in the different groups after 15 weeks of treatment. (A) Controls; (B) vehicle‐treated group; (C) low‐dose leuprorelin group; (D) high‐dose leuprorelin group. Macroscopically, prostates of the control and vehicle‐treated rats showed irregular surfaces with no apparent nodule or mass formations. Treatment of rats with low and high doses of leuprorelin markedly reduced the gross weights of the prostate and seminal vesicle, with respect to control and vehicle‐treated animals, without any significant difference between the two dose groups.
Table 1.
Statistical significance of the absolute and relative weight of urogenital organs (prostate, seminal vesicles and urinary bladder) as well as different prostatic lobes of PB/SV40 Tag transgenic rats treated with low and high doses of leuprorelin (15 weeks)
| Group | No. of rats | Urogenital organs | Ventral prostate | Dorsolateral prostate | Anterior prostate and seminal vesicles | ||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | ||
| Control | 9 | 5.33 ± 1.34 | 1.05 ± 0.24 | 0.58 ± 0.13 | 0.11 ± 0.02 | 1.48 ± 0.59 | 0.29 ± 0.11 | 3.15 ± 0.73 | 0.62 ± 0.13 |
| Vehicle | 9 | 5.03 ± 0.60 | 1.02 ± 0.16 | 0.48 ± 0.09 | 0.10 ± 0.02 | 1.22 ± 0.20 | 0.25 ± 0.05 | 3.01 ± 0.48 | 0.61 ± 0.12 |
| Low‐dose | 9 | 1.71 ± 0.57* | 0.35 ± 0.12* | 0.14 ± 0.06* | 0.03 ± 0.01* | 0.53 ± 0.14* | 0.11 ± 0.03* | 0.94 ± 0.28* | 0.19 ± 0.05* |
| High‐dose | 9 | 1.97 ± 0.81* | 0.40 ± 0.15* | 0.18 ± 0.07* | 0.04 ± 0.01* | 0.64 ± 0.23* | 0.13 ± 0.05* | 0.93 ± 0.50* | 0.19 ± 0.09* |
Values are mean ± SD. *Bonferroni's test was used for multiple comparisons, P < 0.05 is regarded as significant. Groups sharing the same characters are not significantly different.
Effects of leuprorelin treatment on serum LH, FSH and testosterone in PB/SV40 Tag transgenic rats
PB/SV40 Tag transgenic rats treated with low and high doses of leuprorelin for 15 weeks demonstrated a significant reduction in serum total testosterone level that reached 56.8% and 82.1%, respectively. No significant changes were observed in serum LH or FSH compared to controls.
Effects of leuprorelin treatment on the incidence of neoplastic lesions in the prostate
Prostate lesions in control rats showed marked epithelial proliferation with the formation of irregular glands and luminal bridging to give cribriform patterns. The nuclei demonstrated enlargement and severe atypia, and the lesions were compatible with human adenocarcinomas and were therefore diagnosed as such. Glands with less proliferation were also observed. These exhibited crowding of stratified epithelial cells with irregular spacing and occasional luminal bridging. Although nuclear atypia were severe, basic glandular structures were maintained, similar to normal prostates, and the lesions were diagnosed as prostatic intraepithelial neoplasia (PIN), comparable with the human lesions.( 9 )
Adenocarcinomas were composed of atypical cells with many mitoses forming glandular and cribriform structures. Histopathological examination revealed a 100% incidence of prostate adenocarcinomas in the ventral, lateral and anterior lobes at 20 weeks of age in the control and vehicle treated groups (Table 2), whereas the incidence was 55.6% and 66.7% in the dorsal prostate lobes, respectively. Low and high doses of leuprorelin significantly reduced the incidence of prostatic adenocarcinomas in the ventral and lateral lobes (11.1 and 33.3%, and 11.1 and 22.2%, respectively) while causing non‐significant change in the anterior lobe, with respect to controls. As for the dorsal prostate, complete inhibition of prostatic adenocarcinoma development was observed. However, no significant differences were found regarding the incidence of prostatic adenocarcinoma in the different prostatic lobes between the two dose groups.
Table 2.
Incidence of prostatic adenocarcinomas in the different prostatic lobes of PB/SV40 Tag transgenic rats treated with low and high doses of leuprorelin (15 weeks)
| Group | No of rats | Ventral | Lateral | Dorsal | Anterior | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LG PIN | HG PIN | AC | LG PIN | HG PIN | AC | LG PIN | HG PIN | AC | PIN | AC | ||
| Control | 9 | 0 | 0 | 9 (100%) | 0 | 0 | 9 (100%)** | 0 | 4 (44.4%) | 5 (55.6%) | 0 | 9 (100%) |
| Vehicle | 9 | 0 | 0 | 9 (100%) | 0 | 0 | 9 (100%) | 0 | 3 (33.3%) | 6 (66.7%) | 0 | 9 (100%) |
| Low‐dose | 9 | 3 (33.3%) | 5 (55.6%) | 1 (11.1%)* | 1 (11.1%) | 7 (77.8%) | 1 (11.1%)* | 2 (22.2%) | 7 (77.8%) | 0* | 3 (33.3%) | 6 (66.7%) |
| High‐dose | 9 | 2 (22.2%) | 4 (44.4%) | 3 (33.3%)* | 2 (22.2%) | 5 (55.6%) | 2 (22.2%)*, ** | 3 (33.3%) | 6 (66.7%) | 0*, ** | 2 (22.2%) | 7 (77.8%) |
P value is significant at the 0.05 level by Scheffe's analysis. Groups sharing the same characters are not significantly different.
**One case was diagnosed as small cell carcinoma. Percentage is shown in parentheses. AC, adenocarcinoma; HG PIN, high‐grade prostatic intraepithelial; LG PIN, low‐grade prostatic intraepithelial neoplasia.
Atrophic glands were also observed following treatment of transgenic rats with both doses of leuprorelin (Fig. 2), characterized by reduced epithelium and infiltration of inflammatory cells, most frequently observed in the high‐dose group. Small cell carcinomas were also found in the lateral prostates of two rats (in the control and high‐dose groups) and in the dorsal prostate of one rat (in the high‐dose group).
Figure 2.
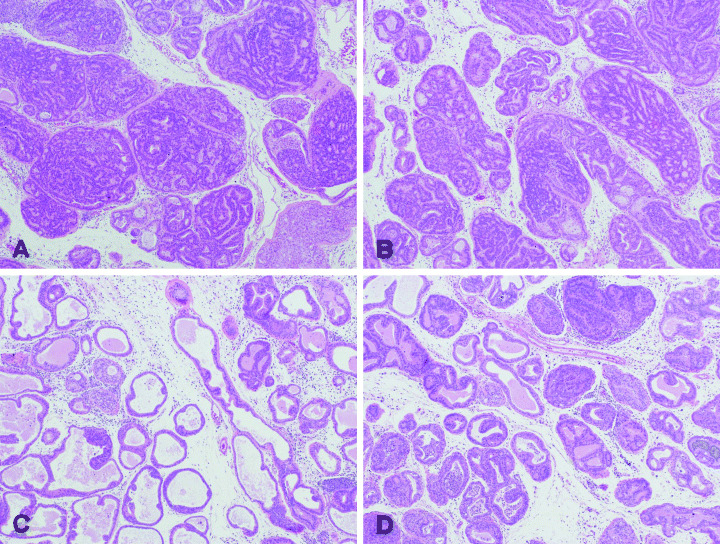
Photomicrographs showing the histopathology of the ventral prostates of (A) control rats; (B) vehicle‐treated group; (C) low‐dose treated group; and (D) high‐dose treated group. (A) and (B) show well‐differentiated adenocarcinoma composed of atypical epithelial cells forming glandular and cribriform structures. (C) and (D) show that intraepithelial proliferation was markedly decreased and the relative volume of stroma was increased, prostatic intraepithelial neoplasia (PIN) is evident. Atrophic glands characterized by reduced epithelium with fibrosis and infiltration of inflammatory cells (including neutrophils, lymphocytes and macrophages) are also shown (hematoxylin and eosin, × 40).
Effects of leuprorelin treatment on androgen receptor and SV40 Tag protein expression
Expression of SV40 Tag and AR proteins was detected in almost all nuclei of the atypical epithelial cells of different prostatic lobes in the control and vehicle treated groups. Treatment of PB/SV40 Tag transgenic animals with low and high doses of leuprorelin acetate significantly reduced SV40 Tag expression in the ventral, dorsal and lateral lobes as well as AR expression in the dorsal prostate, compared to controls. Both SV40 Tag and AR expression was slightly decreased in the anterior prostate following leuprorelin treatment.
Effects of leuprorelin treatment on DNA synthesis in the ventral prostate of leuprorelin‐treated rats
Subcutaneous administration of leuprorelin at low and high doses for 15 weeks to male PB/SV40 Tag transgenic rats caused significant parallel reduction in DNA synthesis in the epithelial cells of the ventral prostates compared to controls, as demonstrated by the decrease in the BrdU labeling indices that reached 61.2% and 59.4%, respectively. However, no significant change in the BrdU labeling index was found between the two dose groups (Fig. 3).
Figure 3.
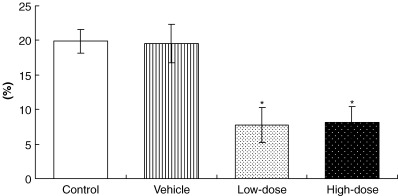
Statistical significance of DNA synthesis (BrdU labeling indices) in epithelial cells of the ventral prostates of control, vehicle‐ and leuprorelin‐treated PB/SV40 Tag transgenic rats. *P < 0.05 versus control and vehicle groups (Bonferroni's test). Values are mean ± SD.
Effects of leuprorelin treatment on quantitative expression of androgen receptor and SV40 Tag in the ventral prostate
RT‐PCR results revealed that administration of low and high doses of leuprorelin for 15 weeks to PB/SV40 Tag transgenic rats produced a significant parallel reduction in the relative mRNA expression of SV40 Tag in the ventral prostates (87.3 and 80.0%, respectively) (Fig. 4), whereas AR expression was not significantly changed in comparison with controls (data not shown).
Figure 4.
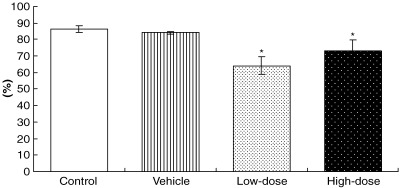
Statistical significance of relative mRNA expression levels of SV40 Tag proteins compared with cyclophilin expression in the ventral prostates of control, vehicle‐ and leuprorelin‐treated PB/SV40 Tag transgenic animals. *P < 0.05 versus control and vehicle groups (Bonferroni's test). Values are mean ± SE.
Effect of leuprorelin administration on the quantitative expression of LHRH‐R in the pituitaries of PB/SV40 Tag transgenic rats
Expression of LHRH‐R was reduced in the pituitaries of leuprorelin‐treated animals, compared to untreated transgenic controls (Fig. 5). However, no significant differences were recorded in the aforementioned parameters with respect to untreated transgenic animals.
Figure 5.
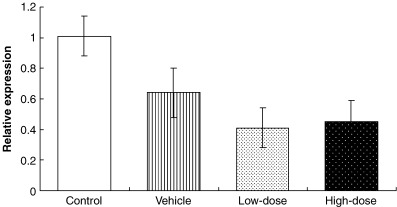
Relative mRNA expression level of luteinizing hormone‐releasing hormone receptor (LHRH‐R) compared with cyclophilin expression in the pituitaries of untreated, vehicle‐ and leuprorelin‐treated PB/SV40 Tag transgenic animals.Values are mean ± SE.
Effects of leuprorelin treatment on gene expression profiles in the ventral prostate of PB/SV40 Tag transgenic rats
Microarray analyses revealed 390 overexpressed and 655 downregulated genes (annotated and easily classified examples). Representative results are summarized in 3, 4.
Table 3.
Representative profile of some downregulated genes (<two‐fold with respect to controls) in the ventral prostates of PB/SV40 Tag transgenic rats following treatment with a low leuprorelin dose
| Function | GenBank no. | Gene name | Fold change |
|---|---|---|---|
| Apoptosis | NM_012922 | Caspase 3 (Casp3) | 0.48 |
| NM_053420 | BCL2/adenovirus E1B 19 Kda‐interacting protein 3 (Bnip3) | 0.47 | |
| NM_134334 | Cathepsin D (Ctsd) | 0.40 | |
| NM_173114 | Prostatic androgen‐repressed message‐1 (PARM‐1) | 0.39 | |
| NM_012588 | Insulin‐like growth factor binding protein 3 (Igfbp3) | 0.36 | |
| NM_022277 | Caspase 8 (Casp8) | 0.33 | |
| NM_031328 | B‐cell CLL/lymphoma 10 (Bcl10) | 0.32 | |
| NM_031735 | Serine/threonine kinase 3 (Stk3) | 0.31 | |
| NM_017312 | Bcl‐2‐realted ovarian killer protein (Bok) | 0.26 | |
| NM_021752 | Apoptosis inhibitor (Api2) | 0.25 | |
| NM_031700 | Claudin 3 (Cldn3) | 0.22 | |
| NM_031775 | Caspase 6 (Casp6) | 0.20 | |
| NM_031098 | Rho‐associated kinase beta (Rock1) | 0.05 | |
| Angiogenesis and invasion | NM_133523 | Matrix metalloproteinase 3 (MMP3) | 0.38 |
| U68726 | Neogenin | 0.34 | |
| NM_031055 | Matrix metalloproteinase 9 (MMP9) | 0.31 | |
| NM_012671 | Transforming growth factor alpha (TGFA) | 0.27 | |
| NM_022221 | Neutrophil collagenase (MMP8) | 0.26 | |
| NM_022603 | Growth factor binding protein 1 (Fgfbp1) | 0.23 | |
| NM_022266 | Connective tissue growth factor (Ctgf) | 0.20 | |
| Cell cycle and growth | NM_171991 | Cyclin B1 (Ccnb1) | 0.49 |
| NM_012704 | Prostaglandin E receptor 3 (Ptger3) | 0.49 | |
| NM_053464 | Spermidine synthase (Srm) | 0.42 | |
| NM_053677 | Protein kinase Chk2 (Rad53) | 0.37 | |
| NM_019219 | Retinoblastoma‐binding protein 9 (Rbbp9) | 0.37 | |
| NM_019296 | Cell division cycle 2 homolog A (Cdc2a) | 0.36 | |
| NM_013015 | Prostaglandin D2‐synthase (Ptgds) | 0.35 | |
| NM_080400 | Checkpoint kinase 1 homolog (Chek 1) | 0.32 | |
| NM_021740 | Prothymosin alpha (Ptma) | 0.28 | |
| NM_031094 | Retinoblastoma‐like 2 (Rbl2) | 0.25 | |
| NM_199501 | Cyclin dependent kinase 2 (Cdk2) | 0.25 | |
| NM_021583 | Prostaglandin E synthase (Ptges) | 0.25 | |
| NM_052981 | Cyclin H (Ccnh) | 0.24 | |
| NM_022381 | Proliferating cell nuclear antigen (Pcna) | 0.17 | |
| Cell signaling | NM_177933 | Sel1 (Suppressor of lin‐12) 1 homolog (Sel1h) | 0.49 |
| NM_017020 | Interleukin 6 receptor (Il6r) | 0.48 | |
| NM_017071 | Insulin receptor (Insr) | 0.47 | |
| NM_012747 | Signal transducer and activator of transcription 3 (Stat3) | 0.46 | |
| NM_130405 | Src associated in mitosis (Sam68) | 0.46 | |
| NM_022532 | v‐raf murine sarcoma 3611 viral oncogene homolog 1 (Araf1) | 0.45 | |
| L26267 | Nuclear factor Kappa B p105 subunit mRNA (NFkB) | 0.44 | |
| NM_012514 | Breast cancer 1 (Brca1) | 0.43 | |
| NM_057211 | Kruppel‐like factor 9 (Klf9) | 0.43 | |
| NM_017218 | Avian erythroblastosis oncogene B3 (Erbb3) | 0.41 | |
| NM_031514 | Janus kinase 2 (Jak2) | 0.38 | |
| NM_013145 | Guanine nucleotide binding protein, alpha inhibiting 1 (Gnai1) | 0.37 | |
| AF231407 | Calmodulin III (Calm3) | 0.34 | |
| NM_031338 | Ca++/calmodulin‐dependent protein kinase kinase beta (Cam2KK) | 0.32 | |
| NM_017198 | p21‐activated kinase 1 (Pak1) | 0.28 | |
| NM_012499 | Adenomatosis polyposis coli (Apc) | 0.28 | |
| NM_053777 | Mitogen activated protein kinase 8 interacting protein (Mapk8ip) | 0.27 | |
| NM_033230 | v‐akt murine thymoma viral oncogene homolog 1 (Akt1) | 0.26 | |
| NM_031143 | Diacylglycerol kinase zeta (Dgkz) | 0.23 | |
| NM_013022 | Rho‐associated coiled‐coil forming kinase 2 (Rock2) | 0.18 | |
| NM_053357 | Beta‐catenin (Catnb) | 0.10 | |
| Replication, DNA repair, transcription and translation | NM_053857 | Eukaryotic translation initiation factor 4E binding protein 1 (Eif4ebp1) | 0.49 |
| NM_031772 | RNA polymerase I (Rpo1–4) | 0.45 | |
| NM_171995 | Damage‐specific DNA binding protein 1 (Ddb1) | 0.45 | |
| XM_234239 | DNA repair endonuclease | 0.44 | |
| NM_012866 | Nuclear transcription factor‐Y gamma (Nfyc) | 0.44 | |
| NM_021662 | DNA polymerase delta, catalytic subunit (Pold1) | 0.43 | |
| NM_053480 | DNA polymerase alpha subunit II (Pola2) | 0.42 | |
| NM_031340 | Timeless homolog (Timeless) | 0.41 | |
| NM_133609 | Eukaryotic translation initiation factor2B, subunit 3 (Eif2b3) | 0.41 | |
| NM_022397 | Ribonucleoprotein F (Hnrpf) | 0.39 | |
| NM_138873 | Nibrin (Nbn, p95) | 0.39 | |
| NM_031058 | Mismatch repair protein (Msh2) | 0.32 | |
| NM_031107 | S6 protein kinase (Rsk‐1) | 0.30 | |
| NM_031599 | Eukaryotic translation initiation factor 2 alpha kinase 3 (Eif2ak3) | 0.29 | |
| NM_053528 | DNA polymerase gamma (Polg) | 0.23 | |
| NM_017141 | DNA polymerase beta (Polb) | 0.21 | |
| NM_138866 | Initiation factor (eIF‐2be) | 0.17 | |
| AJ011608 | DNA polymerase alpha subunit IV primase | 0.05 | |
| Secretory activity | NM_012836 | Carboxypeptidase D (cpd) | 0.49 |
| NM_017284 | Proteasome subunit, beta type 2 (Psmb2) | 0.48 | |
| NM_022219 | Alpha 1,3‐fucosyltransferase (Fuc‐T) | 0.46 | |
| NM_024151 | ADP‐ribosylation factor 4 (Arf4) | 0.41 | |
| NM_053406 | Protein O‐mannosyltransferase 1 (Pomt1) | 0.33 | |
| AF102262 | N‐acetylglucosamine galactosyltransferase (beta1‐4GT) | 0.29 | |
| NM_021869 | Syntaxin 7 (Stx7) | 0.27 | |
| NM_031722 | Coated vesicle membrane protein | 0.24 | |
| NM_019364 | Vesicle transport‐related | 0.22 | |
| Metabolism | NM_012941 | Cytochrome P450, subfamily 51 (Cyp51) | 0.49 |
| NM_013134 | 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase (Hmgcr) | 0.47 | |
| NM_080886 | Sterol‐C4‐methyloxidase‐like (Sc4mol) | 0.43 | |
| NM_012621 | 6‐phosphofructo‐2‐kinase/fructose‐2,6‐biphosphatase 1 (Pfkfb1) | 0.36 | |
| NM_053291 | Phosphoglycerate kinase 1 (Pgk1) | 0.36 | |
| NM_024381 | Glycerol kinase (Gyk) | 0.33 | |
| NM_031118 | Acyl‐coenzyme A: cholesterolacyltransferase (Soat1) | 0.31 | |
| NM_023104 | Acetoacetyl‐CoA synthetase | 0.31 | |
| NM_017136 | Squalene epoxidase (Sqle) | 0.30 | |
| NM_030992 | Phospholipase D1 (Pld1) | 0.28 | |
| NM_012851 | Hydroxysteroid 17β‐dehydrogenase 1 (Hsd17b1) | 0.26 | |
| NM_198738 | Phosphoserine aminotransferase 1 (Psat1) | 0.21 | |
| NM_172062 | Prolyl 4‐hydroxylase α subunit (P4ha1) | 0.18 | |
| NM_031043 | Glycogenin (Gyg) | 0.14 | |
| NM_021751 | Prominin (Prom) | 0.14 | |
| Miscellaneous | NM_053946 | Implantation‐associated protein (IAG2) | 0.49 |
| NM_012548 | Endothelin 1 (Edn1) | 0.47 | |
| NM_199266 | Cystatin related protein 2 | 0.44 | |
| NM_022391 | Pituitary tumor‐transforming 1 (Pttg1) | 0.43 | |
| NM_022298 | Alpha‐tubulin (Tuba1) | 0.40 | |
| NM_031821 | Serum‐inducible kinase (Snk) | 0.38 | |
| NM_173102 | Tubulin, beta (Tubb5) | 0.38 | |
| NM_199370 | Keratin 8 (Krt8) | 0.30 | |
| NM_012715 | Adrenomedullin (Adm) | 0.17 | |
| NM_175759 | Kallikrein, submaxillary gland S3 (rK9, K1k9) | 0.15 | |
| NM_012718 | Androgen regulated 20 KDa protein (Andpro) | 0.08 |
Table 4.
Representative profile of some overexpressed genes (>two‐fold with respect to controls) in the ventral prostates of PB/SV40 Tag transgenic rats following treatment with a low leuprorelin dose
| Miscellaneous | NM_053968 | Metallothionein 3 (Mt3) | 7.66 |
| NM_012657 | Serine protease inhibitor (Spin2b) | 6.79 | |
| NM_145774 | Rab38, member of RAS oncogene family | 3.93 | |
| NM_012774 | Glypican 3 (Gpc3) | 3.57 | |
| NM_012580 | Inhibin alpha (Inha) | 3.50 | |
| NM_144737 | Flavin‐containing monooxygenase 2 (Fmo2) | 3.03 | |
| NM_012662 | Seminal vesicle protein 4 (Svp4) | 2.82 | |
| NM_012789 | Dipeptidyl peptidase 4 (Dpp4) | 2.74 | |
| NM_024136 | Epididymal retinoic acid‐binding protein (Erabp) | 2.39 | |
| NM_080479 | Melanoma antigen, family D, 2 (Maged2) | 2.35 | |
| NM_053348 | Fetuin beta (Fetub) | 2.30 | |
| NM_139104 | Estrogen‐regulated protein CBL20, 204 KD | 2.23 | |
| NM_199119 | DEAD (Asp‐Glu‐Ala‐Asp) box polypeptide 24 (Ddx24) | 2.09 | |
| NM_012880 | Superoxide dismutase 3 (Sod3) | 2.04 |
Discussion
The present study demonstrated clear inhibitory effects of a LHRH agonist, leuprorelin acetate, on prostate oncogenesis in PB/SV40 Tag transgenic rats, in line with the conclusion that androgen ablation therapy continues to be the best approach for treatment of disseminated carcinomas of the prostate in the earliest androgen‐responsive stages. Inhibition was achieved without any significant changes in total bodyweights and absolute or relative liver weights, so detrimental toxic effects were lacking. Histopathological examination revealed that treatment of 5‐week old PB/SV40 Tag transgenic rats for 15 weeks with leuprorelin significantly reduced prostate adenocarcinoma progression in the ventral and lateral lobes, whereas complete inhibition was observed in the dorsal lobe in comparison with controls (Table 2). As shown, there are apparent lobe differences; however, the reasons are unknown. Our findings are in good agreement with previously reported studies demonstrating the inhibitory effect of leuprorelin treatment on the growth of the Dunning R 3327 androgen‐sensitive rat prostatic tumor transplanted into adult male Copenhagen rats,( 13 ) as well as the growth of male genital organs (testis, seminal vesicles and prostate) in intact Sprague Dawley male rats.( 11 ) Multiple comparison analysis revealed that the action of leuprorelin was not enhanced at the higher dose, which is consistent with previously reported studies of leuprorelin dose dependence.( 8 , 11 )
Treatment of transgenic rats with low and high doses of leuprorelin produced a significant decrease in serum total testosterone level, but over 50% testosterone remained, while non‐significant changes were recorded in serum LH and FSH levels, suggesting direct inhibitory effects on testicular steroidogenesis rather than indirect action through the pituitary‐gonadal axis. These findings correlated well with the lack of any significant change in mRNA expression for LHRH receptors in the pituitaries of treated rats. Earlier animal studies revealed inhibition of testicular steroidogenesis in intact rats following leuprorelin treatment, with a subsequent decrease in the relative weight of rat reproductive organs.( 6 , 14 ) Probable direct inhibitory effects of the drug on the prostate gland might exist in this transgenic strain, as evidenced by the detection of LHRH receptor mRNA expression in the ventral prostates of control and leuprorelin‐treated animals by RT‐PCR (data not shown). This possibility clearly warrants further investigation.
It is important to note that prostate cancer development and progression in the prostate adenoma in the TRAMP model as well as in our transgenic model is under the regulation of the androgen‐dependent probasin promoter, which directs prostate‐specific epithelial expression of the SV40 T antigen, an oncoprotein that interacts with retinoblastoma and p53 tumor‐suppressor gene products.( 9 , 15 ) Immunohistochemical and RT‐PCR findings for SV40 Tag oncoprotein expression in the prostates of leuprorelin‐treated animals showed a remarkable significant reduction in the ventral as well as dorsolateral lobes. DNA synthesis and proliferation of epithelial cells in neoplastic lesions in the ventral prostates were significantly reduced in leuprorelin‐treated rats, as revealed by BrdU immunostaining and morphometric analysis of the percentage of relative epithelial areas. The lack of effects on AR protein expression as well as relative AR mRNA expression in the ventral prostates of leuprorelin‐treated transgenic animals could be simply interpreted as reflecting reduction in serum androgen levels above the nadir level that would completely suppress production of ARs. We have already reported the effect of surgical castration on prostate tumor development in transgenic rats. Castration at an early stage induced an immature prostate gland structure and completely suppressed development of any neoplastic lesions, while castration at a late stage, that is, at the age of 20 weeks when adenocarcinomas had already developed, induced marked apoptosis of tumor cells leading to complete disappearance of carcinomas.( 9 ) Compared to the previous data with surgical castration, the present data demonstrate suppression effects of leuprorelin to be rather mild and lobe specific. If suppression of testosterone was largely responsible, such lobe specificity would not be expected as with surgical castration. Those findings support our speculation that suppression of tumor development in the present work was partly due to specific effects of the LH‐RH agonist.
Our results thus suggest that inhibition of prostatic adenocarcinoma development in the ventral, dorsal and lateral prostates of PB/SV40 Tag transgenic rats by leuprorelin treatment was mainly due to downregulation of SV40 Tag oncoprotein expression and partly due to reduction of the serum androgen level. In addition, some androgen‐independent mechanisms resulting in reduction of cell proliferation and regression of prostate cancers might be involved. The present microarray analysis indicated that many kinds of genes, including examples involved in apoptosis, angiogenesis, the cell cycle and growth, were influenced by leuprorelin.
In conclusion, the LHRH agonist leuprorelin acts to inhibit prostate carcinogenesis in PB/SV40 Tag transgenic rats by multiple mechanisms including reduction of testosterone biosynthesis, suppression of SV40 Tag oncoprotein expression and alteration in the expression of many genes that are critically involved in the control of cell proliferation and cell cycle progression, transcription and translation, signaling, angiogenesis and invasion, metabolism and cytoskeleton formation. This study also confirmed the suitability of the rat SV40 Tag model for prostate cancer chemoprevention and chemotherapeutic studies.
Acknowledgments
This work was performed through collaboration between the Japanese Government represented by Professor Tomoyuki Shirai (Department of Experimental Pathology and Tumor Biology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan) and the Egyptian Government represented by Professor Fawzia M. Refaie (Department of Biochemistry, Faculty of Science, Ain Shams University, Cairo, Egypt). The authors would like to thank Takeda Chemical Industries (Osaka, Japan) for providing the leuprorelin acetate microcapsule sustained‐release preparation.
References
- 1. Stewart SL, King JB, Thompson TD, Friedman C, Wingo PA. Cancer mortality surveillance – United States, 1990–2000. MMWR Surveill Summ 2004; 53: 1–108. [PubMed] [Google Scholar]
- 2. Lau HL, Zhu XM, Leung PC et al. Detection of mRNA expression of gonadotropin‐releasing hormone and its receptor in normal and neoplastic rat prostates. Int J Oncol 2001; 19: 1193–201. [DOI] [PubMed] [Google Scholar]
- 3. Tieva A, Bergh A, Damber JE. The clinical implications of the difference between castration, gonadotrophin releasing‐hormone (GnRH) antagonists and agonist treatment on the morphology and expression of GnRH receptors in the rat ventral prostate. BJU Int 2003; 91: 227–33. [DOI] [PubMed] [Google Scholar]
- 4. Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone‐releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology 1999; 140: 5250–6. [DOI] [PubMed] [Google Scholar]
- 5. Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone‐releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol 2000; 163: 623–9. [PubMed] [Google Scholar]
- 6. Okada H, Doken Y, Ogawa Y, Toguchi H. Sustained suppression of the pituitary‐gonadal axis by leuprorelin three‐month depot microspheres in rats and dogs. Pharm Res 1994; 11: 1199–203. [DOI] [PubMed] [Google Scholar]
- 7. Periti P, Mazzei T, Mini E. Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet 2002; 41: 485–504. [DOI] [PubMed] [Google Scholar]
- 8. Chrisp P, Sorkin EM, Leuprorelin. A review of its pharmacology and therapeutic use in prostatic disorders. Drugs Aging 1991; 1: 487–509. [DOI] [PubMed] [Google Scholar]
- 9. Asamoto M, Hokaiwado N, Cho YM et al. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 2001; 61: 4693–700. [PubMed] [Google Scholar]
- 10. Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A. Sex hormone dependency of diethylnitrosamine‐induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res 2001; 92: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gotanda K, Shinbo A, Okada M et al. Effects of combination therapy with a luteinizing hormone‐releasing hormone agonist and chlormadinone acetate on rat prostate weight and plasma testosterone levels. Prostate Cancer Prostatic Dis 2003; 6: 66–72. [DOI] [PubMed] [Google Scholar]
- 12. Kawabe M, Shibata MA, Sano M et al. Decrease of prostaglandin E2 and 5‐bromo‐2′‐deoxyuridine labeling but not prostate tumor development by indomethacin treatment of rats given 3,2′‐dimethyl‐4‐aminobiphenyl and testosterone propionate. Jpn J Cancer Res 1997; 88: 350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ichikawa T, Akimoto S, Shimazaki J. Effect of leuprolide on growth of rat prostatic tumor (R 3327) and weight of male accessory sex organs. Endocrinol Jpn 1988; 35: 181–7. [DOI] [PubMed] [Google Scholar]
- 14. Ogawa Y, Okada H, Heya T, Shimamoto T. Controlled release of LHRH agonist, leuprolide acetate, from microcapsules: serum drug level profiles and pharmacological effects in animals. J Pharm Pharmacol 1989; 41: 439–44. [DOI] [PubMed] [Google Scholar]
- 15. Asamoto M, Hokaiwado N, Cho YM, Shirai T. Effects of genetic background on prostate and taste bud carcinogenesis due to SV40 T antigen expression under probasin gene promoter control. Carcinogenesis 2002; 23: 463–7. [DOI] [PubMed] [Google Scholar]


