Abstract
Y‐box binding protein‐1 (YB‐1) is a member of the cold shock protein family and functions in transcription and translation. Many reports indicate that YB‐1 is highly expressed in tumor cells and is a marker for tumor aggressiveness and clinical prognosis. Here, we show clear evidence that YB‐1 is expressed in the angiogenic endothelial cells of various tumors, such as glioblastoma, esophageal cancer, gastric cancer, colon cancer, and lung cancer, as well as in tumor cells. YB‐1 was highly expressed in glomeruloid microvascular endothelial cells of brain tumors and microvessels in the desmoplastic region around multiple solid tumors. On the other hand, no or low YB‐1 expression was observed in normal angiogenic endothelial cells from fetal kidney, newborn lung, and placenta. The endothelial cells in inflammatory regions of granulomas were also weakly labeled. Knockdown of YB‐1 expression by small‐interfering RNA induced G1 cell cycle arrest and inhibited the growth of human umbilical vein endothelial cells stimulated by growth factors. Taken together, YB‐1 plays an important role in the growth of not only tumor cells but also tumor‐associated endothelial cells, suggesting that YB‐1 is a promising target for cancer therapy. (Cancer Sci 2010)
Tumors require a vascular supply for their rapid growth indicating that targeted disruption of tumor angiogenesis is a promising strategy for cancer therapy.( 1 , 2 ) VEGF plays a critical role in tumor angiogenesis, and blockage of the VEGF pathway inhibits tumor growth.( 3 ) Anti‐angiogenic therapy combined with standard chemotherapy improved the overall survival of cancer patients.( 4 ) However, anti‐angiogenic therapy alone did not provide a significantly improved outcome, suggesting that biological processes other than angiogenesis are involved in tumor growth. Several groups have identified specific markers in tumor‐associated endothelial cells.( 5 , 6 )
YB‐1 is involved in the proliferation and drug resistance of cancer cells.( 7 , 8 , 9 , 10 , 11 ) YB‐1 is highly expressed in various tumors and its expression is closely correlated with an unfavorable clinical outcome.( 8 , 12 , 13 )
Here, we provide the first evidence that YB‐1 is significantly expressed in tumor‐associated endothelial cells and is also involved in their growth. However, no or low YB‐1 expression was observed in angiogenesis associated with inflammation or normal physiological processes.
Taken together, these findings indicate that YB‐1 might be a highly promising molecular target for cancer therapy, because YB‐1 expression is prevalent within tumor vessels as well as in cancer cells.
Materials and Methods
Preparation of human tissue samples. Normal human tissue samples were obtained from three autopsy cases and from nontumor portions of surgically resected specimens. For human neoplastic and inflammatory tissue samples, several cases of surgically resected tissues were examined in the Department of Pathology and Cell biology at the University of Occupational and Environmental Health in Kitakyushu, Japan. These cases were classified according to the World Health Organization Histological Typing of each tissue. The diagnosis was re‐evaluated and confirmed by at least three board‐certified surgical pathologists who had examined formalin‐fixed, paraffin‐embedded tissue sections stained with H&E or other appropriate immunohistochemical stains. This study protocol was approved by the committee of University of Occupational and Environmental Health.
Immunohistochemistry. The specimens were fixed in 20% formaldehyde and embedded in paraffin. Four micrometer‐thick tissue sections were deparaffinized, dehydrated with graded xylene and alcohol, and incubated in 3% hydrogen peroxide for 5 min at room temperature to eliminate endogenous peroxidase activity.
The anti‐YB‐1 antibody has been described previously.( 14 ) Antigen retrieval for the anti‐GFAP and the anti‐Ki‐67 antibodies was performed by microwaving and then autoclaving the samples, for 15 min each, in 0.1 mol/L citrate buffer (pH 6.0). The sections were incubated with one of the following antibodies: mouse monoclonal anti‐GFAP antibody (1:100; Dako, Glostrup, Denmark), mouse monoclonal anti‐CD34 antibody (1:50; Immunotech, Marseilles, France), or mouse monoclonal anti‐Ki‐67 antibody (1:50; Dako, Copenhagen, Denmark). Staining was visualized using the Envision plus system (Dako) followed by counterstaining with hematoxylin. For CD34 and YB‐1 double staining, the mouse‐AP + rabbit HRP polymer detection kit (Biocare Medical, Concord, CA, USA) was used.
Cell culture. HUVECs (HUVEC‐Umbil Vein, Pooled Cells) were purchased from Lonza Walkersville (Walkersville, MD, USA) and maintained in the manufacturer’s recommended medium with growth factors. HUVECs were used between passages 3 and 6.
Proliferation assay of HUVEC. The following 25‐bp oligonucleotides of double‐stranded RNA were commercially generated (Invitrogen, Carlsbad, CA, USA): 5′‐UUUGCUGGUAAUUGCGUGGAGGACC‐3′ (sense) and 5′‐GGUCCUCCACGCAAUUACCAGCAAA‐3′ (antisense); YB‐1 small‐interfering RNA (siRNA) #1, 5′‐UGGAUAGCGUCUAUAAUGGUUACGG‐3′ (sense) and 5′‐CCGUAACCAUUAUAGACGCUAUCCA‐3′ (antisense); YB‐1 siRNA #2. HUVECs were seeded into 12‐well plates at a density of 5.0 × 104 cells per well, and transfected with 10 pmol of siRNAs as previously described.( 15 ) Twenty‐four hours after transfection was set as Day 0. The cells were collected with trypsin and counted daily with a Coulter‐type cell size analyzer (CDA‐500; Sysmex Corp., Kobe, Japan).
Flow cytometry. HUVEC (1 × 106 cells) were seeded in 90‐mm plates, transfected with 200 pmol of indicated siRNAs, and cultured. After 72 h, cells were collected, washed twice with ice‐cold PBS with 0.1% BSA, and resuspended in 70% ethanol. After washing twice with ice‐cold PBS, cells were resuspended on PBS with 0.1% BSA, incubated with RNase (Sigma‐Aldrich, St. Louis, MO, USA), and stained with propidium iodide (Sigma‐Aldrich). Cells were analyzed using an EpicsXL‐MCL flow cytometer (Beckman‐Coulter, Miami, FL, USA).
Western blotting. HUVECs (1 × 105 cells) were seeded in 35‐mM plates and transfected with 20 pmol of indicated siRNAs. After 72 h, cells were lysed in a lysis buffer comprising 50 mM Tris/HCl (pH 8.0), 1 mM EDTA, 120 mM NaCl, 0.5% Nonidet P‐40, and 1 mM PMSF. The lysates were centrifuged at 21 000 g for 10 min at 4°C and the supernatants (25 μg) were separated on 10% (w/v) SDS gels and transferred to a PVDF microporous membrane (Millipore, Bedford, MA, USA). The membrane was immunoblotted with anti‐YB‐1 antibody( 14 ) at a 1:10 000 dilution, or with an anti‐β‐actin antibody (A5441; Sigma‐Aldrich) at a 1:10 000 dilution, for 1 h, and then incubated with HRP‐conjugated anti‐rabbit IgG or anti‐mouse IgG for 40 min. Detection was preformed using enhanced chemiluminescence (Amersham, Piscataway, NJ, USA). The protein expression levels were assessed numerically using a Multi Gauge version 3.0 (Fujifilm, Tokyo, Japan).
Statistical analysis. Pearson’s correlation was used for statistical analysis, and significance was set at the 5% level.
Results
YB‐1 expression in brain tumors. GBM is a severe disease with a poor prognosis and often reappears.( 16 , 17 , 18 ) We first investigated YB‐1 expression in adult GBM. YB‐1 expression in brain tumors has been reported.( 12 , 19 ) However, immunohistochemical analysis of YB‐1 expression in adult brain tumors has not been previously reported. Immunohistochemistry demonstrated YB‐1 expression in all 26 GBM cases (data not shown). Two typical cases are shown in Figure 1. Glioblastoma cells highly express GFAP, which is a diagnostic biomarker of GBM.( 18 ) No GFAP was observed in vessels, including glomeruloid bodies, which are an assured histological feature of GBM.( 16 , 20 ) Tumor vessels including glomeruloid bodies are well stained by CD34. Interestingly, YB‐1 expression was also found in both tumor and tumor vessels (Fig. 1, row A). As shown in Case 2 (Fig. 1, row B), CD34 expression was found in all vessels, but YB‐1 expression was only found in small tumor vessels around large vessels. Ki‐67‐positive cells were also found in peripheral tumor vessels, indicating that YB‐1 is expressed in angiogenic endothelial cells. The clear presence of YB‐1 expression in endothelial cells was further confirmed under ×400 magnification (Fig. 1, rows C,D).
Figure 1.
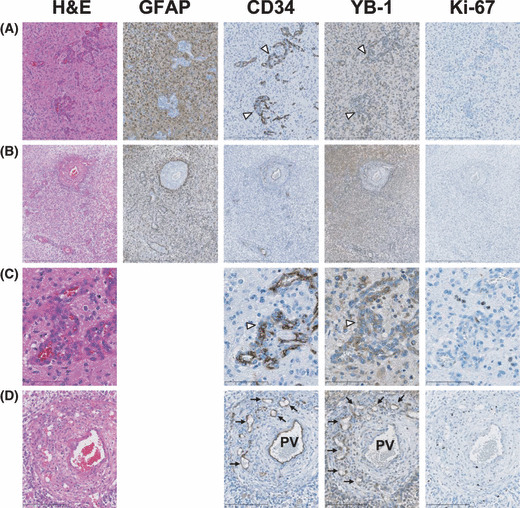
Immunohistochemical staining for glial fibrillary acidic protein (GFAP), CD34, Y‐box binding protein‐1 (YB‐1), and Ki‐67 in two glioblastoma cases. Consecutive paraffin sections from Case 1 (magnification: [A] ×40, [C] ×400) and Case 2 (magnification: [B] ×40, [D] ×400) were stained with H&E and immunohistochemically stained with anti‐GFAP, CD34, YB‐1, and Ki‐67 antibodies. Glomeruloid bodies are indicated by open‐arrow heads (A,C). Tumor vessels sprouting from pre‐existing vessels are indicated by arrows (D). PV indicates pre‐existing vessels (D).
YB‐1 expression in other solid tumors. Many studies have shown that YB‐1 is highly expressed in various solid tumors such as those of the breast, lung, colon, ovary, and soft tissues.( 8 ) However, there have been no descriptions regarding YB‐1 expression in tumor vessels. We investigated YB‐1 expression in four solid tumors derived from esophagus, lung, stomach, and colon. We examined several cases for each tumors and present typical immunohistochemical staining in Figure 2. High levels of YB‐1 expression were reproducibly observed in the cancer cells of these solid tumors (Fig. 2, Tum). Next, we carefully observed YB‐1 expression in the desmoplastic stroma region, which is composed of fibroblasts, inflammatory cells, extracellular matrix, and blood vessels. We found that YB‐1 was significantly expressed in the endothelial cells of tumor vessels (Fig. 2, Des and Fig. 3), which were also positively stained by the anti‐CD34 antibody (2, 3). Double immunohistochemical staining further confirmed that CD34‐positive cells were also positive for YB‐1 in GBMs and two solid tumors (Fig. 4).
Figure 2.
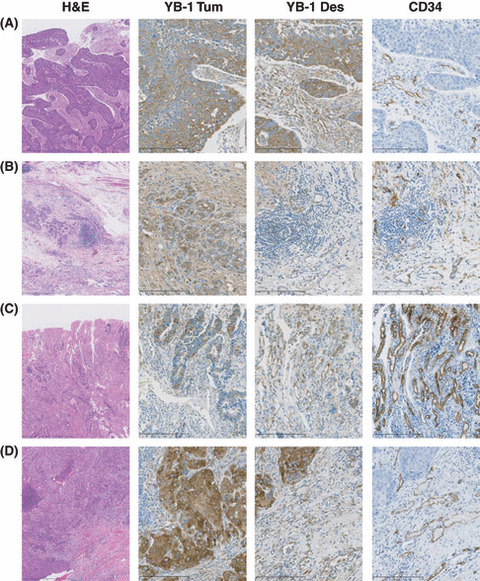
Immunohistochemical staining of Y‐box binding protein‐1 (YB‐1) and CD34 in various solid tumors. (A) Esophageal squamous cell carcinoma; (B) gastric adenocarcinoma; (C) colon adeno‐carcinoma; and (D) lung adenocarcinoma. These carcinomas were stained with H&E (magnification: ×40) and immunohistochemically stained with anti‐YB‐1 and CD34 antibodies (magnification: ×100). Tum and Des indicate tumor lesion and desmoplastic stroma, respectively.
Figure 3.
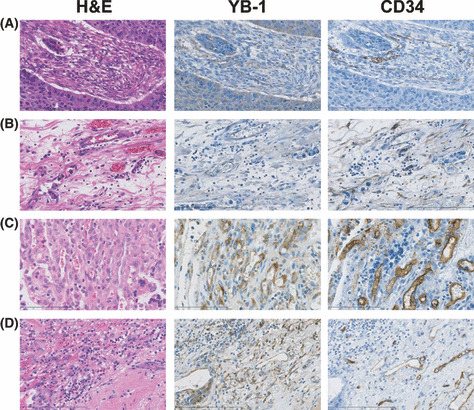
Immunohistochemical staining of Y‐box binding protein‐1 (YB‐1) and CD34 in desmoplastic stroma. H&E staining and immunohistchemical staining sections of Figure 2 (YB‐1 Des and CD34) were examined at ×400 magnification. (A–D) Esophageal squamous cell carcinoma, gastric adenocarcinoma, colon adenocarcinoma, and lung adenocarcinoma, respectively.
Figure 4.
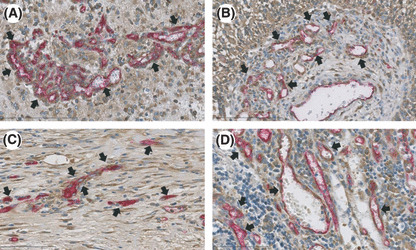
Y‐box binding protein‐1 (YB‐1) and CD34 expression using double immunohistochemical staining. (A) Glomeruloid bodies of glioblastoma multiforme (GBM); (B) sprouting capillaries in pre‐existing vessel of GBM; (C) esophageal squamous cell carcinoma; (D) colon adenocarcinoma. 3,3′‐Diaminobenzidine (DAB) was used as chromogen for YB‐1 staining (brown color) and Vulcan fast red was used for CD34 staining (red color). Arrows indicate neovessels with endothelial cells positively stained with both YB‐1 and CD34.
YB‐1 expression and nontumorigenic angiogenesis. Tumor‐specific inhibition of angiogenesis is a most important therapeutic strategy for cancer patients. To investigate whether YB‐1 could be used as a tumor‐specific endothelial marker, we examined YB‐1 expression in endothelial cells of normal tissue and granulation tissue with inflammation. As shown in Figure 5, YB‐1 expression was weakly detected in endothelial cells from fetal tissue, newborn lung, and placenta, which are normal, angiogenic tissues. On the other hand, YB‐1 was expressed, albeit weakly, in the endothelial cells of the inflammatory area (Fig. 6).
Figure 5.
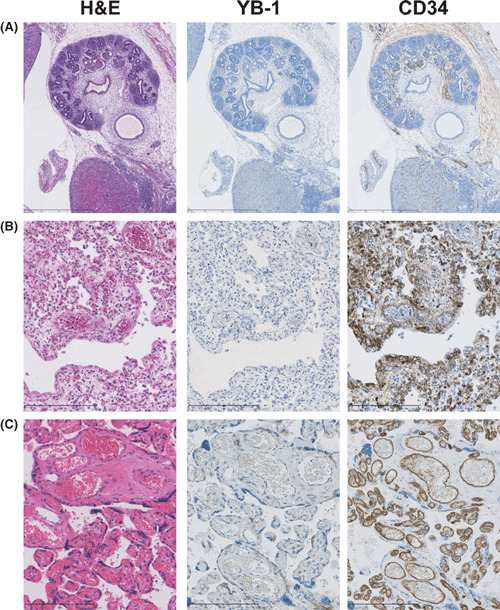
Immunohistochemical staining of Y‐box binding protein‐1 (YB‐1) and CD34 in normal tissues. (A) Renal tissue obtained from an aborted fetus (10 weeks of gestation; magnification: ×40), (B) fetal (25 weeks of gestation; magnification: ×200) lung tissue obtained at autopsy and (C) placental tissue (35 weeks of gestation; magnification: ×200). These normal tissues were stained with H&E and immunohistochemically stained with anti‐YB‐1 and CD34 antibodies.
Figure 6.
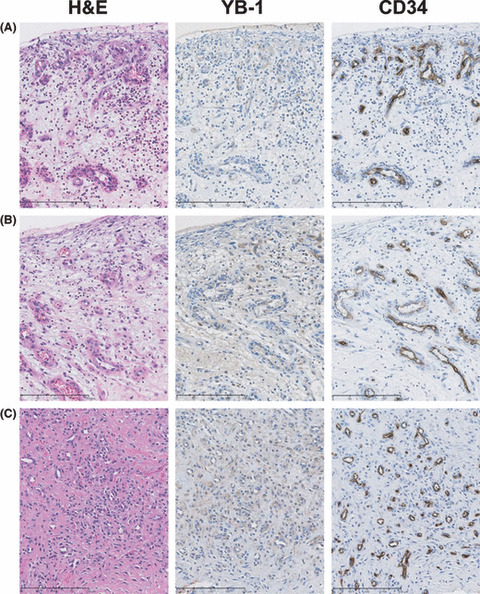
Immunohistochemical staining of Y‐box binding protein‐1 (YB‐1) and CD34 in inflammatory granulation tissues. (A) Uterine cervical mucosa; (B) vaginal mucosa; and (C) articular synovial tissue. These inflammatory granulation tissues were stained with H&E and immunohistochemically stained with anti‐YB‐1 and CD34 antibodies (magnification: ×200).
YB‐1‐dependent growth of HUVEC. No YB‐1 expression was observed in the umbilical veins at multiple gestational stages (20, 23, 24, 26, 27, 37, 38 or 39 weeks) (data not shown). However, we have previously shown that YB‐1 is expressed in HUVEC, which are derived from normal tissues.( 21 ) We next investigated whether YB‐1 expression is involved in the growth of HUVEC. A specific siRNA for YB‐1 effectively down‐regulated YB‐1 expression by 14–27% compared with the control siRNA (Fig. 7A,B). As shown in Figure 7(C), down‐regulation of YB‐1 significantly abolished the growth factor‐dependent growth of HUVEC. The analysis of cell cycle profiles was done after transfection with a YB‐1‐specific siRNA #2 (Fig. 7D). An increase of G1‐phase cells and decrease of S‐phase cells were observed, but a marked increase of sub‐G1 population of cells was not, indicating that YB‐1 knockdown induces G1 cell cycle arrest, but does not induce apoptosis in HUVEC.
Figure 7.
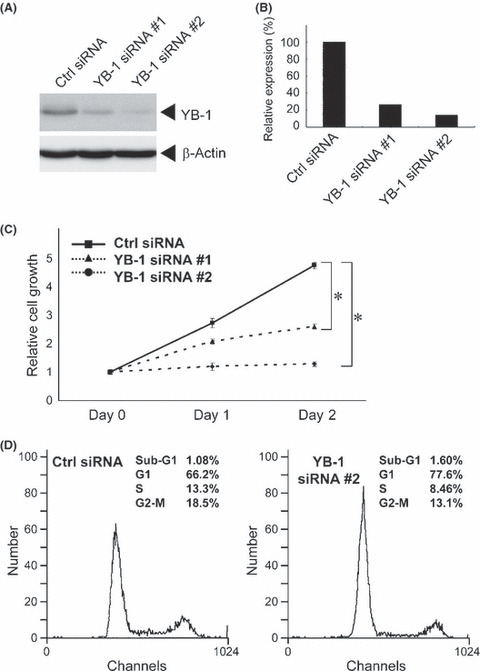
Y‐box binding protein‐1 (YB‐1)‐dependent growth of human umbilical endothelial cells. (A) 1 × 105 human umbilical vein endothelial cell (HUVEC) were transiently transfected with 20 pmol of control (Ctrl) scramble siRNA or YB‐1 siRNA. After 72 h, whole cell lysates (25 μg) were subjected to SDS‐PAGE, and western blotting was performed using anti‐YB‐1 or anti‐β‐actin antibodies. (B) YB‐1 expression levels were normalized to β‐actin. YB‐1 expression in cells transfected with control siRNA was set to 100%. (C) 5 × 104 HUVECs were transiently transfected with 10 pmol of control (Ctrl) siRNA or YB‐1 siRNAs. Twenty‐four hours after transfection was set as Day 0. Cells were counted after the indicated number of days. The results were normalized to cell numbers at Day 0. Points represent the mean of at least three independent experiments; bars show the SD. *P < 0.001. (D) HUVECs were treated with indicated siRNAs. After 72 h, cells were collected and used for propidium iodide staining. DNA contents in single cell were measured.
Discussion
Chemotherapy is one of the main therapeutic strategies for solid tumors. Anticancer agents comprise a wide variety of chemicals that interfere with the basic pathways of cell growth.( 22 , 23 ) Molecular‐targeted therapies for solid tumors are a new paradigm for cancer chemotherapy, but their usefulness is still limited due to a cytostatic response or resistance. In other words, drug resistance is a barrier to not only traditional chemotherapy, but also to molecular‐targeted therapy. The tumor microenvironment is involved in the induction, selection, and rapid growth of cancer cells,( 24 , 25 ) and it is thought to be a promising target for solid tumor treatment.( 26 ) Angiogenesis is crucial for the growth of all solid tumors except pancreatic cancer.( 1 , 27 , 28 ) Anti‐angiogenic cancer therapies are a promising strategy to avoid drug resistance, but their efficacy is still limited. The combination of anti‐angiogenic agents and chemotherapy provides therapeutic synergy and has improved the clinical outcome for solid tumors.( 3 ) However, many questions remain as to how to maximize the efficiency.
YB‐1 is an important protein for brain development and Yb‐1 null‐mutant mice show embryonic lethality.( 29 ) YB‐1 has multiple biological functions in both the nucleus and the cytoplasm.( 7 , 30 ) YB‐1 expression closely correlates with cell proliferation and it is up‐regulated in cancer cells; overexpression of YB‐1 is a hallmark of cancer. Up‐regulation of YB‐1 is observed in various solid tumors and its expression level inversely correlates with clinical outcome.( 12 , 13 , 14 ) Nuclear expression of YB‐1 is significantly correlated with poor prognosis in cancer patients.( 8 , 13 ) YB‐1 also has oncogenic potential itself.( 31 ) Here, we showed that YB‐1 is highly expressed in brain tumors. We also found that YB‐1 is significantly expressed in glomeruloid bodies. These are not tumor cells because the cells in glomeruloid bodies express CD34 but not GFAP. Instead, YB‐1 is expressed in endothelial cells, as shown in Figure 1. We have previously shown that YB‐1 expression correlates with chemoresistance.( 11 ) Glioma‐associated endothelial cells have also been shown to be chemoresistant,( 32 ) suggesting that YB‐1 expression in glomeruloid bodies might be responsible for the chemoresistance. In addition, endothelial cells significantly express YB‐1 in tumor vessels in the desmoplastic stroma of various solid tumors (2, 3, 4). Almost no or low expression of YB‐1 was observed in angiogenic endothelial cells in normal tissues and regions exhibiting inflammation (5, 6). The differences in angiogenic stimuli between normal/inflammatory angiogenic tissues and tumor‐associated stroma may account for difference in the level of YB‐1 expression. Various angiogenic stimuli may differentially induce YB‐1 expression.
Nuclear YB‐1 increases expression of several genes involved in cell growth such as epidermal growth factor receptor.( 11 , 33 ) This indicates that nuclear YB‐1 functions as a transcription factor to accelerate cancer cell growth. We have recently reported that YB‐1 positively regulates CDC6 expression and promotes cell cycle progression in human cancer cell lines.( 34 ) Although immunohistochemical analysis shows that YB‐1 expression is mainly observed in the cytoplasm of tumor‐associated endothelial cells, it is possible that cytoplasmic YB‐1 may translocate into nuclei at a specific phase of cell cycle to promote growth in normal cells. Further studies are required to provide insight into YB‐1 function in normal cells. Silencing of YB‐1 expression induces a G1‐phase cycle arrest of tumor cell growth, suggesting that YB‐1 represents a promising molecular target for cancer therapy.( 9 ) Here, we show that down‐regulation of YB‐1 also induces G1 cell cycle arrest and significantly reduces the growth rate of human endothelial cells (Fig. 7). These data indicate that YB‐1 is a novel and specific biomarker of proliferative endothelial cells in tumor vessels. Because YB‐1 possesses pleiotropic functions, it may be difficult to design compounds to specifically inhibit YB‐1 activity. Nevertheless, therapeutic experiments using xenografts and anti‐RNA drugs are warranted for validation of YB‐1 as a molecular target.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AP
Alkaline phosphatase
- BSA
Bovine serum albumin
- GBM
Glioblastoma multiforme
- GFAP
Glial fibrillary acidic protein
- H&E
Hematoxylin–eosin
- HRP
Horseradish peroxidase
- HUVEC
Human umbilical vein endothelial cell
- PBS
Phosphate‐buffered saline
- PMSF
Phenylmethylsulfonyl fluoride
- PVDF
Polyvinylidene difluoride
- VEGF
Vascular endothelial cell growth factor
- YB‐1
Y‐box binding protein‐1
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Scientific Research from the Ministry for Education, Culture, Sports, Science and Technology of Japan (17016075), UOEH Grant for Advanced Research, The Vehicle Racing Commemorative Foundation. We thank Ms H. Isagai for technical assistance.
References
- 1. Folkman J. Angiogenesis. Annu Rev Med 2006; 57: 1–18. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 4: 273–86. [DOI] [PubMed] [Google Scholar]
- 3. Ellis ML, Hicklin JD. VEGF‐targeted therapy: mechanisms of anti‐tumour activity. Nat Rev Cancer 2008; 8: 579–91. [DOI] [PubMed] [Google Scholar]
- 4. Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther 2008; 7: 3670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carson‐Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res 2001; 61: 6649–55. [PubMed] [Google Scholar]
- 6. Croix BS, Rago C, Velculescu V et al. Gene expressed in human tumor endothelium. Science 2000; 289: 1197–202. [DOI] [PubMed] [Google Scholar]
- 7. Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y‐box‐binding protein, YB‐1. Bioessays 2003; 25: 691–8. [DOI] [PubMed] [Google Scholar]
- 8. Kuwano M, Oda Y, Izumi H et al. The role of nuclear Y‐box binding protein 1 as a global marker in drug resistance. Mol Cancer Ther 2004; 3: 1485–92. [PubMed] [Google Scholar]
- 9. Shiota M, Izumi H, Onitsuka T et al. Twist promotes tumor cell growth through YB‐1 expression. Cancer Res 2008; 68: 98–105. [DOI] [PubMed] [Google Scholar]
- 10. Torigoe T, Izumi H, Ishiguchi H et al. Cisplatin resistance and transcription factors. Curr Med Chem Anticancer Agents 2005; 5: 15–27. [DOI] [PubMed] [Google Scholar]
- 11. Kohno K, Uchiumi T, Niina I et al. Transcription factors and drug resistance. Eur J Cancer 2005; 41: 2577–86. [DOI] [PubMed] [Google Scholar]
- 12. Faury D, Nantel A, Dunn SE et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB‐1 expression in tumors. J Clin Oncol 2007; 25: 1196–208. [DOI] [PubMed] [Google Scholar]
- 13. Oda Y, Ohishi Y, Saito T et al. Nuclear expression of Y‐box‐binding protein‐1 correlates with P‐glycoprotein and topoisomerase IIα expression, and with poor prognosis in synovial sarcoma. J Pathol 2003; 199: 251–8. [DOI] [PubMed] [Google Scholar]
- 14. Ohga T, Koike K, Ono M et al. Role of the human Y box‐binding protein YB‐1 in cellular sensitivity to the DNA‐damaging agents Cisplatin, Mitomycin C and Ultraviolet Light. Cancer Res 1996; 56: 4224–8. [PubMed] [Google Scholar]
- 15. Igarashi T, Izumi H, Uchiumi T et al. Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene 2007; 26: 4749–60. [DOI] [PubMed] [Google Scholar]
- 16. Kleihues P, Nakazato Y, Burger PC et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System, 4th edn. Lyon: IARC Press, 2007; 33–49. [Google Scholar]
- 17. Ohgaki H, Dessen P, Jourde B et al. Genetic pathways to glioblastoma: a population‐based study. Cancer Res 2004; 64: 6892–9. [DOI] [PubMed] [Google Scholar]
- 18. Jung CS, Foerch C, Schänzer A et al. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain 2007; 130: 3336–41. [DOI] [PubMed] [Google Scholar]
- 19. Gao Y, Fotovati A, Lee C et al. Inhibition of Y‐box binding protein‐1 shows the growth of glioblastoma multiforme and sensitizes to temozolomide independent O6‐methylguanine‐DNA methyltransferase. Mol Cancer Ther 2009; 8: 3276–84. [DOI] [PubMed] [Google Scholar]
- 20. Sundberg C, Nagy JA, Brown LF et al. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor‐164 gene delivery. Am J Pathol 2001; 158: 1145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukuda T, Ashizuka M, Nakamura T et al. Characterization of the 5′‐untranslated region of YB‐1 mRNA and autoregulation of translation by YB‐1 protein. Nucleic Acids Res 2004; 32: 611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004; 10: 789–99. [DOI] [PubMed] [Google Scholar]
- 23. Blume‐Jensen P, Hunter T. Oncogenic kinase signalling. Nature 2001; 411: 355–65. [DOI] [PubMed] [Google Scholar]
- 24. Liotta LA, Kohn EC. The microenvironment of the tumour‐host interface. Nature 2001; 411: 375–59. [DOI] [PubMed] [Google Scholar]
- 25. Borovski T, Verhoeff JJ, Ten Cate R et al. Tumor microvasculature supports proliferation and expansion of glioma‐propagating cells. Int J Cancer 2009; 125: 1222–30. [DOI] [PubMed] [Google Scholar]
- 26. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 27. Chu GC, Kimmelman AC, Hezel AF et al. Stromal biology of pancreatic cancer. J Cell Biochem 2007; 101: 887–907. [DOI] [PubMed] [Google Scholar]
- 28. Olive KP, Jacobetz MA, Davidson CJ et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324: 1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uchiumi T, Fotovati A, Sasaguri T et al. YB‐1 is important for an early stage embryonic development: neural tube formation and cell proliferation. J Biol Chem 2006; 281: 40440–9. [DOI] [PubMed] [Google Scholar]
- 30. Kuwano M, Uchiumi T, Hayakawa H et al. The basic and clinical implications of ABC transporters, Y‐box‐binding protein‐1 (YB‐1) and angiogenesis‐related factors in human malignancies. Cancer Sci 2003; 94: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergmann S, Royer‐Pokora B, Fietze E et al. YB‐1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res 2005; 65: 4078–87. [DOI] [PubMed] [Google Scholar]
- 32. Virrey JJ, Golden EB, Sivakumar W et al. Glioma‐associated endothelial cells are chemoresistant to temozolomide. J Neurooncol 2009; 95: 13–22. [DOI] [PubMed] [Google Scholar]
- 33. Wu J, Lee C, Yocom D et al. Disruption of the Y‐box binding protein‐1 results in suppression of the epidermal growth factor receptor and HER‐2. Cancer Res 2006; 68: 4872–79. [DOI] [PubMed] [Google Scholar]
- 34. Basaki Y, Taguchi K, Izumi H et al. Y‐box binding protein‐1 promotes cell cycle progression through CDC6‐dependent pathway in human cancer cells. Eur J Cancer 2010; 46: in press. [DOI] [PubMed] [Google Scholar]


