Abstract
Gene silencing associated with aberrant DNA methylation of promoter CpG islands is one mechanism through which several genes may be inactivated in human cancers. Cyclin D2, a member of the D‐type cyclins, implicated in cell cycle regulation, differentiation and malignant transformation, is inactivated due to aberrant DNA methylation in several human cancers. In the present study, we examined the promoter methylation status and expression of Cyclin D2 in human epithelial ovarian cancer, and then determined the relationship between methylation status and various clinicopathological variables. Twelve ovarian cancer cell lines and 71 surgical specimens were examined by methylation‐specific polymerase chain reaction and quantitative reverse transcription–polymerase chain reaction to evaluate the methylation status and expression of the Cyclin D2 gene. The relationship between methylation status and various clinicopathological variables was evaluated using statistical analysis. Aberrant methylation of Cyclin D2 was present in five of 12 ovarian cancer cell lines and 16 of 71 primary ovarian cancer tissues. In five cell lines with methylation, expression of the Cyclin D2 gene tended to be lower than in cell lines without methylation. In ovarian cancer tissues, methylation bands were detected in 16 of 71 cases. The methylation status of Cyclin D2 was associated with advanced stage and a residual tumor size (>2 cm) (P = 0.027 and P = 0.031, respectively). Based on univariate analysis, patients with aberrant methylation of the Cyclin D2 promoter had a significantly worse chance of disease‐free survival than those without methylation (P = 0.021). Our results suggest that aberrant promoter methylation of the Cyclin D2 gene is significantly associated with patient prognosis in epithelial ovarian cancer. (Cancer Sci 2007; 98: 380 –386)
Epithelialovarian cancer is the most common and deadliest gynecological malignancy in developed countries. Early stages of ovarian cancer are generally asymptomatic and difficult to detect. By the time clinical diagnosis is made, most patients have widespread tumor dissemination.( 1 ) Despite a high response rate to first‐line chemotherapy, the prognosis of these women is poor, with an overall 5‐year survival rate of only 10–20%.( 1 , 2 )
Epigenetic alterations, changes that affect gene expression but not the gene sequence itself, are believed to be one mechanism by which tumor suppressor genes are inactivated in human cancers.( 3 , 4 ) In particular, hypermethylation of cytosine residues in CpG islands leads to heritable gene silencing via the formation of a repressive chromatin structure.( 5 , 6 ) Studies of DNA hypermethylation in human ovarian cancer have identified some key genes as targets for epigenetic downregulation, including some hormone receptors,( 7 ) cytokines, cell signaling intermediates, adhesion molecules,( 8 ) DNA damage checkpoint genes,( 9 ) and regulators of the cell cycle.( 10 ) The cell cycle regulators, notably the cyclins, have the potential to function as oncogenes when regulated inappropriately.
The cyclins are a family of proteins that dictate transitions between phases of the cell cycle by regulating the activity of their downstream effectors, the cyclin‐dependant kinases (cdk). The D‐type cyclins, D1, D2 and D3, play a critical role in early checkpoint regulation of the G1 phase of the cell cycle. They activate cdk4 and cdk6, leading to the phosphorylation of the retinoblastoma tumor suppressor protein (Rb). This, in turn, dissociates Rb from the transcription factor E2F, thereby permitting DNA transcription. Given the critical role of the D‐type cyclins in cell cycle regulation, their abnormal or untimely expression could disrupt the normal cell cycle, resulting in cell proliferation.( 11 ) In fact, Cyclin D1 is considered by some to be a putative protooncogene, as it is overexpressed in a number of tumor types, including breast cancer, thyroid carcinoma, stomach cancer and lymphomas.( 12 ) Aberrant expression of Cyclin D2 has also been demonstrated in human ovarian granulose cell tumors and testicular germ cell tumor cell lines.( 13 )
Although well known for their proliferation‐promoting activity, the D‐type cyclins (notably D2) also have growth‐inhibitory effects. Cyclin D2 has been shown to be dramatically upregulated under conditions of growth arrest in human and murine fibroblasts. Furthermore, transient overexpression of Cyclin D2 efficiently inhibits cell cycle progression and DNA synthesis. This suggests that an alternative role for Cyclin D2 may be to promote exiting from the cell cycle and maintenance of a non‐proliferative state.( 14 ) The expression of Cyclin D2 is frequently lost in human breast cancers, gastric cancers, lung cancers and ovarian granulose cell tumors. This loss of expression is the result of promoter hypermethylation.( 10 , 15 , 16 , 17 , 18 )
In the present study, we examined the promoter methylation status and gene expression of Cyclin D2 in human epithelial ovarian cancer cell lines. We also evaluated the correlation between methylation status of the Cyclin D2 promoter and various clinicopathological parameters in patients with epithelial ovarian cancer.
Materials and Methods
Cell lines. Twelve ovarian carcinoma cell lines were used. OVCAR3, SKOV3 (both adenoarcinomas), Caov3, OV90 (both serous adenocarcinoma), TOV21G, ES2 (both clear cell adenocarcinoma) and TOV112D (endometrioid adenocarcinoma) were purchased from American Type Culture Collection. JHOS2, JHOS3, HTOA (all serous adenocarcinoma), OMC3 (mucinous adenocarcinoma) and JHOC5 (clear cell adenocarcinoma) were purchased from Riken Cell Bank (Tsukuba). Cell lines were maintained in DMEM/F12 medium (Invitrogen), supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen), and incubated in a 5% CO2 atmosphere at 37°C.
Surgical specimens and clinical data. The research protocol was approved by the Ethics Committee of Tohoku University Graduate School of Medicine, Sendai, Japan. We examined 71 ovarian cancer specimens obtained from patients treated between 1988 and 2002 at Tohoku University Hospital, Sendai, Japan. All specimens were retrieved from the surgical pathology files at Tohoku University Hospital. Informed consent was obtained from each patient. Specimens were fixed in 10% formalin and embedded in paraffin. Patient age, performance status on admission, histology, stage, grade, residual tumor after primary surgery, and overall survival were obtained from a chart review. The median follow‐up time for patients was 59 months (range, 4–120 months). Performance status was defined according to the WHO criteria.( 19 ) Histology, stage and grading followed the FigO criteria.( 20 ) Residual tumor was defined as the amount of unresectable tumor left following primary volume reductive surgery. Optimal volume reduction was achieved when the residual tumor was less than 2 cm. Patients with a residual tumor greater than 2 cm were considered to have suboptimal volume reduction. Overall survival was calculated from the time of initial surgery to death or the date of the last contact. Survival times of patients still alive or lost to follow‐up were censored as of December 2002.
An ovarian tissue obtained from a 50‐year‐old woman who had received surgical treatment for benign uterine tumor was used as a normal ovarian tissue for methylation‐specific polymerase chain reaction (MSP) and reverse transcription–polymerase chain reaction (RT‐PCR).
Methylation‐specific polymerase chain reaction. The methylation status of the samples was assessed using MSP as described previously.( 21 ) Genomic DNA from ovarian cancer cell lines was extracted using the AquaPure Genomic DNA kit (Bio‐Rad). Genomic DNA from ovarian tumor specimens was extracted from paraffin blocks. For each tissue, the presence of carcinoma was confirmed on a H&E stained section. For DNA extraction, three 5‐µm tissue sections from the same block were scraped from the slide and treated with Dexpat (Takara). The quality and integrity of the DNA were evaluated in terms of the A260/280 ratio. Genomic DNA (1 µg) was treated with sodium bisulfite using a CpGenome DNA modification kit (Intergen) according to the manufacturer's protocol. Amplification was conducted in a 20‐µL reaction volume containing 2 µL of 10× ExTaq buffer, 1.5 µL of 2.5 mM MgCl2, 1 mM of each primer, 1.5 mL of 2.5 mM dNTPs, and 1 unit of Takara ExTaq polymerase (Takara). The reaction was cycled for 40 cycles, each of which consisted of denaturation at 95°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 45 s, followed by a 7‐min extension at 72°C. The primers used were 5′‐AGAGTATGTGTTAGGGTTGATT‐3′ and 5′‐ACATCCTCACCAACCCTCCA‐3′ (−1431 to −1326, 106‐bp) for the unmethylated reaction (U), and 5′‐GGCGGATTTTATCGTAGTCG‐3′ and 5′‐CTCCACGCTCGATCCTTCG‐3′ (−1404 to −1304, 101‐bp) for the methylated reaction (M).( 18 ) Universal unmethylated human genomic DNA (Intergen) was used as a positive control for the unmethylated reaction. Universal methylated human male genomic DNA (Intergen) was used as a positive control for the methylated reaction. Reaction products were separated by electrophoresis on 3% agarose gel, stained with ethidium bromide, and visualized under ultraviolet light.
Quantitative RT‐PCR. Total RNA was isolated from cells by phenol–chloroform extraction using Isogen reagent (Nippon Gene). RNA was treated with RNase‐free DNase (Roche Diagnostics; 1 µg/µL) for 2 h at 37°C, followed by heat inactivation at 65°C for 10 min. Total RNA (5 µg) was reversed transcribed using the Superscript II first‐strand synthesis system (Invitrogen) with random hexamers according to the manufacturer's protocol. Quantitative polymerase chain reaction (PCR) was carried out using an iCycler system (Bio‐Rad). For the determination of Cyclin D2 cDNA content, a 25‐µL reaction mixture consisting of 23 µL iQSYBR Green MasterMix, 1 µL of each primer and 1 µL of cDNA template was cycled as follows: 2‐min denaturation at 90°C, 30‐s annealing at either 60°C (for Cyclin D2) or 62°C (for β‐actin), and 1.5‐min extension at 72°C. Primers for PCR reactions were as follows: Cyclin D2‐F, 5′‐TACTTCAAGTGCGTGCAGAAGGAC‐3′ and Cyclin D2‐R, 5′‐TCCCACACTTCCAGTTGCGATCAT‐3′;( 22 ) and β‐actin‐F, 5′‐CCAACCGCGAGAAGATGAC‐3′ and β‐actin‐R, 5′‐GGAAGGAAGGCTGGAAGAGT‐3′.( 23 )β‐Actin primers were utilized as an internal positive control and Cyclin D2 expression level was calculated by dividing the quantity obtained for Cyclin D2 by the quantity obtained for β‐actin. Two independent RT‐PCR reactions were carried out for each sample.
5‐Aza‐2′‐deoxycitidine and trichostatin A treatment. To confirm that epigenetic change contributed to loss of Cyclin D2 gene expression, we assessed the effect of 5‐aza‐2′‐deoxycitidine (5azaC) (Sigma), a demethylating agent, and trichostatin A (TSA) (Sigma), a histone deacetylase inhibitor, on Cyclin D2 mRNA expression and cell growth of ovarian cancer cell lines by quantitative RT‐PCR and cell count, respectively.
Ovarian cancer cell lines (OMC3, OVCAR3, JHOS2, JHOC5 and SKOV3) were cultured at a point of 70% confluence in 10‐cm cell dishes. They were treated with 1.0 µM 5azaC for 3 or 5 days. They were also treated with 0.5 µM TSA.( 24 , 25 ) We set up TSA treatment times of 4, 8, 16 and 32 h, and the treatments for 8 and 16 h appeared the most effective for gene expression compared to control culture (data not shown). Total RNA was prepared at each time point and the expression of Cyclin D2 mRNA was analyzed by quantitative RT‐PCR. Furthermore, we investigated the effects of these chemical agents on cell growth of ovarian cancer cell lines by cell count at each time point.
Immunohistochemistry. For the purpose of investigating cell proliferation we examined the immunohistochemical expression of Ki‐67 in ovarian cancer tissue. Immunohistchemical analysis was carried out with the streptavidin–biotin amplification method using the NX/ES IHC system (Ventana Medical Systems). Monoclonal antibody for Ki‐67 (MIB‐1) was purchased from DAKO. For antigen retrieval, the slides ware heated in an autoclave at 120°C for 5 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dyhydrate [pH 6.0]). The dilution of primary antibody was 1:50. Scoring of Ki‐67 in carcinoma cells was counted independently by two of the authors (M. S. and J. A.), and the percentage of immnunoreactivity in at least 500 carcinoma cells (i.e. the labeling index) was determined.
Statistical analysis. Statistical analysis was carried out using Stat View 5.0 software (SAS Institute). The correlation between the Cyclin D2 mRNA expression level and methylation status was assessed using the Mann–Whitney U‐test. The statistical significance between methylation status and various clinicopathological parameters was evaluated using Friedman's χ2 r‐test and the Mann–Whitney U‐test. A univariate analysis of prognostic significance for prognostic factors was carried out using the log‐rank test after each survival curve was obtained by the Kaplan–Meier method. Multivariate analysis was carried out using the Cox regression model to evaluate the predictive power of each variable independently. All patients who could be assessed were included in the intention‐to‐treat analysis. A result was considered significant when the P‐value was less than 0.05.
Results
Methylation status of the Cyclin D2 gene in ovarian cancer cell lines and tissues. Bands corresponding to methylated Cyclin D2 were detected in five of 12 cell lines, three of which also contained the unmethylated band, as shown in Fig. 1. The methylated band was detected in two of five cell lines derived from serous adenocarcinoma (Caov3, OV90), in one of three cell lines from clear cell carcinoma (ES2), in the one mucinous adenocarcinoma (OMC3), but not in the endometrioid adenocarcinoma. The normal ovarian tissue was negative for the methylated band. The methylated band was detected in 16 of the 71 surgical specimens (6/26 serous, 4/23 clear cell, 3/15 endometrioid and 3/7 mucinous adenocarcinoma), as shown in Table 1.
Figure 1.

Methylation status of the Cyclin D2 gene in ovarian cancer cell lines and a normal ovarian tissue. The 101‐bp bands in the ‘Methylated’ lanes indicate the presence of methylated alleles of the Cyclin D2 gene. The 106‐bp bands in the ‘Unmethylated’ lanes correspond to the unmethylated alleles. Methylation status is denoted as follows: +, methylated alleles with or without unmethylated alleles; –, purely unmethylated alleles. M‐DNA, universal methylated human male genomic DNA, was used for positive control of methylated reaction. U‐DNA, universal unmethylated fetal genomic DNA, was used for positive control of unmethylated reaction.
Table 1.
Patient characteristics and cyclin D2 methylation status
| Variable | n | Cyclin D2 methylation | P‐value | ||
|---|---|---|---|---|---|
| + | – | % | |||
| Age (years) | |||||
| <50 | 29 | 8 | 21 | 27.6 | |
| ≥50 | 42 | 8 | 34 | 19 | NS |
| Performance status† | |||||
| 0–1 | 51 | 9 | 42 | 17.6 | |
| 2–4 | 19 | 7 | 12 | 36.8 | NS |
| FigO stage | |||||
| I, II | 35 | 4 | 31 | 2.9 | |
| III, IV | 36 | 12 | 24 | 33.3 | 0.027 |
| Histological type of adenocarcinoma | |||||
| Serous | 26 | 6 | 20 | 23.1 | |
| Endometrioid | 15 | 3 | 12 | 20 | |
| Mucinous | 7 | 3 | 4 | 75 | |
| Clear cell | 23 | 4 | 19 | 17.4 | NS |
| Grade | |||||
| 1 | 24 | 5 | 19 | 20.8 | |
| 2 | 22 | 7 | 15 | 31.8 | |
| 3 | 17 | 3 | 14 | 17.6 | NS |
| Residual tumor size (cm) | |||||
| <2 | 47 | 7 | 40 | 14.9 | |
| ≥2 | 24 | 9 | 15 | 37.5 | 0.031 |
| Ki‐67 labeling index (median) | 21.6 | 23.6 | 20.4 | NS | |
0, asymptomatic and fully active; 1, symptomatic, fully ambulatory, restricted in physically strenuous activity; 2, symptomatic, ambulatory, capable of self‐care, more than 50% of walking hours are spent out of bed; 3, symptomatic, limited self‐care, more than 50% of time is spent in bed, but not bedridden; 4, completely disabled, no self‐care, bedridden.
Expression of the Cyclin D2 gene in ovarian cancer cell lines and normal ovarian tissue. The expression of the Cyclin D2 gene in the cell lines is presented in Fig. 2. Quantitative RT‐PCR was carried out and the ratio of Cyclin D2 to β‐actin was calculated to allow for comparison among the cell lines. The median value of relative Cyclin D2 gene expression in cell lines with methylation (0.015) tended to be lower than that in cell lines without methylation (0.03), although the difference was not significant (P = 0.19, Mann–Whitney U‐test). The expression level of the Cyclin D2 gene in normal ovarian tissue was relatively high compared with ovarian cancer cell lines.
Figure 2.

Expression of the Cyclin D2 gene in ovarian cancer cell lines and normal ovarian tissue. Two independent reverse transcription–polymerase chain reactions were carried out for each sample, and the ratio of Cyclin D2: β‐actin was calculated and normalized with the level of normal ovarian tissue. Methylation status is indicated in the same way as in Fig. 1.
Effects of 5azaC and TSA treatment on methylated cell lines. To confirm that promoter methylation contributed to the loss of Cyclin D2 gene expression, we assessed the effect of 5azaC, a demethylating agent, on Cyclin D2 mRNA expression by quantitative RT‐PCR. OMC3 and OVCAR3 cells, which were positive for the methylated band in MSP, were treated. From MSP analysis OMC3 had only methylated alleles, but OVCAR3 had both methylated and unmethylated alleles. We also assessed the effect of TSA, a histone deacetylase inhibitor, to investigate whether another epigenetic change, histone deacetylation, contributed to the silencing of Cyclin D2 gene expression. Treatment of OMC3 cells with 5azaC for 5 days led to a 2.64‐fold increase in expression (Fig. 3a). Treatment of OVCAR3 cells with 5azaC for 5 days resulted in a 222‐fold increase in expression (Fig. 3b). Treatment with TSA also contributed to re‐expression of the Cyclin D2 gene in OMC3 and OVCAR3 cells (2.3‐fold and 119‐fold, respectively) (Fig. 3). These results suggested that the decreased expression of Cyclin D2 in these cell lines was related to epigenetic change, including DNA methylation or histone deacetylation.
Figure 3.
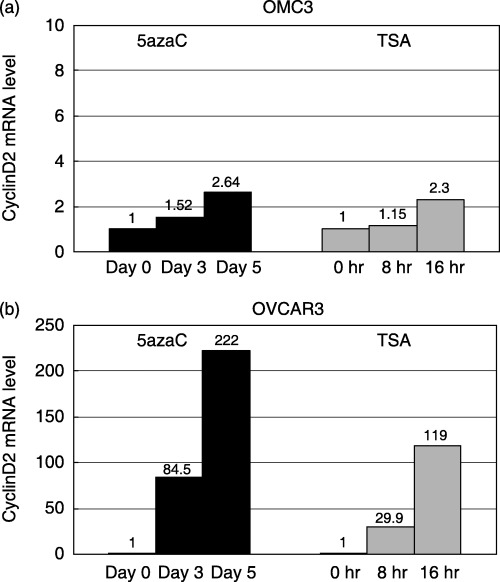
Expression level of the Cyclin D2 gene as determined by quantitative reverse transcription–polymerase chain reaction in OMC3 and OVCAR3 cells following treatment with (a) 5‐aza‐2′‐deoxycitidine (5azaC) or (b) trichostatin A (TSA). The ratio of Cyclin D2 : β‐actin was calculated and normalized with the level before treatment.
The effects of 5azaC and TSA on cell growth are summarized in Fig. 4. Compared with cell growth in control culture, cell growth with 5azaC or TSA treatment was suppressed in each culture. These chemical agents resulted in inhibition of cell growth in these ovarian cancer cell lines simultaneous with re‐expression of the Cyclin D2 gene.
Figure 4.

Cell number of OMC and OVCAR3 cells following treatment with (a) 5‐aza‐2′‐deoxycitidine (5azaC) or (b) trichostatin A (TSA). *Control treatment with medium alone.
Effects of 5azaC and TSA treatment on unmethylated cell lines. In the MSP and quantitative RT‐PCR analyses, expression of the Cyclin D2 gene was decreased in some cell lines without promoter methylation. We assessed the effect of 5azaC or TSA treatment in these cell lines (JHOS2, JHOC5 and SKOV3) to investigate the participation of epigenetic change in the silencing of this gene. Treatment of JHOS2 cells with TSA resulted in higher re‐expression than treatment with 5azaC (Fig. 5a). Treatment of JHOC5 cells with TSA for 16 h resulted in an 84.4‐fold increase in expression, and treatment with 5azaC also led to a 137‐fold increase in expression (Fig. 5b). As for SKOV3 cells, treatment with TSA did not increase the expression of this gene. These results suggest that histone deacetylation may contribute to silencing of the Cyclin D2 gene in JHOS2 and JHOC5 cells, but not in SKOV3.
Figure 5.

Expression level of the Cyclin D2 gene as determined by quantitative reverse transcription–polymerase chain reaction in (a) JHOS2, (b) JHOC5 and (c) SKOV3 cells following treatment with 5‐aza‐2′‐deoxycitidine (5azaC) or trichostatin A (TSA). The ratio of Cyclin D2 : β‐actin was calculated and normalized with the level before treatment.
Correlation between clinicopathological parameters and methylation status of Cyclin D2 in epithelial ovarian cancer. The clinicopathological parameters relative to the methylation status of Cyclin D2 are presented in Table 1. Methylation status was significantly associated with advanced stage and residual tumor size >2 cm. There was no association between methylation status and age, performance status, histological type, histological grade or Ki‐67 labeling index
The results of the univariate analysis of prognostic significance for each variable with respect to survival are summarized in 2, 3. Of the clinicopathological parameters evaluated, performance status, stage, histological grade and residual tumor size were significantly associated with disease‐free and overall survival. The methylation status of Cyclin D2 was significantly associated with disease‐free survival; the cases with methylation had significantly worse rates of disease‐free survival than those without methylation (Fig. 6; P = 0.021). With regard to overall survival, methylated cases had a worse prognosis than unmethylated cases, but the difference was not significant (Fig. 7; P = 0.063). In multivariate analysis, methylation status of cyclin D2 turned out not to be an independent prognostic factor (data not shown).
Table 2.
Univariate analysis of disease‐free survival
| Variable | P‐value |
|---|---|
| Cyclin D2 methylation status | 0.0212 |
| Age | 0.6657 |
| Performance status | <0.0001 |
| FigO stage | 0.0001 |
| Histological type | 0.4709 |
| Grade | 0.1332 |
| Residual tumor | 0.0008 |
Table 3.
Univariate analysis of overall survival
| Variable | P‐value |
|---|---|
| Cyclin D2 methylation status | 0.0625 |
| Age | 0.4195 |
| Performance status | 0.0003 |
| FigO stage | 0.0003 |
| Histological type | 0.0637 |
| Grade | 0.1983 |
| Residual tumor | 0.0016 |
Figure 6.

Association between Cyclin D2 promoter methylation status and disease‐free survival in patients with epithelial ovarian cancer.
Figure 7.

Association between Cyclin D2 promoter methylation status and overall survival in patients with epithelial ovarian cancer.
Discussion
Aberrant promoter methylation is found in many types of human cancer and is a common mechanism for transcriptional inactivation of various genes, including tumor suppressor genes, DNA repair genes, cell cycle regulatory genes and apoptosis‐related genes. In the present study, we determined the Cyclin D2 promoter methylation status of several ovarian cancer cell lines and ovarian cancer surgical specimens, measured the levels of Cyclin D2 gene expression in ovarian cancer cell lines and linked the methylation status of the Cyclin D2 promoter to various clinical and pathological variables in ovarian cancer patients.
From MSP and quantitative RT‐PCR analysis, there was a trend towards a reduction in gene expression in the presence of hypermethylation; however, this association was not significant, and it was suggested that expression of the Cyclin D2 gene in ovarian cancer cell lines, as a whole, was considerably low in comparison with that in normal ovarian tissue. There was an increase in Cyclin D2 gene expression following the 5azaC treatment of cell lines with promoter methylation of the Cyclin D2 gene in MSP. However, TSA or 5azaC treatment of the cell lines without methylation in MSP resulted in re‐expression of the Cyclin D2 gene. Together with these findings, it is suggested that some epigenetic changes, including promoter methylation or histone deacetylation, might contribute to silencing of the Cyclin D2 gene in epithelial ovarian cancer cell lines. The re‐expression by treatment with 5azaC in the unmethylated cell lines JHOS2 and JHOC5 suggests that the Cyclin D2 gene may be secondary re‐expressed owing to activating other suppressed gene by promoter methylation with treatment of 5azaC, or there is a possibility that aberrant methylation did exist but in a different region of the Cyclin D2 promoter to that which we analyzed. Further investigation and data regarding the acetylation status of histones, a different DNA methylation analysis to decipher the MSP results, and DNA methylation of the transcription factor of Cyclin D2 are needed to supplement our hypothesis.
Epithelial ovarian cancer cell growth following treatment with 5azaC or TSA was suppressed in OMC3 and OVCAR3 cell lines. Treatment with these chemical agents resulted in inhibition of cell growth as well as re‐expression of the Cyclin D2 gene. However, another tumor suppressor gene was also re‐expressed by these treatments, and these chemicals could have cell toxicity in itself( 26 , 27 , 28 ). The present data suggests that 5azaC and TSA could be therapeutic agents targeting epigenetic changes in epithelial ovarian cancer, and epigenetic gene silencing of the Cyclin D2 gene could used as a marker of tumor growth.
The D‐type cyclins are early checkpoint regulators at the G1 phase of the cell cycle. Although well known for their proliferation‐promoting activity, the D‐type cyclins also have growth‐inhibitory effects.( 14 ) Thus, decreased expression of Cyclin D2 could result in abnormal cell proliferation and contribute to malignant transformation. Indeed, Cyclin D2 gene silencing secondary to DNA promoter methylation has been demonstrated in several human cancers.( 15 , 16 , 17 , 29 ) Cyclin D2 promoter hypermethylation has also been detected in nearly half of breast cancers and is associated with gene silencing. Cyclin D2 hypermethylation has also been demonstrated in small cell and non‐small cell lung cancer tumor tissues and cell lines,( 17 ) and in approximately half of gastric cancer specimens.( 16 ) In the present study, 22.5% of the surgical specimens and 41.7% of the cell lines had aberrant Cyclin D2 promoter hypermethylation. Our results, though somewhat higher than what has been reported for ovarian granulosa cell tumors,( 10 ) are similar to the percentages seen in several other cancers. Howver, some reports say that aberrant methylation of the Cyclin D2 promoter is an early event in tumorigenesis, as is suggested by its presence in ductal carcinoma in situ in breast cancer and its absence in normal ducts;( 15 , 18 , 29 ) however, this epigenetic change was associated with advanced ovarian cancer in the present study. Our results suggest that aberrant methylation of this gene could be related to tumor progression rather than tumorigenesis of epithelial ovarian cancer.
A number of biological tumor variables, such as DNA ploidy, steroid hormone receptor status and the expression of certain oncogenes, are associated with prognosis in epithelial ovarian cancer.( 30 , 31 , 32 ) The promoter methylation status of several genes, such as 14‐3‐3 sigma, BRCA1, hMLH1 and TMS1, has been used to predict poor survival in epithelial ovarian cancer patients.( 9 , 24 , 33 , 34 , 35 ) In the present study, Cyclin D2 promoter methylation was significantly associated with advanced stage, a larger residual tumor size and poor prognosis. Because there was a trend toward the repression of gene expression in the presence of promoter hypermethylation in ovarian cancer cell lines, we presume that Cyclin D2 gene silencing might occur in primary tissues with methylation, though the levels of the Cyclin D2 gene have not been analyzed in this study. These results suggest that the aberrant promoter methylation of Cyclin D2, or decreased expression of this gene caused by methylation, may be associated with aggressive biological characteristics, and may play a significant role in disease progression in epithelial ovarian cancer.
The contribution of Cyclin D2 to the pathophysiology of epithelial ovarian cancer is not known at a rudimentary level. Though numerous studies have classified it as an oncogene, our data and that of others strongly supports the hypothesis that it functions as a tumor suppressor gene. Further studies are needed to better clarify the relationship between Cyclin D2 gene expression level and its function as either an oncogene or a tumor suppressor. A deeper understanding of the role of D‐type cyclins in ovarian cancer tumor biology could provide a foundation on which to base new diagnostic tests or molecular therapies.
Acknowledgment
We thank Setsuya Aiba (Department of Dermatology, Tohoku University Graduate School of Medicine) for technical assistance related to quantitative RT‐PCR analysis.
References
- 1. Akahira J, Yoshikawa H, Shimizu Y et al. Prognostic factors of stage IV epithelial ovarian cancer: a multicenter retrospective study. Gynecol Oncol 2001; 81: 398–403. [DOI] [PubMed] [Google Scholar]
- 2. Bonnefoi H, A’Hern RP, Fisher C et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol 1999; 17: 767–75. [DOI] [PubMed] [Google Scholar]
- 3. Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet 1999; 21: 163–7. [DOI] [PubMed] [Google Scholar]
- 4. Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res 2001; 61: 3225–9. [PubMed] [Google Scholar]
- 5. Kass SU, Pruss D, Wolffe AP. How does DNA methylation repress transcription? Trends Genet 1997; 13: 444–9. [DOI] [PubMed] [Google Scholar]
- 6. Razin A, Ceder H. DNA methylation and gene expression. Microbiol Rev 1991; 55: 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Doherty AM, Church SW, Russell SHE et al. Methylation status of oestrogen receptor‐α gene promoter sequences in human ovarian epithelial cell lines. Br J Cancer 2002; 86: 282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rathi A, Virmani AK, Schorge JO et al. Methylation profiles of sporadic ovarian tumor and nonmalignant ovaries from high‐risk women. Clin Cancer Res 2002; 8: 3324–31. [PubMed] [Google Scholar]
- 9. Akahira J, Sugihashi Y, Suzuki T et al. Decreased expression of 14‐3‐3sigma is associated with advanced disease in human epithelial ovarian cancer: its correlation with aberrant DNA methylation. Clin Cancer Res 2004; 10: 2687–93. [DOI] [PubMed] [Google Scholar]
- 10. Dhillon VS, Shahid M, Husain SA. CpG methylation of the FHIT, FANCF, cyclin‐D2, BRCA2 and RUNX3 genes in granulose cell tumors (GCTs) of ovarian origin. Mol Cancer 2004; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messague J. G1 cell‐cycle control and cancer. Nature 2004; 432: 298–306. [DOI] [PubMed] [Google Scholar]
- 12. Zhang P. The cell cycle and development: redundant roles of cell cycle regulators. Curr Opin Cell Biol 1999; 11: 655–62. [DOI] [PubMed] [Google Scholar]
- 13. Sicinski P, Donaher JL, Geng Y et al. Cyclin D2 is an FSH‐responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384: 470–4. [DOI] [PubMed] [Google Scholar]
- 14. Meyyappan M, Wong H, Hull C, Raibowol KT. Increased expression of cyclin D2 during multiple status of growth arrest in primary established cells. Mol Cell Biol 1998; 18: 3163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Evron E, Umbricht CB, Korz D et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res 2001; 61: 2782–7. [PubMed] [Google Scholar]
- 16. Yu J, Leung WK, Ebert MPA et al. Absence of cyclin D2 expression is associated with promoter hypermethylation in gastric cancer. Br J Cancer 2003; 88: 1560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virmani A, Rathi A, Heda S et al. Aberrant methylation of the cyclin D2 promoter in primary small cell, nonsmall cell lung and breast cancers. Int J Cancer 2003; 107: 341–5. [DOI] [PubMed] [Google Scholar]
- 18. Fackler MJ, McVeigh M, Evron E et al. DNA methylation of RASSF1A, HIN‐1, RAR‐b, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer 2003; 107: 970–5. [DOI] [PubMed] [Google Scholar]
- 19. WHO. Handbook for Reporting Results of Cancer Treatment. WHO Publication No. 48. Geneva: WHO, 1979. [Google Scholar]
- 20. Shimizu Y, Kamoi H, Amada S et al. Toward the developing of a universal grading system for ovarian epithelial carcinoma. Prognostic significance of histopathologic features − problems involved in the architectural grading system. Gynecol Oncol 1998; 70: 2–12. [DOI] [PubMed] [Google Scholar]
- 21. Herman JG, Graff JR, Myohanen S et al. Methylation‐specific PCR: a novel PCR assay for methylation status of CpG islamds. Proc Natl Acad Sci USA 1996; 93: 9821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi DS, Yoon S, Lee EY et al. Characterization of cyclin D2 expression in human endometrium. Gynecol Invest 2002; 9: 41–6. [DOI] [PubMed] [Google Scholar]
- 23. Akahira J, Suzuki T, Ito K et al. Differential expression of progesterone receptor isoform A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn J Cancer Res 2002; 93: 807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akahira J, Sugihashi Y, Ito K et al. Promoter methylation status and expression of TMS1 gene in human epithelial ovarian cancer. Cancer Sci 2004; 95: 40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamikihara T, Arima T, Kato K et al. Epigenetic silencing of the imprinted gene ZAC by DNA methylation is an early event in the progression of human ovarian cancer. Int J Cancer 2005; 115: 690–700. [DOI] [PubMed] [Google Scholar]
- 26. Schwartsmann G, Fernandes MS, Schaan MD et al. Decitabine (5‐Aza‐2′‐deoxycytidine; DAC) plus daunorubicin as a first line treatment in patients with acute myeloid leukemia: preliminary observations. Leukemia 1997; 11: S28–31. [PubMed] [Google Scholar]
- 27. Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5‐aza‐2′‐deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res 1998; 58: 95–101. [PubMed] [Google Scholar]
- 28. Juttermann R, Li E, Jaenisch R. Toxicity of 5‐aza‐2′‐deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA 1994; 91: 11 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evron E, Dooley WC, Umbricht CB et al. Detection of breast cancer cells in ductal lavage fluid by methylation‐specific PCR. Lancet 2001; 357: 1335–6. [DOI] [PubMed] [Google Scholar]
- 30. Silvestrini R, Daidone MG, Veneroni S et al. The clinical predictivity of biomarkers of stage III–IV epithelial ovarian cancer in a prospective randomized treatment protocol. Cancer 1998; 82: 159–67. [PubMed] [Google Scholar]
- 31. Akahira J, Inoue T, Suzuki T et al. Progesterone receptor isoforms A and B in human epithelial ovarian carcinoma: immunohistochemical and RT‐PCR studies. Br J Cancer 2000; 83: 1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berchuck A, Kamel A, Whitaker R et al. Overexpression of HER‐2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res 1990; 50: 4087–91. [PubMed] [Google Scholar]
- 33. Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol 2006; 101: 403–10. [DOI] [PubMed] [Google Scholar]
- 34. Gifford G, Paul J, Vasey PA et al. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin Cancer Res 2004; 10: 4420–6. [DOI] [PubMed] [Google Scholar]
- 35. Terasawa K, Sagae S, Toyota M et al. Epigenetic inactivation of TMS1/ASC in ovarian cancer. Clin Cancer Res 2004; 10: 2000–6. [DOI] [PubMed] [Google Scholar]


