Abstract
There is a continuous debate regarding the classification of thyroid follicular lesions and the term “well‐differentiated tumor of uncertain malignant potential (WDT‐UMP)” was recently introduced to cover this problematic spectrum of tumors. The objective of this study was to reappraise WDT‐UMP using morphological, immunochemical, and molecular analysis and to shed more light on encapsulated thyroid follicular‐patterned tumors. A total of 30 cases of WDT‐UMP with equivocal papillary thyroid carcinoma‐type nuclear changes (PTC‐N) or focal unequivocal PTC‐N were examined. As a control, follicular adenoma (n = 29), follicular carcinoma (n = 8), hyalinizing trabecular adenoma (n = 5), and PTC (n = 48) were included. HBME‐1, cytokeratin 19, and galectin‐3 were positive in 12 (40.0%), 10 (33.3%) and 11 (36.7%) cases of WDT‐UMP, respectively. According to the positivity of those markers, significant differences were obtained between WDT‐UMP and PTC encapsulated common type (P = 0.028, 0.010, and 0.004, respectively), infiltrative follicular variant (P = 0.020, 0.026, and 0.008, respectively), and infiltrative common type (P = 0.004, 0.001, and 0.005, respectively), but not between WDT‐UMP and follicular adenoma or follicular carcinoma. BRAFV600E mutation was absent but RET/PTC1 rearrangement was found in only two (6.7%) cases of WDT‐UMP. None of the 20 patients with WDT‐UMP developed recurrence, with an average follow‐up of 80 months. These findings indicate that WDT‐UMP has a favorable outcome and is distinct from PTC in morphological, immunohistochemical, and molecular characteristics. We propose that WDT‐UMP should be classified as “well‐differentiated tumor with uncertain behavior”. (Cancer Sci 2011; 102: 288–294)
Papillary thyroid carcinoma‐type nuclear changes (PTC‐N) are the most reliable morphological features in the diagnosis of PTC, which include nuclear enlargement, nuclear overlapping, nuclear clearing, nuclear grooves, and cytoplasmic pseudoinclusions. In published reports and textbooks, nuclear changes are the diagnostic criteria for malignancy in thyroid tumor, regardless of whether or not the tumor has a capsule, is invasive, or has a papillary growth pattern.( 1 ) However, there are exceptions, and PTC‐N are not evident in columnar cell variant or cribriform‐morular variant PTC.( 2 , 3 ) They can be also found in benign lesions, such as hyalinizing trabecular adenoma (HTA) and Hashimoto thyroiditis.( 4 , 5 ) We doubt that these PTC‐N are the golden standard of malignancy, although the majority of PTC do have them. Many pathologists are also aware that the distinctions between follicular adenoma (FTA), encapsulated PTC, and follicular carcinoma (FTC) are not always clear‐cut. When PTC‐N are equivocal or incomplete, significant disagreement occurs in the diagnosis of those tumors.( 6 , 7 , 8 , 9 , 10 , 11 , 12 ) Therefore, in 2000, Williams( 13 ) proposed a new diagnostic terminology, that is, WDT‐UMP for capsulated follicular‐pattern tumors with equivocal PTC‐N and without definite invasion. This group of tumors has been suggested to be borderline in nature, although there is no direct proof for this concept at the present time.( 8 , 14 , 15 )
The purpose of this study was to reappraise the clinicopathological, immunohistochemical, and molecular features of WDT‐UMP and to establish a new viewpoint on this controversial entity.
Materials and Methods
Patients and evaluation of the morphological features of WDT‐UMP. The surgical pathology files of 2648 cases with thyroid lesions, who were treated between 1990 and 2009, were reviewed from the database of the Department of Human Pathology, Wakayama Medical University (Wakayama, Japan). An approval was obtained from Wakayama Medical University ethics committees and the patients gave their written informed consent. The data regarding age, sex, presentation, gross pathology, and outcome were obtained by reviewing clinical files and contacting the referring clinicians.
Hematoxylin–eosin‐stained slides of all patients were reviewed with attention to PTC‐N by two pathologists (Z. L. and K. K.). Thirty encapsulated follicular‐patterned tumors with equivocal PTC‐N and without capsular or vascular invasion, well demarcated from the surrounding thyroid parenchyma, were selected as WDT‐UMPs according to the following criteria, which was modified from those proposed by Williams.( 13 ) When PTC‐N were observed equivocally, such as only nuclear clearing and nuclear grooves without nuclear pseudoinclusions, we classified them as WDT‐UMP (n = 18). When unequivocal PTC‐N were seen in the entire part of the tumor we classified them as encapsulated follicular variant PTC (EFV‐PTC; n = 2). When unequivocal PTC‐N were found in only part of the tumor we classified them as WDT‐UMP (n = 12), as explained in Figure 1. Therefore, the incidence of WDT‐UMP in the thyroid specimens was 1.1%; 501 cases (18.9%) of conventional PTC were examined in the same period. The cellularity of the tumor cells was evaluated according to the criteria proposed by Thompson et al. ( 16 ) One thousand tumor cells were counted randomly for each case and the nuclei size was evaluated by the ratio of tumor cells compared with normal thyroid follicular cells as described by Mai et al. ( 17 )
Figure 1.
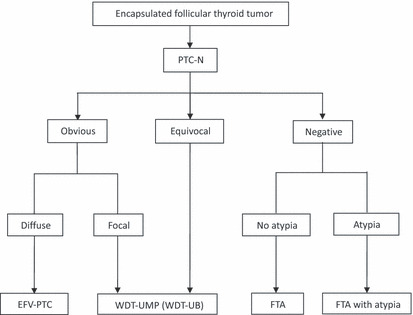
Nomenclature for encapsulated thyroid follicular tumors (modified with permission from Williams’ proposal( 13 )). EFV‐PTC, encapsulated follicular variant papillary thyroid carcinoma; FTA, follicular thyroid adenoma; PTC‐N, papillary thyroid carcinoma‐type nuclear changes; WDT‐UB, well‐differentiated tumor with uncertain behavior; WDT‐UMP, well‐differentiated tumor of uncertain malignant potential.
As the control groups, FTA (n = 16), HTA (n = 5), infiltrative common type PTC (C‐PTC; n = 24), and encapsulated common type PTC (EC‐PTC; n = 10) were selected according to the criteria of the World Health Organization classification.( 1 ) The follicular variant PTCs (FV‐PTCs) were selected by strictly following the criteria of entire or almost complete follicular pattern and with clear‐cut PTC‐N, as proposed by LiVolsi et al. ( 18 , 19 ) Encapsulated follicular‐patterned tumor with diffuse and unequivocal PTC‐N but without definite invasion were included as EFV‐PTC (n = 2) (Fig. 1). Those with definite invasion or metastasis were included as infiltrative follicular variant PTC (IFV‐PTC; n = 12).
Immunohistochemical staining. Five‐micrometer‐thick paraffin sections of all tumors were dewaxed, rehydrated in graded alcohols, and processed using a Dako EnVision detection kit (DakoCytomation, Carpinteria, CA, USA). Briefly, antigen retrieval was carried out as follows: galectin‐3 (GAL‐3) was heated in a microwave oven five times in 3‐min period in 10 mM Tris–EDTA buffer (10 mM Tris base, 1 mM EDTA solution, 0.05% Tween‐20; pH 9.0); trypsin solution digestion was carried out (pH 7.8) for cytokeratin 19 (CK19) for 30 min at 37°C; and no antigen retrieval was used for HBME‐1. Endogenous peroxidase activity was blocked with a 1.7% H2O2‐methanol solution for 30 min, then the slides were incubated in 10% normal goat serum for 30 min to prevent non‐specific binding. They were incubated overnight at 4°C with a primary antibody at an appropriate dilution: HBME‐1 (1:100, clone HBME‐11; DakoCytomation), GAL‐3 (1:100, clone Glectin‐3; DakoCytomation). and CK19 (1:100, clone b170; Vision BioSystems Novocastra, Newcastle upon Tyne, UK). Positive controls were PTC for GAL‐3 and HBME‐1 and colon mucosa for CK19. Phosphate‐buffered saline was used instead of the first antibody as negative controls.
Detection of BRAFV600E mutation and RET/PTC1 rearrangement. To further examine the molecular features, DNA sequencing was used to analyze BRAFV600E mutation and RET/PTC1 rearrangement for all WDT‐UMPs. DNA and RNA was extracted from the formalin‐fixed, paraffin‐embedded tissues according to the method described by Jing et al. ( 20 ) Briefly, two sections of 10‐μm thickness were taken from each paraffin block and deparaffinized by xylene. The tissues for DNA extraction were digested with proteinase K (0.5 mg/mL; Qiagen, Tokyo, Japan) at 55°C overnight. After extraction with phenol/chloroform, genomic DNA was precipitated and 100 ng DNA was used in PCR. All tumor samples were analyzed for the thymine (T) to adenine (A) missense mutation at nucleotide 1799 in exon 15 of the BRAF gene, as described in our previous study.( 21 )
Total RNA was extracted using the Ultraspec RNA isolation system (Biotecx, Houston, TX, USA). Two micrograms of total RNA was reverse transcribed by Superscript II (Invitrogen, Carlsbad, CA, USA) in a 25 μL total reaction volume containing RT buffer random hexamers (Invitrogen), deoxynucleotide triphosphate, and RNase inhibitor (Roche Applied Science, Indianapolis, IN, USA). Two sets of PCR primers were synthesized by Integrated DNA Technologies (Operon Biotechnologies, Tokyo, Japan) to detect the RET/PTC1 rearrangement.( 21 )
All PCR products were purified using a QIAEX II gel extraction kit (Qiagen). Amplified DNA fragments were sequenced using a DNA sequencing kit (Bigdye Terminator v3.0 CycleSequencing Ready Reaction; Applied Biosystems, Foster City, CA, USA) and subjected to direct sequencing in both directions. The sequencing results were analyzed by ABI Prism DNA sequencing analysis software (Applied Biosystems).
Scoring of immunostaining and statistical analysis. Immunoreactivity was scored using a semiquantitative scoring method. A case was scored as positive only when strong signals in the cytoplasm or along cell membranes were detected for GAL‐3, HBME‐1, and CK19, respectively. Based on the evaluation of the heterogeneous positive distribution and the differing intensity of the simultaneous staining, all the cases were scored as negative, focally positive (+, <25%), positive (++, 25–50%), or diffusely positive (+++, more than 75%).
The data were analyzed by SPSS for Windows, version 14.0 (SPSS, Chicago, IL, USA). Associations between categorical variables were evaluated by the chi‐square or Fisher’s exact tests. All statistical tests were two‐sided. Probability values <0.05 were considered statistically significant.
Results
A summary of the clinicopathological information of the patients with WDT‐UMP is shown in Table 1. The patients included 25 females and five males, with a female:male ratio of 5:1. Age at surgery ranged from 17 to 80 years old with an average of 50 ± 16 (mean ± SD) years. The WDT‐UMP ranged in size from 1.0 to 4.6 cm, with an average size of 2.0 ± 0.9 cm (mean ± SD). Ten cases were originally diagnosed as adenomatous nodule, 20 cases as FTA, and only simple excision or lobectomy was done; lymph node dissection was not carried out. No invasion to thyroid parenchyma or lymph node metastasis (LNM) was identified in any of the cases at surgery. Twenty cases were available for follow‐up analyses at a range of 35–206 months (average, 81 months), and all of these patients were alive at latest follow‐up without recurrence (Table 1).
Table 1.
Clinicopathological features and immunohistochemical scores of well‐differentiated thyroid tumors of uncertain malignant potential (or well‐differentiated thyroid tumors of uncertain behavior)
| Case | Age (years) | Gender | Tumor size (cm) | Nuclei size (T/N) | Nuclear clearing | Nuclear grooves (%) | Follow‐up (months) | HBME‐1 | CK19 | GAL‐3 | BRAF mutation | RET/PTC1 rearrangement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | F | 4.6 | 3 | + | 3.0 | −† | +++ | − | − | − | − |
| 2 | 58 | M | 3.0 | 3 | + | 3.0 | −† | ++ | − | ++ | − | − |
| 3 | 47 | F | 3.0 | 2 | + | 1.0 | −† | − | − | − | − | − |
| 4 | 43 | F | 1.3 | 3 | + | 1.0 | 206 | ++ | ++ | ++ | − | − |
| 5 | 63 | F | 1.7 | 2 | + | 3.0 | 185 | − | − | − | − | − |
| 6 | 29 | M | 2.0 | 3 | + | 1.0 | 130 | − | + | − | − | − |
| 7 | 41 | F | 2.8 | 3 | + | 1.0 | −† | − | − | − | − | − |
| 8 | 36 | F | 1.1 | 3 | + | 3.0 | −† | − | − | − | − | − |
| 9 | 44 | F | 2.0 | 3 | + | 3.0 | 109 | +++ | − | − | − | − |
| 10 | 33 | F | 1.8 | 3 | + | 3.0 | −† | ++ | − | − | − | − |
| 11 | 50 | F | 1.0 | 3 | + | 3.0 | 89 | − | − | − | − | − |
| 12 | 44 | F | 1.5 | 3 | + | 3.0 | 87 | +++ | − | + | − | − |
| 13 | 35 | F | 0.9 | 3 | + | 3.0 | 74 | ++ | +++ | +++ | − | − |
| 14 | 46 | F | 1.6 | 2 | + | 1.0 | −† | +++ | + | ++ | − | − |
| 15 | 73 | F | 2.7 | 2 | + | 3.0 | 71 | − | − | − | − | − |
| 16 | 17 | F | 3.2 | 2 | + | 1.0 | 68 | − | − | − | − | − |
| 17 | 30 | F | 1.0 | 2 | + | 3.0 | 66 | − | − | − | − | − |
| 18 | 52 | F | 1.3 | 3 | + | 1.0 | 65 | ++ | − | − | − | − |
| 19 | 50 | F | 2.2 | 2 | + | 1.0 | 61 | − | +++ | − | − | − |
| 20 | 44 | M | 2.5 | 2 | + | 3.0 | 61 | +++ | +++ | +++ | − | − |
| 21 | 38 | F | 2.8 | 3 | + | 3.0 | 56 | − | − | − | − | − |
| 22 | 74 | F | 2.5 | 3 | + | 1.0 | 56 | − | +++ | − | − | − |
| 23 | 73 | F | 2.8 | 3 | + | 3.0 | 56 | +++ | − | ++ | − | + |
| 24 | 41 | F | 1.1 | 3 | + | 3.0 | 56 | − | − | − | − | − |
| 25 | 68 | M | 2.8 | 2 | + | 3.0 | −† | − | − | − | − | − |
| 26 | 80 | F | 1.5 | 2 | + | 1.0 | 43 | − | − | − | − | − |
| 27 | 64 | F | 2.6 | 2 | + | 1.0 | 42 | − | − | ++ | − | − |
| 28 | 35 | F | 1.5 | 3 | + | 3.0 | 35 | ++ | + | ++ | − | − |
| 29 | 53 | M | 1.0 | 3 | + | 3.0 | −† | − | ++ | ++ | − | + |
| 30 | 80 | F | 1.3 | 2 | + | 1.0 | −† | − | +++ | +++ | − | − |
†Follow‐up was not available. −, negative; +, positive; CK19, cytokeratin 19; F, female; GAL‐3, galectin‐3; M, male, T/N, nuclei size of the tumor cells versus that of normal thyroid follicular cells.
The interpretation of what constituted PTC‐N varies among pathologists.( 11 , 13 , 22 , 23 ) In this study, the normal thyroid follicular cells were small, oval, or round and arranged regularly (Fig. 2A). In FV‐PTC, the tumor cells were arranged in a follicular pattern with typical PTC‐N, including nuclear clearing/ground glass, pseudoinclusions, and nuclear grooves. The nuclei were nearly always overlapping or crowded (Fig. 2B). All WDT‐UMPs had an intact capsule (Fig. 2C), the follicles contained colloid and were lined by cuboidal or oval epithelium in variable sizes. The epithelial cells had polarity and were crowded but rarely overlapping. The nuclei size was 2–4 times of that of normal thyroid follicular cells, as shown in Figure 2D. The nuclei were located in the middle or apical of the cytoplasm, and they showed clear nuclei different from the hyperchromatic nuclei of FTA (Fig. 2E). Nuclear grooves were observed in 1–3% of the tumor cells. However, pseudoinclusion was rare or absent in all 30 cases because of our diagnosis criteria. In contrast, approximately 3–8% of nuclear pseudoinclusions were observed in FV‐PTC (Fig. 2B) and HTA (Fig. 2F).
Figure 2.
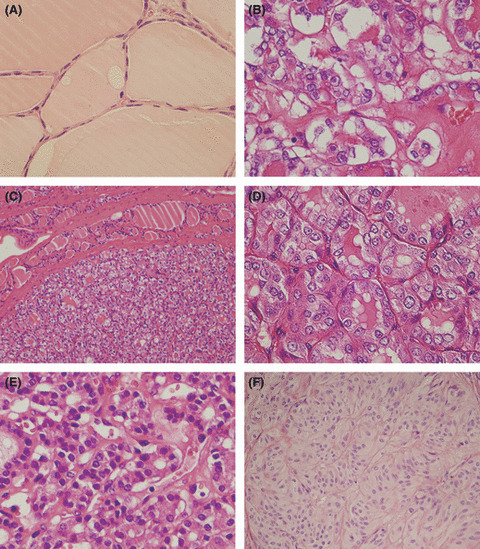
Comparison of morphology in different follicular thyroid lesions. (A) Normal thyroid epithelium. (B) Follicular variant papillary thyroid carcinoma, which shows typical nuclear changes of papillary thyroid carcinoma. (C,D) Well‐differentiated tumor of uncertain behavior, which shows 2–4‐times enlargement of nuclei size, ground glass features, and 3–4% of nuclear grooves, but pseudoinclusions are rare or absent. (E) Follicular thyroid adenoma, which shows 1–4‐times enlargement of nuclei size, the obvious difference is that the nuclei are hyperchromatic. (F) Hyalinizing trabecular adenoma, which is encapsulated, with trabecular growth pattern, polygonal and elongated cells, pseudoinclusions, and cytoplasmic yellow bodies. There is an accumulated hyaline substance in the basement membrane between trabeculae and alveoli. Original magnification: (C,F) ×100; (A,B,D,E) ×200.
Immunostaining of HBME‐1, GAL‐3, and CK19 showed different reactivity among the six groups of follicular tumor (1, 2). In WDT‐UMPs, 12 (40.0%) cases showed positive reaction with HBME‐1, 10 (33.3%) cases for CK19, and 11 (36.7%) cases for GAL‐3. As summarized in Table 1, there were five cases that were reactive with all three markers, three more cases were positive for HBME‐1 and GAL‐3, and two more cases were positive for CK19 and GAL‐3. However, no further cases were positive for HBME‐1 and GAL‐3. Overall, the reaction was much more extensive and intense in WDT‐UMP (Fig. 3A) than in FTA (Fig. 3B). No reaction with HBME‐1 or CK19 was observed in the five HTAs, however, GAL‐3 was focally reactive with the tumor cells in one case. In FV‐PTC, all three markers were diffusely reactive with the tumor cells, and were more intense in the cytoplasm than the reactivity in WDT‐UMP (Fig. 3C). Univariate analysis revealed that statistically significant differences were obtained between WDT‐UMP and three groups of PTCs (EC‐PTC, IFV‐PTC, and C‐PTC) according to the three markers, but no significant difference was observed between WDT‐UMP and FTA (Fig. 4). The comparison between WDT‐UMP and HTA, EFV‐PTC was not effective because of the case limitation of HTA and EFV‐PTC.
Table 2.
Immunohistochemistry of HBME‐1, CK19 and GAL‐3 in thyroid tumors
| Groups | n (Frequency, %) | ||
|---|---|---|---|
| HBME‐1 | CK19 | GAL‐3 | |
| WDT‐UMP (WDT‐UB) (n = 30) | 12 (40.0) | 10 (33.3) | 11 (36.7) |
| PTC (n = 48) | |||
| EC‐PTC (n = 10) | 8 (80.0) | 8 (80.0) | 9 (90.0) |
| C‐PTC (n = 27) | 19 (70.4) | 19 (70.4) | 18 (66.7) |
| EFV‐PTC (n = 2) | 2 (100.0) | 1 (50.0) | 1 (50.0) |
| IFV‐PTC (n = 9) | 8 (88.9) | 7 (77.8) | 8 (88.9) |
| FTA (n = 29) | 6 (20.7) | 9 (31.0) | 7 (24.1) |
| FTC (n = 8) | 4 (50.0) | 3 (37.5) | 3 (37.5) |
| HTA (n = 5) | 0 (0.0) | 0 (0.0) | 1 (20%) |
CK19, cytokeratin 19; GAL‐3, galectin‐3.
Figure 3.
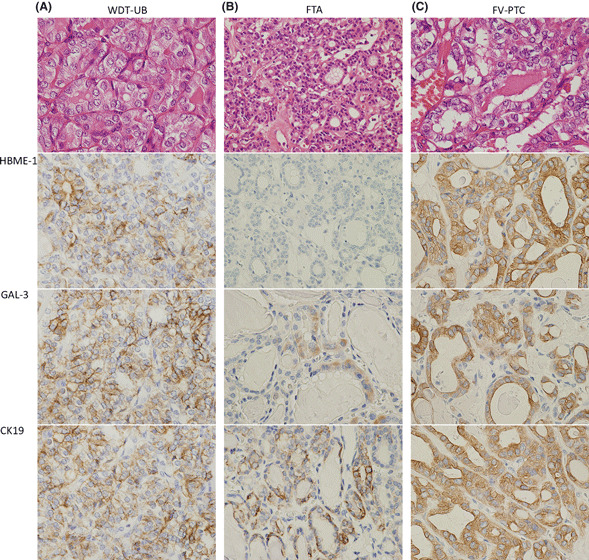
Immunohistochemistry of HBME‐1, GAL‐3, CK19 in well‐differentiated tumor of uncertain behavior (WDT‐UB), follicular thyroid adenoma (FTA), and follicular variant papillary thyroid carcinoma (FV‐PTC). (A) In WDT‐UB, the reaction of the three markers increased, but was mainly in the cell membrane, and the staining was less intense. (B) In FTA, only occasional reaction with the three markers was found. (C) In infiltrative FV‐PTC, all of the three markers showed intense and diffuse reaction with the carcinoma cells. Original magnification: (A,C) ×200; (B) ×100.
Figure 4.
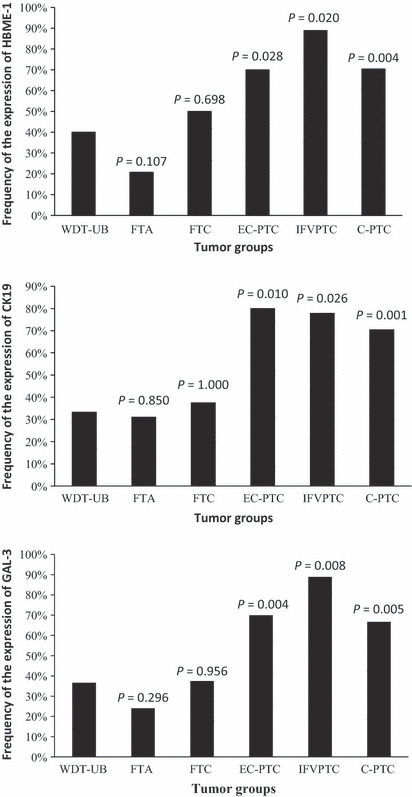
Different expression of HBME‐1, cytokeratin 19 (CK19), and galectin‐3 (GAL‐3) in well‐differentiated tumor of uncertain behavior (WDT‐UB) and the other five groups of thyroid tumors. P‐values obtained using the chi‐square or Fisher’s exact test. C‐PTC, infiltrative common type papillary thyroid carcinoma; EC‐PTC, encapsulated common type papillary thyroid carcinoma; FTA, follicular thyroid adenoma; FTC, follicular thyroid carcinoma; IFV‐PTC, infiltrative follicular variant papillary thyroid carcinoma.
BRAFV600E mutation was absent in all cases. However, RET/PTC1 rearrangement was indicated in two (6.7%) cases of WDT‐UMP (Table 1, Fig. 5). These findings indicated that BRAFV600E mutation is absent in WDT‐UMP, but RET/PTC1 rearrangement may occur in a few cases.
Figure 5.
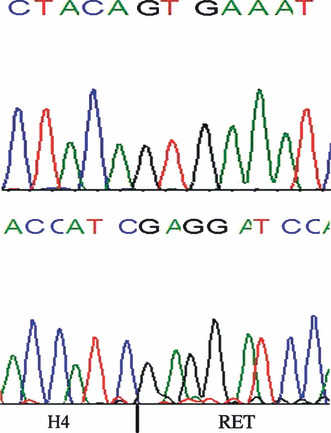
Sequence analysis of BRAF exon 15 in one patient (case 7) showing the wild‐type sequence (top panel), and of RET/PTC1 rearrangement (case 23) harboring H4/ret rearrangement (bottom panel).
Discussion
As emphasized recently by the debate regarding the terminology for encapsulated follicular tumors with equivocal PTC‐N, an important impetus for overdiagnosis of WDT‐UMP or EFV‐PTC is the litigation climate, whereby the pathologists make the diagnosis using lax criteria to avoid being sued after missing the malignancy. However, this diagnosis method causes overtreatment to a certain extent and results in psychological burden for the patient.
On the basis of the criteria proposed by Williams,( 13 ) we developed an intimate description of the nuclear changes of WDT‐UMP, including 2–4 times nuclear enlargement, 1–3% nuclear grooves and rare or absent nuclear pseudoinclusions. Papillary carcinoma‐type nuclear changes are the most important morphology when diagnosing non‐invasive encapsulated follicular thyroid tumors. As can be seen in Figure 1: if PTC‐N are negative, the classification should be FTA; if PTC‐N are equivocal, it should be WDT‐UMP; if PTC‐N are unequivocal but focal, it should be WDT‐UMP; and if PTC‐N are unequivocal but diffuse, it should be EFV‐PTC. Thus, our concept of WDT‐UMP contains both Williams’ WDT‐UMP and encapsulated follicular tumors with focal PTC‐N, therefore, a new terminology of “well‐differentiated tumor with uncertain behavior (WDT‐UB)” is proposed to distinguish it from that of Williams. However, morphological comparison between FTA, WDT‐UMP, and EFV‐PTC may be confusing, because it rests on the pathologist’s perception of the nuclear characteristics, which unfortunately is subjective.( 12 ) Observer variations occur when assessing these lesions and up to 61% of these types of tumors often meet diagnostic discrepancy even among expert pathologists.( 7 , 11 , 12 , 24 ) Because of the good biological behavior, low LNM, and almost no recurrence and no patient death after lobectomy,( 25 ) Chan recommended that strict criteria should be applied in the diagnosis of these tumors.( 12 )
Galectin‐3, HBME‐1, and CK19 have been shown to be sensitive markers for diagnosis of PTC( 26 , 27 , 28 ) and were applied to further examine the immunochemical features of WDT‐UB in this study. The positive frequency of GAL‐3, CK19, and HBME‐1 was 36.7%, 33.3%, and 40.0%, respectively, which was consistent with previous reports.( 6 , 15 , 29 , 30 ) Surprisingly, no statistical difference was observed between WDT‐UB and FTA or FTC for the positive frequencies of these markers, which indicates that it is difficult to discriminate WDT‐UB from the FTA/FTC group with these markers. However, significant differences were found between WDT‐UB and each PTC group, except EFV‐PTC. This observation suggests that WDT‐UB may be a distinct entity different from EC‐PTC, IFV‐PTC, and C‐PTC.
In addition to the immunochemical findings, molecular characteristics of WDT‐UB confirmed the absence of BRAFV600E mutation and the rare occurrence of RET/PTC1 rearrangement, which is consistent with previous reports.( 6 , 14 , 31 ) BRAFV600E mutation has been shown to be an infrequent event in FV‐PTC( 32 , 33 , 34 , 35 ) and absent in benign thyroid lesions,( 6 , 31 , 34 , 36 , 37 ) but RET/PTC1 rearrangement has been found in both PTC and benign lesion, such as Hashimoto thyroiditis.( 38 ) These results suggest that examination of BRAF mutation and RET/PTC1 rearrangement is not reliable to differentiate WDT‐UMP from benign thyroid lesions.
In the present study, we clearly showed that WDT‐UB behave as if they are benign tumors, even if they have PTC‐N, either focal or equivocal. Although the case number is small, none in our study had invasion or cervical LNM in our 30 cases at surgery, and no recurrence was shown in 20 cases at a median follow‐up of 80 months, which is completely different from EFV‐PTC previously reported.( 25 , 39 )
In conclusion, we have introduced details of the histological criteria of WDT‐UMP, which included the focal PTC‐N tumor group in addition to the tumor with diffuse but equivocal PTC‐N. Well‐differentiated tumor of uncertain malignant potential has a favorable outcome and shows distinct morphological, immunohistochemical, and molecular features compared to C‐PTC. We rename both WDT‐UMP and non‐invasive encapsulated follicular patterned thyroid tumors with focal PTC‐N as a borderline tumor of WDT‐UB, which share PTC‐N to a certain extent.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgments
Dr Liu is a PhD student at Wakayama Medical University, who is on exchange from the School of Medicine, Shandong University, and supported by scholarships from the China Scholarship Council (2007102087) and Wakayama Medical University.
References
- 1. DeLellis RA, LIoyd RV, Heitz PU, Eng C. World Health Organization Classification of Tumours: Pathology and genetics of tumours of Endocrine Organs. Lyon: IARC press, 2004. [Google Scholar]
- 2. Cameselle‐Teijeiro J, Chan JK. Cribriform‐morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis‐associated thyroid carcinoma? Mod Pathol 1999; 12: 400–11. [PubMed] [Google Scholar]
- 3. Gaertner EM, Davidson M, Wenig BM. The columnar cell variant of thyroid papillary carcinoma. Case report and discussion of an unusually aggressive thyroid papillary carcinoma. Am J Surg Pathol 1995; 19: 940–7. [DOI] [PubMed] [Google Scholar]
- 4. Asioli S, Erickson LA, Lloyd RV. Solid cell nests in Hashimoto’s thyroiditis sharing features with papillary thyroid microcarcinoma. Endocr Pathol 2009; 20: 197–203. [DOI] [PubMed] [Google Scholar]
- 5. Carney JA, Hirokawa M, Lloyd RV, Papotti M, Sebo TJ. Hyalinizing trabecular tumors of the thyroid gland are almost all benign. Am J Surg Pathol 2008; 32: 1877–89. [DOI] [PubMed] [Google Scholar]
- 6. Hofman V, Lassalle S, Bonnetaud C et al. Thyroid tumours of uncertain malignant potential: frequency and diagnostic reproducibility. Virchows Arch 2009; 455: 21–33. [DOI] [PubMed] [Google Scholar]
- 7. Elsheikh TM, Asa SL, Chan JK et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 2008; 130: 736–44. [DOI] [PubMed] [Google Scholar]
- 8. Kakudo K, Bai Y, Katayama S et al. Classification of follicular cell tumors of the thyroid gland: analysis involving Japanese patients from one institute. Pathol Int 2009; 59: 359–67. [DOI] [PubMed] [Google Scholar]
- 9. Baloch ZW, Livolsi VA. Follicular‐patterned lesions of the thyroid: the bane of the pathologist. Am J Clin Pathol 2002; 117: 143–50. [DOI] [PubMed] [Google Scholar]
- 10. Serra S, Asa SL. Controversies in thyroid pathology: the diagnosis of follicular neoplasms. Endocr Pathol 2008; 19: 156–65. [DOI] [PubMed] [Google Scholar]
- 11. Hirokawa M, Carney JA, Goellner JR et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 2002; 26: 1508–14. [DOI] [PubMed] [Google Scholar]
- 12. Chan JK. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2002; 117: 16–8. [DOI] [PubMed] [Google Scholar]
- 13. Williams ED. Guest editorial: two proposals regarding the terminology of thyroid tumors. Int J Surg Pathol 2000; 8: 181–3. [DOI] [PubMed] [Google Scholar]
- 14. Fusco A, Chiappetta G, Hui P et al. Assessment of RET/PTC oncogene activation and clonality in thyroid nodules with incomplete morphological evidence of papillary carcinoma: a search for the early precursors of papillary cancer. Am J Pathol 2002; 160: 2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Papotti M, Rodriguez J, De Pompa R, Bartolazzi A, Rosai J. Galectin‐3 and HBME‐1 expression in well‐differentiated thyroid tumors with follicular architecture of uncertain malignant potential. Mod Pathol 2005; 18: 541–6. [DOI] [PubMed] [Google Scholar]
- 16. Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the English literature. Cancer 2001; 91: 505–24. [DOI] [PubMed] [Google Scholar]
- 17. Mai KT, Yazdi HM, Perkins DG, Commons AS, Thomas J. Papillary thyroid carcinoma and related thyroid neoplastic lesions: a light microscopic study with emphasis on nuclear changes. Tumori 2000; 86: 238–49. [DOI] [PubMed] [Google Scholar]
- 18. LiVolsi VA. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer 2003; 98: 1997; author reply. [DOI] [PubMed] [Google Scholar]
- 19. Zidan J, Karen D, Stein M, Rosenblatt E, Basher W, Kuten A. Pure versus follicular variant of papillary thyroid carcinoma: clinical features, prognostic factors, treatment, and survival. Cancer 2003; 97: 1181–5. [DOI] [PubMed] [Google Scholar]
- 20. Jing X, Nakamura Y, Nakamura M et al. Detection of Epstein–Barr virus DNA in gastric carcinoma with lymphoid stroma. Viral Immunol 1997; 10: 49–58. [DOI] [PubMed] [Google Scholar]
- 21. Zuo H, Nakamura Y, Yasuoka H et al. Lack of association between BRAF V600E mutation and mitogen‐activated protein kinase activation in papillary thyroid carcinoma. Pathol Int 2007; 57: 12–20. [DOI] [PubMed] [Google Scholar]
- 22. Kakudo K, Katoh R, Sakamoto A et al. Thyroid gland: international case conference. Endocr Pathol 2002; 13: 131–4. [DOI] [PubMed] [Google Scholar]
- 23. Rosai J. The encapsulated follicular variant of papillary thyroid carcinoma: back to the drawing board. Endocr Pathol 2010; 21: 7–11. [DOI] [PubMed] [Google Scholar]
- 24. Lloyd RV, Erickson LA, Casey MB et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol 2004; 28: 1336–40. [DOI] [PubMed] [Google Scholar]
- 25. Piana S, Frasoldati A, Di Felice E, Gardini G, Tallini G, Rosai J. Encapsulated well‐differentiated follicular‐patterned thyroid carcinomas do not play a significant role in the fatality rates from thyroid carcinoma. Am J Surg Pathol 2010; 34: 868–72. [DOI] [PubMed] [Google Scholar]
- 26. Barut F, Onak Kandemir N, Bektas S, Bahadir B, Keser S, Ozdamar SO. Universal markers of thyroid malignancies: galectin‐3, HBME‐1, and cytokeratin‐19. Endocr Pathol 2010; 21: 80–9. [DOI] [PubMed] [Google Scholar]
- 27. Barroeta JE, Baloch ZW, Lal P, Pasha TL, Zhang PJ, LiVolsi VA. Diagnostic value of differential expression of CK19, Galectin‐3, HBME‐1, ERK, RET, and p16 in benign and malignant follicular‐derived lesions of the thyroid: an immunohistochemical tissue microarray analysis. Endocr Pathol 2006; 17: 225–34. [DOI] [PubMed] [Google Scholar]
- 28. Nasr MR, Mukhopadhyay S, Zhang S, Katzenstein AL. Immunohistochemical markers in diagnosis of papillary thyroid carcinoma: utility of HBME1 combined with CK19 immunostaining. Mod Pathol 2006; 19: 1631–7. [DOI] [PubMed] [Google Scholar]
- 29. Bukhari U, Sadiq S, Kehar SI. Differential expression of CK 19 in follicular adenoma, well‐differentiated tumour of uncertain malignant potential (WDT‐UMP) and follicular variant of papillary carcinoma. J Pak Med Assoc 2009; 59: 15–8. [PubMed] [Google Scholar]
- 30. Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of HBME1, galectin‐3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol 2006; 126: 700–8. [DOI] [PubMed] [Google Scholar]
- 31. Fontaine JF, Mirebeau‐Prunier D, Franc B et al. Microarray analysis refines classification of non‐medullary thyroid tumours of uncertain malignancy. Oncogene 2008; 27: 2228–36. [DOI] [PubMed] [Google Scholar]
- 32. Nikiforova MN, Kimura ET, Gandhi M et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003; 88: 5399–404. [DOI] [PubMed] [Google Scholar]
- 33. Trovisco V, Vieira de Castro I, Soares P et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol 2004; 202: 247–51. [DOI] [PubMed] [Google Scholar]
- 34. Di Cristofaro J, Marcy M, Vasko V et al. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: association of N‐ras mutation in codon 61 with follicular variant. Hum Pathol 2006; 37: 824–30. [DOI] [PubMed] [Google Scholar]
- 35. Rivera M, Ricarte‐Filho J, Knauf J et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol 2010; 23: 1191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical‐pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 2003; 120: 71–7. [DOI] [PubMed] [Google Scholar]
- 37. Arora N, Scognamiglio T, Lubitz CC et al. Identification of borderline thyroid tumors by gene expression array analysis. Cancer 2009; 115: 5421–31. [DOI] [PubMed] [Google Scholar]
- 38. Rhoden KJ, Unger K, Salvatore G et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto’s thyroiditis share low‐level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab 2006; 91: 2414–23. [DOI] [PubMed] [Google Scholar]
- 39. Baloch ZW, LiVolsi VA. Encapsulated follicular variant of papillary thyroid carcinoma with bone metastases. Mod Pathol 2000; 13: 861–5. [DOI] [PubMed] [Google Scholar]


