Abstract
Aberrant crypt foci (ACF) in colorectal mucosa are the earliest known morphological precursors to colorectal cancer and can be subclassified as dysplastic, heteroplastic (non‐dysplastic), and mixed types. Serrated adenoma (SA) is a polyp with serrated architecture and dysplasia, and can be subclassified as traditional SA or sessile SA. Sessile SA is thought to be preneoplastic and differs from most lesions in the traditional SA category because of their flat morphology and general lack of cytological dysplasia. Serrated polyps include hyperplastic polyps (HP), SA, and admixed hyperplastic‐adenomatous polyps and are considered a morphological continuum encompassing heteroplastic ACF, HP, admixed hyperplastic‐adenomatous polyps, and SA. Recent studies have uncovered other developmental pathways including a heteroplastic ACF‐HP/SA‐carcinoma sequence and a heteroplastic ACF–adenoma–carcinoma sequence. Heteroplastic ACF histopathologically resemble HP and SA. Sporadic HP are usually present in the left colon, are small, and are considered benign. However, adenocarcinoma arising in the setting of colorectal HP or SA, especially in patients with hyperplastic polyposis, has been described. The relationship between heteroplastic ACF, HP, and colorectal cancer is less certain than that of dysplastic ACF. Here, we discuss the current understanding of genetic and epigenetic alterations in the development of colorectal cancer. Our goal is to provide a conceptual framework for understanding the heteroplastic ACF–HP/SA–carcinoma sequence. (Cancer Sci 2008; 99: 1071–1076)
Precursor lesions for colorectal cancer
In general, colorectal polyps are classified as adenomas or hyperplastic polyps (HP).( 1 ) Most colorectal cancers (CRC) develop from adenomatous polyps (tubular adenoma) and show morphological and genetic progression through an adenoma–carcinoma sequence, even in hereditary colorectal cancer syndromes.( 2 , 3 , 4 )
Sporadic HP are usually present in the left colon, are small, and are generally regarded as harmless lesions with no potential for malignancy.( 5 , 6 ) However, adenocarcinoma arising in the setting of colorectal HP or serrated adenomas (SA), especially in patients with hyperplastic polyposis (HPP), which is characterized by the presence of numerous HP or large HP, have been described.( 7 , 8 , 9 , 10 , 11 ) Indeed, patients with HPP have an increased risk of CRC.( 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ) Serrated polyps (SP) include HP, SA, and admixed hyperplastic‐adenomatous polyps (AHAP),( 19 , 20 ) and are considered a morphological continuum encompassing non‐dysplastic (heteroplastic) aberrant crypt foci (ACF), HP, AHAP, and SA.( 21 ) SA are composed of dysplastic epithelium but with the sawtooth configuration that is typical of HP.( 20 ) SA are subclassified as traditional SA and sessile SA.( 22 , 23 ) Sessile SA is thought to be preneoplastic and differs from most lesions in the traditional SA category because of its flat morphology and general lack of cytological dysplasia.( 22 , 23 ) The incidence of SA is reported to be 0.8–4.9% of colorectal tumors.( 24 , 25 ) In addition, the incidence of carcinoma in SA is reported to be 1.5–19.2%, which is equal to or lower than the incidence in tubular adenoma.( 24 )
Recent studies have proposed a heteroplastic ACF–HP/SA–carcinoma sequence as an alternative pathway to the typical adenoma–carcinoma sequence.( 8 , 9 , 10 , 11 , 21 ) In addition, another pathway, a heteroplastic ACF–adenoma–carcinoma sequence, has also been proposed.( 26 , 27 ) In some pathways in colorectal tumorigenesis, genetic and epigenetic alterations have been well postulated.( 28 , 29 , 30 ) However, genetic and epigenetic changes related to morphological progression, including the heteroplastic ACF–HP/SA–carcinoma sequence and the heteroplastic ACF–adenoma–carcinoma sequence have not been well characterized because of the complexity of the genetic and epigenetic events underlying these pathways. In the present review, we discuss the current knowledge regarding genetic and epigenetic alterations in the development of CRC. The goal is to provide a conceptual framework to understand the heteroplastic ACF–HP/SA–carcinoma sequence.
Molecular pathways
In the adenoma–carcinoma sequence, a series of tumor‐suppressor genes on chromosomes 5q (APC), 17p (p53), and 18q (DCC, SMAD2, and DPC4/SMAD4) are inactivated by mutations and chromosomal deletions.( 2 , 31 , 32 ) Deletions of chromosome 1p are found in early as well as advanced stages of colorectal tumorigenesis.( 33 , 34 , 35 ) Frequent activating mutations in the K‐ras oncogene are thought to arise in large preneoplastic adenomas.( 2 ) BRAF mutations tend to occur in a mutually exclusive relationship with K‐ras mutations.( 19 , 36 , 37 , 38 ) BRAF mutations have been found in HP, SA (including some from patients with HPP) and colorectal carcinomas.( 39 , 40 , 41 )
A subset of CRC show a characteristic mutator phenotype that leads to microsatellite instability (MSI) and mutations of other genes, such as TGFβRII ( 42 ) and BAX.( 43 ) This mutator phenotype usually results from inactivation of mismatch repair genes such as hMSH2 and hMLH1.( 44 , 45 , 46 , 47 ) A subset of familial CRC (hereditary non‐polyposis CRC) result from germline mutations in the mismatch repair genes.( 2 , 47 ) However, approximately 15–20% of non‐familial (sporadic) CRC have MSI because of epigenetic inactivation of hMLH1.( 48 , 49 , 50 )
Aberrant methylation of CpG islands occurs in genetic diseases such as fragile‐X syndrome,( 51 ) in aging cells,( 52 ) and in neoplasia.( 53 , 54 ) Examples of this process in CRC include inactivation of the cell cycle regulator p16,( 55 ) the DNA repair gene MGMT,( 56 ) the mismatch repair gene hMLH1,( 57 ) and APC.( 58 ) A distinct pathway for colorectal tumorigenesis has been described, termed CpG island methylator phenotype (CIMP).( 29 ) Tumors with this phenotype are characterized by a high degree of concordant CpG island methylation, which affects most of the genes known to be methylated in this tumor type.( 29 )
Given that genetic (mutations) and epigenetic (CIMP) changes are common in CRC, both could arise independently of each other, such that CRC might contain an accumulation of genetic and epigenetic changes that occurred randomly and were selected for stochastically during progression. Alternatively, epigenetic changes may mark a distinct group of tumors that have a distinct etiology and molecular profile.
Dysplastic ACF
A dysplastic ACF–adenoma–carcinoma sequence is well recognized.( 59 ) ACF in colorectal mucosa are the earliest known morphological precursors to CRC.( 26 , 60 , 61 , 62 , 63 , 64 ) The histopathology of human ACF varies but can be subclassified as dysplastic, heteroplastic (non‐dysplastic), and mixed type.( 59 , 60 ) Dysplastic ACF resemble adenomas and are more common in familial adenomatous polyposis (FAP), which is the result of germline mutation of the APC gene, than in patients with sporadic colorectal neoplasia (Table 1). In addition to dysplasia, these ACF are characterized by abnormal epithelial proliferation in the upper aspects of the crypts, lack of methylation and mutations of K‐ras, and the presence of APC mutations in dysplastic ACF from FAP patients but not patients with sporadic CRC (Fig. 1; Table 1).( 19 , 26 , 59 , 64 ) As shown in Table 1, mutation of APC was found in 8/8 (100%) dysplastic ACF from FAP but in only 5/28 (18%) and 0/5 (0%) dysplastic ACF from sporadic CRC. In contrast, mutation of K‐ras was observed predominantly in dysplastic ACF from sporadic CRC compared with dysplastic ACF from FAP (2/4 [50%] and 18/28 [64%] vs 0/24 [0%]; Table 1). Although CpG island methylation of the MINT1, MINT2, MINT31, p16, MGMT, and hMLH1 loci tended to be more frequent in dysplastic ACF from sporadic CRC than in dysplastic ACF from FAP (3/4 [75%] vs 2/24 [8%]; Table 1), further analyses are needed because the number of dysplastic ACF from sporadic CRC analyzed was too small.
Table 1.
Reported frequencies of genetic and epigenetic alterations in dysplastic aberrant crypt foci (ACF)
| Alteration | Dysplastic ACF | Author | |
|---|---|---|---|
| From FAP (%) | From sporadic CRC (%) | ||
| APC mutation | 18 (5/28) | Otori et al.( 64 ) | |
| 100 (8/8) | 0 (0/5) | Takayama et al.( 26 ) | |
| K‐ras mutation | 0 (0/24) | 50 (2/4) | Chan et al.( 59 ) |
| 64 (18/28) | Otori et al.( 64 ) | ||
| 13 (1/8) | 64 (7/11) | Takayama et al.( 26 ) | |
| BRAF mutation | 0 (0/21) | 0 (0/3) | Beach et al.( 19 ) |
| Chromosome 1p loss | 0 (0/24) | 0 (0/3) | Chan et al.( 59 ) |
| MGMT methylation | 5 (1/20) | 25 (1/4) | Chan et al.( 59 ) |
| CIM | 8 (2/24) | 75 (3/4) | Chan et al.( 59 ) |
| CIMP‐high | 4 (1/24) | 25 (1/4) | Chan et al.( 59 ) |
CIM, CpG island methylation of MINT1, MINT2, MINT31, p16, MGMT and/or hMLH1; CIMP, CpG island methylator phenotype; CRC, colorectal cancer; FAP, familial adenomatous polyposis.
Figure 1.
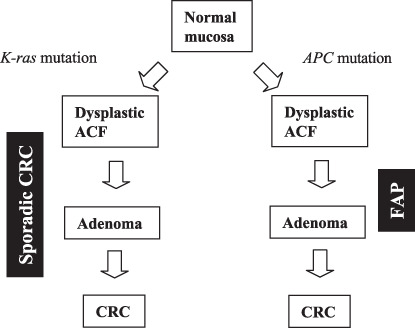
Schematic diagram of the dysplastic aberrant crypt foci (ACF)–adenoma–carcinoma sequence. CRC, colorectal cancer; FAP, familial adenomatous polyposis.
Heteroplastic (non‐dysplastic) ACF
Dysplastic ACF are recognized precursors of CRC, but the relationship of heteroplastic ACF to CRC is less certain.( 26 , 60 , 61 , 62 , 63 , 64 ) The possibility that heteroplastic ACF are precursors of a subset of CRC is supported by several lines of evidence: (1) heteroplastic ACF are clonal;( 65 ) (2) genetic alterations that are common in CRC, such as K‐ras mutation,( 26 , 60 , 61 , 63 , 64 ) chromosome 1p loss,( 59 ) and CpG island methylation,( 59 ) are present in heteroplastic ACF; (3) ACF, adenomas, and carcinomas share similar incidences and anatomical distributions;( 66 , 67 , 68 ) and (4) ACF with mixed heteroplastic and dysplastic components have been described.( 69 ) These findings have suggested the presence of a heteroplastic ACF–adenoma–carcinoma sequence in which K‐ras mutation precedes APC mutation.( 26 , 27 )
An alternative pathway for colorectal carcinogenesis with a heteroplastic ACF–HP/SA–carcinoma sequence has also been proposed.( 8 , 9 , 10 , 11 , 21 ) Because heteroplastic ACF resemble HP histopathologically,( 26 , 60 , 64 ) heteroplastic ACF may be precursors of serrated lesions that progress to CRC. Chan et al. reported that CpG island methylation of the MINT1, MINT2, MINT31, p16, MGMT, and hMLH1 loci is present in approximately half of the heteroplastic ACF in patients with sporadic CRC (Fig. 2; Table 2), whereas CpG island methylation is rare in dysplastic ACF associated with FAP (Table 1).( 59 ) Thus, CpG island methylation may be an early event in colorectal carcinogenesis via a heteroplastic ACF–HP/SA–carcinoma sequence.
Figure 2.
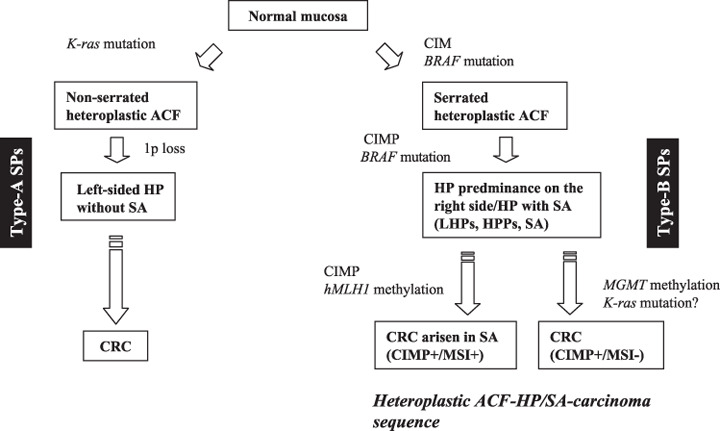
Diagram of pathways to colorectal cancer (CRC) via hyperplastic aberrant crypt foci (ACF). CIM, CpG island methylation; CIMP, CpG island methylator phenotype; HP, hyperplastic polyp; HPP, hyperplastic polyposis; LHP, large hyperplastic polyp; MSI, microsatellite instability; SA, serrated adenoma; SP, serrated polyps.
Table 2.
Reported frequencies of genetic and epigenetic alterations in heteroplastic aberrant crypt foci (ACF)
| Alteration | Heteroplastic ACF | Author | ||
|---|---|---|---|---|
| With sporadic CRC (%) | Serrated | Non‐serrated | ||
| APC mutation | 0 (0/56) | Otori et al.( 64 ) | ||
| 0 (0/8) | Takayama et al.( 26 ) | |||
| K‐ras mutation | 36 (10/28) | Chan et al.( 59 ) | ||
| 88 (28/32) | Takayama et al.( 26 ) | |||
| 93 (50/56) | Otori et al.( 64 ) | |||
| 19 (3/16) | 42 (14/33) | Rosenberg et al.( 70 ) | ||
| BRAF mutation | 4 (1/26) | Beach et al.( 19 ) | ||
| 63 (10/26) | 3 (1/33) | Rosenberg et al.( 70 ) | ||
| Chromosome 1p loss | 10 (2/20) | Chan et al.( 59 ) | ||
| MGMT methylation | 13 (3/24) | Chan et al.( 59 ) | ||
| CIM | 50 (14/28) | Chan et al.( 59 ) | ||
| CIMP‐high | 11 (3/28) | Chan et al.( 59 ) | ||
CIM, CpG island methylation of MINT1, MINT2, MINT31, p16, MGMT and/or hMLH1; CIMP, CpG island methylator phenotype; CRC, colorectal cancer.
Interestingly, serrated hyperplastic ACF has a higher frequency of BRAF mutations than non‐serrated hyperplastic ACF (Table 2).( 70 ) In contrast, the frequency of K‐ras mutations was higher in non‐serrated hyperplastic ACF than in serrated hyperplastic ACF (Table 2).( 70 ) This mutually exclusive relationship between K‐ras and BRAF mutations suggests the existence of distinct pathways to hyperplastic ACF as shown in Figure 2.
Morphological and molecular associations
Previous studies have shown that genetic and epigenetic alterations detected frequently in CRC are present in sporadic HP and in HP, SA, AHAP, tubular adenomas, and carcinomas of patients with HPP.( 10 , 11 , 60 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 ) These alterations include APC and K‐ras mutations, chromosome 1p loss, MSI, and CIMP (Table 3). There is evidence for two CIMP‐positive pathways for colorectal carcinogenesis. One is characterized by methylation of MGMT associated with the MSI‐low phenotype, and the other is characterized by the CIMP‐high phenotype, paucity of K‐ras mutations, and with or without MSI‐high dependent on the methylation status of hMLH1.( 29 , 57 , 79 , 80 , 81 ) Jass et al. described the importance of serrated lesions, low levels of MSI, K‐ras mutation, and MGMT hypermethylation in colorectal carcinogenesis (Fig. 2).( 21 , 82 ) Other investigators have reported that MGMT methylation correlates positively with K‐ras mutation in the traditional pathways but negatively in the SP neoplasia pathways.( 83 ) Further studies are needed to elucidate the association between MGMT methylation and K‐ras mutations in SP.
Table 3.
Reported frequencies of genetic and epigenetic alterations in hyperplastic polyps (HP), serrated polyps (SP), serrated adenoma (SA), admixed hyperplastic‐adenomatous polyps (AHAP) and adenocarcinoma (AC)
| Alteration | HP | SP | SA (%) | AHAP (%) | AC arising in SA (%) | Authors | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predominantly right side (%) | Predominantly left side (%) | P‐value | With HP predominantly right side/HP with SA (type‐B SP) (%) | With HP predominantly left side without SA (type‐A SP) (%) | P‐value | |||||
| APC mutation | 20 (7/35) | Fogt et al.( 77 ) | ||||||||
| 4 (1/26) | Dehari et al.( 76 ) | |||||||||
| K‐ras mutation | 0 (0/34) | 17 (6/36) | 0.025 | 6 (3/51) | 19 (5/26) | 0.111 | 0 (0/4) | 67 (2/3) | Beach et al.( 19 ) | |
| 4 (2/45) | 11 (9/83) | 0.326 | 0 (0/6) | 67 (2/3) | Rashid et al.( 11 ) | |||||
| 2 (1/44) | 14 (8/58) | 0.041 | 5 (3/64) | 17 (8/46) | 0.049 | 25 (2/8) | Chan et al.( 73 ) | |||
| 0 (0/9) | Chan et al.( 41 ) | |||||||||
| 24 (7/29) | 0 (0/11) | O’Brien et al.( 83 ) | ||||||||
| BRAF mutation | 68 (23/34) | 19 (7/36) | <0.0001 | 61 (31/51) | 12 (3/26) | <0.0001 | 75 (3/4) | 33 (1/3) | Beach et al.( 19 ) | |
| 100 (9/9) | Chan et al.( 41 ) | |||||||||
| 62 (18/29) | 82 (9/11) | O’Brien et al.( 83 ) | ||||||||
| Chromosome 1p loss | 4 (2/45) | 17 (14/83) | 0.051 | 0 (0/6) | Rashid et al.( 11 ) | |||||
| 5 (2/44) | 17 (10/58) | 0.064 | 3 (2/64) | 22 (10/46) | 0.004 | 0 (0/8) | Chan et al.( 73 ) | |||
| MGMT methylation | 45 (13/29) | 20 (2/10) | Toyota et al.( 79 ) | |||||||
| CIMP‐high | 76 (26/34) | 19 (7/34) | <0.0001 | 76 (38/50) | 4 (1/25) | <0.0001 | 75 (3/4) | 100 (3/3) | Beach et al.( 19 ) | |
| 77 (34/44) | 19 (11/58) | <0.0001 | 77 (49/64) | 4 (2/46) | <0.0001 | 75 (6/8) | Chan et al.( 73 ) | |||
| 68 (15/22) | Park et al.( 78 ) | |||||||||
| 79 (23/29) | 90 (9/10) | O’Brien et al.( 83 ) | ||||||||
| MSI‐high | 0 (0/45) | 5 (4/83) | 0.297 | 0 (0/6) | 0 (0/3) | Rashid et al.( 11 ) | ||||
| 0 (0/34) | 0 (0/36) | NA | 6 (1/51) | 0 (0/26) | 1.000 | 0 (0/4) | 33 (1/3) | Beach et al.( 19 ) | ||
| 0 (0/44) | 0 (0/58) | NA | 2 (1/64) | 0 (0/46) | 0.500 | 13 (1/8) | Chan et al.( 73 ) | |||
| 9 (2/22) | Park et al.( 78 ) | |||||||||
| 0 (0/29) | 82 (9/11) | O’Brien et al.( 83 ) | ||||||||
Values in bold indicate presence of statistical significance. Serrated polyps include HP, SA, and AHAP. CIMP, CpG island methylator phenotype; MSI, microsatellite instability; NA, not applicable.
Hyperplastic polyps in the right colon differ morphologically from HP in the left colorectum.( 84 ) In addition, Rashid et al. reported differences in topographic expression of p21Waf1/Cip1 cyclin‐dependent kinase inhibitor and Ki‐67 proliferation marker in right‐ and left‐sided HP.( 11 ) The genetic and epigenetic alterations in HP from patients with HP predominantly in the left colorectum without SA and patients with SP without SA (defined as type‐A SP) also differ from HP from patients with HP predominantly in the right colorectum and SP from patients with SA (defined as type‐B SP). Type‐A SP have more K‐ras mutations and loss of 1p but fewer BRAF mutations,( 39 , 40 , 41 ) and lower CpG island methylation than Type‐B SP.( 39 , 73 , 85 ) In contrast, type‐B SP have frequent BRAF mutations and CIMP but infrequent K‐ras mutations and loss of chromosome 1p.( 11 , 39 , 73 , 85 ) BRAF mutations were detected more frequently in tubular adenoma in patients with multiple or large HP and HPP (predominant in type‐B SP) than in those with sporadic adenomas.( 37 ) In addition, the fact that colon cancers derived from SA contain frequent BRAF mutations and MSI( 83 ) (Table 3) suggests that CIMP‐high and BRAF mutation precede MSI in the heteroplastic ACF–HP/SA–carcinoma sequence (Fig. 2). These data provide additional evidence that progression of colorectal carcinogenesis in patients with type‐B SP is distinct from that in patients with type‐A SP.
Conclusion
Although the vast majority of HP never progress to cancer, the molecular and morphological observations described in this review suggest that carcinogenesis may occur via a heteroplastic ACF–HP/SA–carcinoma sequence. Identification of additional molecular markers to predict the malignant potential of HP is needed. A better understanding of molecular carcinogenesis of the colorectum may provide insights that will improve diagnosis and therapy of colorectal tumors.
Acknowledgments
The authors are grateful to Dr Stanley R. Hamilton (M. D. Anderson Cancer Center, Houston, TX, USA) for his help in reading this manuscript.
References
- 1. Hamilton SR, Vogelstein B, Kudo S et al . Carcinoma of the colon and the rectum. In: Hamilton SR, Aaltonen LA, eds. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. Lyon: IARC Press, 2000; 105–19. [Google Scholar]
- 2. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996; 87: 159–70. [DOI] [PubMed] [Google Scholar]
- 3. Fearon ER, Dang CV. Cancer genetics: tumor suppressor meets oncogene. Curr Biol 1999; 9: R62–5. [DOI] [PubMed] [Google Scholar]
- 4. Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 2000; 119: 854–65. [DOI] [PubMed] [Google Scholar]
- 5. Kaye GI, Fenoglio CM, Pascal RR, Lane N. Comparative electron microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. Variations in cellular structure relative to the process of epithelial differentiation. Gastroenterology 1973; 64: 926–45. [PubMed] [Google Scholar]
- 6. Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975; 36: 2251–70. [DOI] [PubMed] [Google Scholar]
- 7. Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst 2001; 93: 1307–13. [DOI] [PubMed] [Google Scholar]
- 8. Jass JR, Cottier DS, Pokos V, Parry S, Winship IM. Mixed epithelial polyps in association with hereditary non‐polyposis colorectal cancer providing an alternative pathway of cancer histogenesis. Pathology 1997; 29: 28–33. [DOI] [PubMed] [Google Scholar]
- 9. Iino H, Jass JR, Simms LA et al . DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 1999; 52: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jass JR, Iino H, Ruszkiewicz A et al . Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut 2000; 47: 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rashid A, Houlihan PS, Booker S, Petersen GM, Giardiello FM, Hamilton SR. Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology 2000; 119: 323–32. [DOI] [PubMed] [Google Scholar]
- 12. Goldman H, Ming S, Hickock DF. Nature and significance of hyperplastic polyps of the human colon. Arch Pathol 1970; 89: 349–54. [PubMed] [Google Scholar]
- 13. Estrada RG, Spjut HJ. Hyperplastic polyps of the large bowel. Am J Surg Pathol 1980; 4: 127–33. [DOI] [PubMed] [Google Scholar]
- 14. Williams GT. Metaplastic (hyperplastic) polyps of the large bowel: benign neoplasms after all? Gut 1997; 40: 691–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCann BG. A case of metaplastic polyposis of the colon associated with focal adenomatous change and metachronous adenocarcinomas. Histopathology 1988; 13: 700–2. [DOI] [PubMed] [Google Scholar]
- 16. Cooke SA. Polyposis coli. The clinical spectrum in adults. S Afr Med J 1978; 53: 454–7. [PubMed] [Google Scholar]
- 17. Cohen SM, Brown L, Janower ML, McCready FJ. Multiple metaplastic (hyperplastic) polyposis of the colon. Gastrointest Radiol 1981; 6: 333–5. [DOI] [PubMed] [Google Scholar]
- 18. Teoh HH, Delahunt B, Isbister WH. Dysplastic and malignant areas in hyperplastic polyps of the large intestine. Pathology 1989; 21: 138–42. [DOI] [PubMed] [Google Scholar]
- 19. Beach R, Chan AO, Wu TT et al . BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol 2005; 166: 1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Longacre TA, Fenoglio‐Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol 1990; 14: 524–37. [DOI] [PubMed] [Google Scholar]
- 21. Jass JR. Serrated route to colorectal cancer: back street or super highway? J Pathol 2001; 193: 283–5. [DOI] [PubMed] [Google Scholar]
- 22. Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology 1996; 110: 748–55. [DOI] [PubMed] [Google Scholar]
- 23. Torlakovic EE, Gomez JD, Driman DK et al . Sessile Serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32: 21–9. [DOI] [PubMed] [Google Scholar]
- 24. Yao T, Ohji Y, Koga Y, Gushima M, Tsuneyoshi M. Incidence of and clinicopathological features of colorectal carcinoma arising from serrated polyps (‘serrated carcinomas’). Stomach Intestine 2007; 42: 299–306. (In Japanese.) [Google Scholar]
- 25. Ikehara H, Saito Y, Kusano C, Matsuda T. Endoscopic daignosis of serrated adenoma using magnifying endoscopy. Stomach Intestine 2007; 42: 307–11. (In Japanese.) [Google Scholar]
- 26. Takayama T, Ohi M, Hayashi T et al . Analysis of K‐ras, APC, and β‐catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology 2001; 121: 599–611. [DOI] [PubMed] [Google Scholar]
- 27. De Filippo C, Caderni G, Bazzicalupo M et al . Mutations of the Apc gene in experimental colorectal carcinogenesis induced by azoxymethane in F344 rats. Br J Cancer 1998; 77: 2148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol 2003; 23: 206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toyota M, Ahuja N, Ohe‐Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999; 96: 8681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong JJ, Hawkins NJ, Ward RL. Colorectal cancer: a model for epigenetic tumorigenesis. Gut 2007; 56: 140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White RL. Tumor suppressing pathways. Cell 1998; 92: 591–2. [DOI] [PubMed] [Google Scholar]
- 32. Jen J, Kim H, Piantadosi S et al . Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 1994; 331: 213–21. [DOI] [PubMed] [Google Scholar]
- 33. Vogelstein B, Fearon ER, Kern SE et al . Allelotype of colorectal carcinomas. Science 1989; 244: 207–11. [DOI] [PubMed] [Google Scholar]
- 34. Lothe RA, Andersen SN, Hofstad B et al . Deletion of 1p loci and microsatellite instability in colorectal polyps. Genes Chromosomes Cancer 1995; 14: 182–8. [DOI] [PubMed] [Google Scholar]
- 35. Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in Dukes’ stages B and C colorectal cancer patients. J Clin Oncol 2003; 21: 820–9. [DOI] [PubMed] [Google Scholar]
- 36. Davies H, Bignell GR, Cox C et al . Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–54. [DOI] [PubMed] [Google Scholar]
- 37. Yuen ST, Davies H, Chan TL et al . Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res 2002; 62: 6451–5. [PubMed] [Google Scholar]
- 38. Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch‐repair status. Nature 2002; 418: 934. [DOI] [PubMed] [Google Scholar]
- 39. Kambara T, Simms LA, Whitehall VL et al . BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 2004; 53: 1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang S, Farraye FA, Mack C, Posnik O, O’Brien MJ. BRAF and KRAS mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol 2004; 28: 1452–9. [DOI] [PubMed] [Google Scholar]
- 41. Chan TL, Zhao W, Leung SY, Yuen ST. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res 2003; 63: 4878–81. [PubMed] [Google Scholar]
- 42. Markowitz S, Wang J, Myeroff L et al . Inactivation of the type II TGF‐β receptor in colon cancer cells with microsatellite instability. Science 1995; 268: 1336–8. [DOI] [PubMed] [Google Scholar]
- 43. Rampino N, Yamamoto H, Ionov Y et al . Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 1997; 275: 967–9. [DOI] [PubMed] [Google Scholar]
- 44. Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993; 363: 558–61. [DOI] [PubMed] [Google Scholar]
- 45. Aaltonen LA, Peltomaki P, Leach FS et al . Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260: 812–16. [DOI] [PubMed] [Google Scholar]
- 46. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993; 260: 816–19. [DOI] [PubMed] [Google Scholar]
- 47. Peltomaki P, De La Chapelle A. Mutations predisposing to hereditary nonpolyposis colorectal cancer. Adv Cancer Res 1997; 71: 93–119. [DOI] [PubMed] [Google Scholar]
- 48. Herman JG, Umar A, Polyak K et al . Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA 1998; 95: 6870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cunningham JM, Christensen ER, Tester DJ et al . Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998; 58: 3455–60. [PubMed] [Google Scholar]
- 50. Hawkins N, Norrie M, Cheong K et al . CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology 2002; 122: 1376–87. [DOI] [PubMed] [Google Scholar]
- 51. Hansen RS, Gartler SM, Scott CR, Chen SH, Laird CD. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum Mol Genet 1992; 1: 571–8. [DOI] [PubMed] [Google Scholar]
- 52. Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet 1994; 7: 536–40. [DOI] [PubMed] [Google Scholar]
- 53. Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998; 72: 141–96. [PubMed] [Google Scholar]
- 54. Jones PA. DNA methylation errors and cancer. Cancer Res 1996; 56: 2463–7. [PubMed] [Google Scholar]
- 55. Herman JG, Merlo A, Mao L et al . Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 1995; 55: 4525–30. [PubMed] [Google Scholar]
- 56. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6‐methylguanine‐DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 1999; 59: 793–7. [PubMed] [Google Scholar]
- 57. Kane MF, Loda M, Gaida GM et al . Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair‐defective human tumor cell lines. Cancer Res 1997; 57: 808–11. [PubMed] [Google Scholar]
- 58. Esteller M, Sparks A, Toyota M et al . Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res 2000; 60: 4366–71. [PubMed] [Google Scholar]
- 59. Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol 2002; 160: 1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol 1997; 28: 1396–407. [DOI] [PubMed] [Google Scholar]
- 61. Pretlow TP, Brasitus TA, Fulton NC, Cheyer C, Kaplan EL. K‐ras mutations in putative preneoplastic lesions in human colon. J Natl Cancer Inst 1993; 85: 2004–7. [DOI] [PubMed] [Google Scholar]
- 62. Heinen CD, Shivapurkar N, Tang Z, Groden J, Alabaster O. Microsatellite instability in aberrant crypt foci from human colons. Cancer Res 1996; 56: 5339–41. [PubMed] [Google Scholar]
- 63. Yamashita N, Minamoto T, Ochiai A, Onda M, Esumi H. Frequent and characteristic K‐ras activation and absence of p53 protein accumulation in aberrant crypt foci of the colon. Gastroenterology 1995; 108: 434–40. [DOI] [PubMed] [Google Scholar]
- 64. Otori K, Konishi M, Sugiyama K et al . Infrequent somatic mutation of the adenomatous polyposis coli gene in aberrant crypt foci of human colon tissue. Cancer 1998; 83: 896–900. [DOI] [PubMed] [Google Scholar]
- 65. Sakurazawa N, Tanaka N, Onda M, Esumi H. Instability of X chromosome methylation in aberrant crypt foci of the human colon. Cancer Res 2000; 60: 3165–9. [PubMed] [Google Scholar]
- 66. Bouzourene H, Chaubert P, Seelentag W, Bosman FT, Saraga E. Aberrant crypt foci in patients with neoplastic and nonneoplastic colonic disease. Hum Pathol 1999; 30: 66–71. [DOI] [PubMed] [Google Scholar]
- 67. Shpitz B, Bomstein Y, Mekori Y et al . Aberrant crypt foci in human colons: distribution and histomorphologic characteristics. Hum Pathol 1998; 29: 469–75. [DOI] [PubMed] [Google Scholar]
- 68. Roncucci L, Modica S, Pedroni M et al . Aberrant crypt foci in patients with colorectal cancer. Br J Cancer 1998; 77: 2343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Otori K, Sugiyama K, Hasebe T, Fukushima S, Esumi H. Emergence of adenomatous aberrant crypt foci (ACF) from hyperplastic ACF with concomitant increase in cell proliferation. Cancer Res 1995; 55: 4743–6. [PubMed] [Google Scholar]
- 70. Rosenberg DW, Yang S, Pleau DC et al . Mutations in BRAF and KRAS differentially distinguish serrated versus non‐serrated hyperplastic aberrant crypt foci in humans. Cancer Res 2007; 67: 3551–4. [DOI] [PubMed] [Google Scholar]
- 71. Jen J, Powell SM, Papadopoulos N et al . Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994; 54: 5523–6. [PubMed] [Google Scholar]
- 72. Otori K, Oda Y, Sugiyama K et al . High frequency of K‐ras mutations in human colorectal hyperplastic polyps. Gut 1997; 40: 660–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol 2002; 160: 529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bardi G, Johansson B, Pandis N et al . Cytogenetic aberrations in colorectal adenocarcinomas and their correlation with clinicopathologic features. Cancer 1993; 71: 306–14. [DOI] [PubMed] [Google Scholar]
- 75. Lothe RA, Peltomaki P, Meling GI et al . Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993; 53: 5849–52. [PubMed] [Google Scholar]
- 76. Dehari R. Infrequent APC mutations in serrated adenoma. Tohoku J Exp Med 2001; 193: 181–6. [DOI] [PubMed] [Google Scholar]
- 77. Fogt F, Brien T, Brown CA, Hartmann CJ, Zimmerman RL, Odze RD. Genetic alterations in serrated adenomas: comparison to conventional adenomas and hyperplastic polyps. Hum Pathol 2002; 33: 87–91. [DOI] [PubMed] [Google Scholar]
- 78. Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, Wu TT. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol 2003; 162: 815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Toyota M, Ohe‐Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA 2000; 97: 710–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rashid A, Shen L, Morris JS, Issa JP, Hamilton SR. CpG island methylation in colorectal adenomas. Am J Pathol 2001; 159: 1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Whitehall VL, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of O‐6‐methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low‐level DNA microsatellite instability. Cancer Res 2001; 61: 827–30. [PubMed] [Google Scholar]
- 82. Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology 2002; 123: 862–76. [DOI] [PubMed] [Google Scholar]
- 83. O’Brien MJ, Yang S, Mack C et al . Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30: 1491–501. [DOI] [PubMed] [Google Scholar]
- 84. Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27: 65–81. [DOI] [PubMed] [Google Scholar]
- 85. Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut 2004; 53: 573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


