Abstract
The aim of the present study was to clarify the mechanisms of cell death induced by heavy‐ion irradiation focusing on the bystander effect in human lung cancer A549 cells. In microbeam irradiation, each of 1, 5, and 25 cells under confluent cell conditions was irradiated with 1, 5, or 10 particles of carbon ions (220 MeV), and then the surviving fraction of the population was measured by a clonogenic assay in order to investigate the bystander effect of heavy‐ions. In this experiment, the limited number of cells (0.0001–0.002%, 5–25 cells) under confluent cell conditions irradiated with 5 or 10 carbon ions resulted in an exaggerated 8–14% increase in cell death by clonogenic assay. However, these overshooting responses were not observed under exponentially growing cell conditions. Furthermore, these responses were inhibited in cells treated with an inhibitor of gap junctional intercellular communication (GJIC), whereas they were markedly enhanced by the addition of a stimulator of GJIC. The present results suggest that bystander cell killing by heavy‐ions was induced mainly by direct cell‐to‐cell communication, such as GJIC, which might play important roles in bystander responses. (Cancer Sci 2009; 100: 684–688)
It is thought that heavy‐ion radiation therapy is a promising modality of cancer therapy that can deliver higher biological effects on tumors than conventional X‐ray radiation therapy, and may also conserve the function of normal organs because of its excellent dose distribution.( 1 ) Recently, excellent clinical results were reported in the treatment of lung cancer, prostate cancer, cancer of the head and neck, and malignant tumors of bone and soft tissue.( 2 ) Heavy‐ion has high linear energy transfer (LET) with increased relative biological effectiveness (RBE). However, the possible mechanism of increasing cell killing in heavy‐ion therapy is not fully understood. Previous studies have demonstrated that the induction of cell killing of non‐irradiated cells by adjacent cells that are directly irradiated by heavy‐ions is considered to play an important role in radiation‐induced cell death, not only in tumors but also in normal organs. This phenomenon is termed the “bystander effect”, and was reported by Nagasawa and Little in 1992.( 3 ) They reported that increased sister chromatid exchanges were observed in more than 30% of cells when less than 1% of the nuclei of cultured cells were actually traversed by an α‐particle in Chinese hamster ovary cells. It has been proposed that the mediating mechanism involves secreted soluble factors,( 4 , 5 , 6 ) an increase in oxidative stress,( 7 ) plasma membrane‐bound lipid rafts,( 8 ) and gap junctional intracellular communication (GJIC).( 4 , 7 , 9 , 10 , 11 , 12 , 13 ) Gap junctions are membrane channels that connect the cytoplasm of cells and allow direct exchange of small molecules and ions between adjacent cells. GJIC is essential for the maintenance of tissue homeostasis and proliferation and the regulation of embryonic development and differentiation, and it plays a key role in induction of the bystander effect.( 14 , 15 , 16 , 17 ) Here, bystander killing of cells by heavy‐ion irradiation was investigated, focusing on the involvement of GJIC on cell survival, to clarify the underlying mechanisms of the bystander effect in a human lung cancer cell line in vitro.
Materials and Methods
Cell cultures. A549 (a human lung cancer cell line with wild‐type p53) was obtained from the Cell Resource Center for Biomedical Research at the Institute of Development, Aging and Cancer, Tohoku University, Japan. Cells were cultured in RPMI‐1640 medium (Immuno‐Biological Laboratories, Takasaki, Japan) supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria). Two days prior to irradiation experiments, 5 × 105 and 5 × 104 cells were inoculated into a specially designed 35‐mm microbeam dish that was constructed as previously described,( 18 ) to prepare confluent cultures and sparsely populated cultures, respectively. Unless otherwise described, all cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. The size of the cell nucleus and the whole cells was determined to be 14.6 ± 4.3 and 106 ± 24.9 µm2, respectively.
Drug treatment. Prior to irradiation, cells were pretreated for 2 h with 0.1 mM lindane (Sigma, St Louis, MO, USA) and for 48 h with 1 mM 8‐Br‐cAMP (Sigma) to inhibit and stimulate GJIC, respectively.( 11 , 19 ) Control cells were treated with 0.25% dimethyl sulfoxide (DMSO) and 2% phosphate‐buffered saline (PBS) as the solvent for lindane and 8‐Br‐cAMP, respectively. Immediately after microbeam irradiation, the cells were washed once with fresh culture medium and maintained in the absence of these drugs. In parallel, immediately after sham‐irradiation, control cells were washed once with fresh culture medium and maintained in the absence of these drugs. The colony‐formation assay showed that at the concentrations used for these experiments, these drugs were not cytotoxic to A549 cells.
Irradiation. Conditioned medium was removed from the dishes of cell monolayers just prior to irradiation, and the dishes were covered with 8‐µm‐thick Kapton polyimide film (DuPont‐Toray, Tokyo, Japan) to avoid drying, followed by irradiation at room temperature. Immediately after irradiation, fresh medium was added to the dishes, and the cultures were then incubated at 37°C in 95% air/5% CO2. In all of the experiments, the cells for control conditions were sham‐irradiated and manipulated in parallel with the test cells.
For X‐irradiation, cultures were irradiated with X‐rays (0.93 Gy/min) from an X‐ray machine (MBR‐1505; Hitachi, Tokyo, Japan) at 140 kV and 4.5 mA with a 0.5‐mm Al filter.( 20 )
Targeted heavy‐ion irradiation to only a very small fraction of cells within the whole cell population was carried out using microbeams installed at the Takasaki Ion Accelerator for Advanced Radiation Application of Japan Atomic Energy Agency, for which the set‐up and irradiation procedures have been described.( 10 , 18 , 21 , 22 , 23 , 24 , 25 ) By using microbeams collimated through a 20‐µm‐diameter aperture, each of 1, 5, or 25 cells within the whole population was targeted with the precise number of carbon ions (18.3 MeV/u, LET = 103 keV/µm), as described previously.( 10 , 18 , 25 ) In a dish, 1.3 × 106 ± 0.4 × 106 cells were populated in confluent cultures that were then irradiated, and then the fraction of hit cells among the whole population was estimated to be 0.0001, 0.0004, and 0.002% when 1, 5, and 25 cells were targeted. Likewise, in sparse cultures, 7.4 × 104 ± 2.9 × 104 cells/dish were populated, and the fraction of hit cells was estimated to be 0.001, 0.007, and 0.03% when 1, 5, and 25 cells were targeted. In these situations, the vast majority of cells could be thence considered as bystander cells, and the contribution of the effects induced in hit cells to the overall response should be negligible.
Conventional heavy‐ion broadfield irradiation was carried out using broad‐beams of carbon ions (18.3 MeV/u, 108 keV/µm) as previously described.( 25 , 26 , 27 ) The LET at the cell surface was calculated according to the kinetic energy loss, assuming water equivalence. Dose (Gy) was calculated as fluence (ion particles/cm2) × LET (keV/µm) × 1.6 × 10−9. The physical properties of carbon ion broad‐beams and microbeams are shown in Table 1.
Table 1.
Physical properties of carbon ion broadbeam and microbeam
| Energy | LET (keV/µm) | Traversed particle/1 Gy | |
|---|---|---|---|
| Per nucleus | Per cell | ||
| A. Physical properties of carbon ion broadbeam | |||
| 220 MeV | 108 | 0.84 | 6.1 |
| Energy | LET (keV/µm) | Dose (Gy) by a particle to | |
|---|---|---|---|
| A nucleus | A whole cell | ||
| B. Physical properties of carbon ion microbeam | |||
| 220 MeV | 103 | 1.1 | 0.16 |
The average nuclear or whole‐cell cross section of confluent A549 cells was considered to be 14.6 and 106 µm2, respectively.
Clonogenic assay. Cell survival was tested by a clonogenic assay. Figure 1 shows the procedures for cell preparation and irradiation of microbeams. Within 1 h after irradiation, cells were reseeded into triplicate 60‐mm dishes and incubated for 14 days. Then, they were fixed and stained with 2% (w/v) crystal violet in methanol. Only colonies containing greater than 50 cells were counted as survivors. RBE was calculated based on the D10, which was determined as the dose (Gy) required to reduce the surviving fraction to 10%. Comparisons of surviving fractions between two groups were made by unpaired two‐tailed t‐test. A P‐value of 0.05 or less between groups was considered to be significant.
Figure 1.
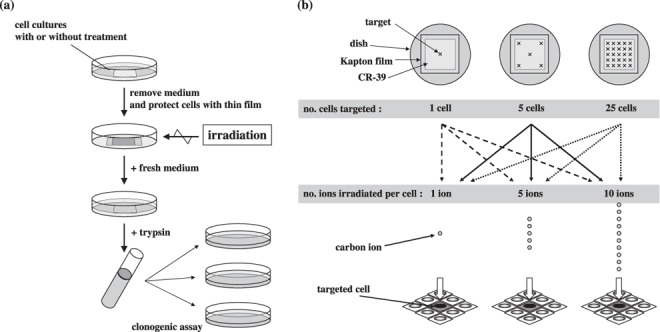
(a) Schema of the protocol of cell preparation and clonogenic assay. (b) Schema of microbeam irradiation. Each of 1, 5, or 25 cells in the whole population was targeted with a precise number of carbon ions.
Results
Survival curves for A549 cells exposed to X‐rays and broad‐beams of carbon ions. Figure 2 shows the dose responses of A549 cells to low LET X‐rays and high LET broad‐beams of carbon ions. The survival curve for carbon ions did not have initial shoulders, and the slope of the survival curve for carbon ions was steeper than that of the curve for X‐rays. The D0, the dose required to decrease the surviving fraction by e (1/e) in the linear range of the survival curve, and D10 X‐ray values were 1.23 and 4.16 Gy, and those of carbon ions were and 1.07 and 2.46 Gy, respectively. Thus, the RBE of carbon ions to X‐rays was calculated to be 1.60 at D10.
Figure 2.
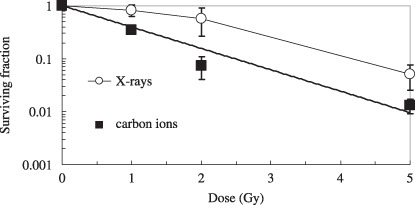
Survival curves for A549 cells exposed to 140 kV X‐rays or broad‐beams of 220 MeV carbon ions, tested by the clonogenic assay. The data are plotted with the means and standard errors of more than three independent experiments.
Microbeam irradiation in confluent culture. Figure 3 shows the surviving fraction of confluent A549 cell cultures where 1–25 cells were irradiated with 1–10 carbon ions. The surviving fraction of the non‐irradiated A549 control was normalized to 1.0 (plating efficiency ranging from 47 to 57%; average ± SD, 51 ± 5%). When five cells (0.0004% of the whole population) or 25 cells (0.002% of the whole population) were individually irradiated by 5 or 10 carbon ions per cell, the surviving fraction was significantly reduced compared with that of the non‐irradiated control cells, whereas the surviving fraction was not significantly different from the non‐irradiated control cells after single‐cell irradiation. The actual reduction rates that were statistically significant were 9% (P = 0.014) for the 5 cells irradiated with 5 ions, 9% (P = 0.021) for the 5 cells irradiated with 10 ions, 12% (P = 0.002) for the 25 cells irradiated with 5 ions, and 14% (P = 0.033) for the 25 cells irradiated with 10 ions.
Figure 3.
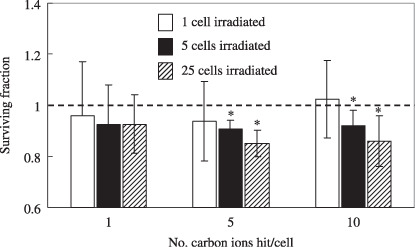
Surviving fraction of confluent cultures of A549 cells where 1–25 cells were irradiated with 1, 5, and 10 carbon ions. The surviving fraction of the non‐irradiated A549 control was normalized as 1.0 (asterisks: P < 0.05 compared with non‐irradiated A549 control). The data are plotted with the means and standard errors of more than three independent experiments. The reduction rates that were statistically significant were as follows: 9% (P = 0.014) for the 5 cells irradiated by 5 ions/cell; 8% (P = 0.021) for the 5 cells irradiated by 10 ions/cell; 12% (P = 0.002) for the 25 cells irradiated by 5 ions/cell; and 14% (P = 0.033) for the 25 cells irradiated by 10 ions/cell (asterisks: P < 0.05 compared with non‐irradiated A549 control).
Microbeam irradiation in low‐density culture. To investigate whether the bystander effect in A549 cells was induced by adjacent cell‐to‐cell communication, the surviving fractions of the confluent and low‐density cultures were compared for the condition of 25 cells irradiated with 5 ions per cell (Fig. 4). As shown in Figure 3, the surviving fraction in the confluent culture was significantly reduced compared with that of the non‐irradiated control cells (reduction of 12%, P = 0.002). On the other hand, when the 25 cells in the low‐density culture where the cells were not allowed to communicate directly with each other were hit by 5 carbon ions, no reduction in the surviving fraction was observed (Fig. 4).
Figure 4.
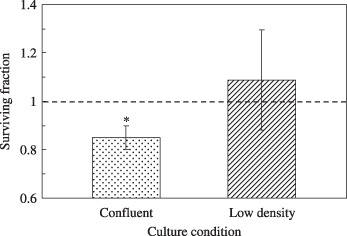
The surviving fraction of low‐density culture (non‐confluent) and confluent culture of A549 cells where 25 cells were irradiated with 5 carbon ions. The surviving fraction of the non‐irradiated A549 control was normalized as 1.0 (asterisk: P < 0.05 compared with non‐irradiated A549 control).
Role of GJIC in cell survival. Figure 5 shows the difference in the surviving fraction of confluent cells cultured with lindane, a GJIC‐suppressing agent, or 8‐Br‐cAMP, a GJIC‐stimulating agent, before irradiation. Twenty‐five cells were targeted by 5 carbon ions per cell in each culture. The surviving fraction of cells treated with lindane and exposed to carbon ions was almost the same as that of non‐irradiated cells (reduction of 3%), and was significantly higher than that of irradiated cells without lindane (P = 0.04). DMSO, a scavenger of reactive oxygen species, was added as the solvent for lindane. The surviving fraction of cells treated with 0.25% DMSO was almost the same as that of irradiated cells without DMSO (Fig. 5). When cells were treated with 8‐Br‐cAMP and exposed to carbon ions, the reduction in the surviving fraction was markedly enhanced (reduction of 21%), and this reduction value was somewhat higher than that of irradiated cells without 8‐Br‐cAMP (P = 0.070).
Figure 5.
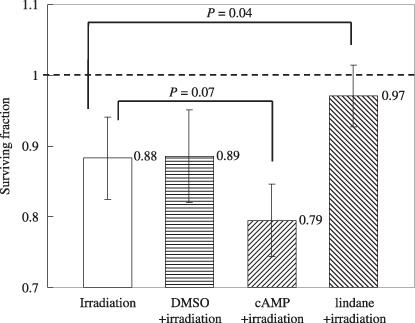
The surviving fraction of confluent cultures of A549 cells where 25 cells were irradiated with 5 carbon ions with or without treatment with lindane (an inhibitor of gap junctional intracellular communication [GJIC]), dimethyl sulfoxide (DMSO) (a solvent of lindane), or 8‐Br‐cAMP (a stimulator of GJIC). The surviving fraction of the non‐irradiated A549 control was normalized as 1.0. The data are plotted with the means and standard errors of more than three independent experiments.
Discussion
So far, heavy‐ion therapy has achieved good cancer controllability in a shorter treatment time while sparing critical normal organs.( 2 ) However, the underlying biological mechanism of cell killing by heavy‐ion irradiation is not fully elucidated. In our study, the cell‐killing effect of heavy‐ion irradiation was investigated by clonogenic assay, focusing on the bystander effect, which is cell killing through signal transduction from irradiated to non‐irradiated bystander cells.
In terms of biological responses of cells to heavy‐ion irradiation, the D10 values for carbon‐ion beams and X‐rays were 2.46 and 4.16 Gy, respectively. Therefore, the calculated RBE was 1.60 at D10 in the present study. Suzuki et al. investigated the RBE values of several human cell lines, including A549, and reported that the RBE values ranged from 2.00 to 3.01 for the 77 keV/µm carbon ion beam of the Heavy Ion Medical Accelerator in Chiba.( 28 ) Our RBE was somewhat smaller than their RBE but there was no significant difference.
It is considered that the bystander effect is an important factor that determines the survival of cells exposed to heavy‐ion beam irradiation in addition to its strong and direct biological effect. Several investigators have elucidated this phenomenon in detail using the microbeam irradiation system, which can individually deliver a precise number of charged particles to the cells, as Nagasawa and Little first reported the cell‐killing effect from irradiated cells to non‐irradiated cells.( 3 , 10 , 25 ) Sawant et al. reported that a 1–40% reduction in the surviving fraction was observed in non‐irradiated cells when 10% of the nuclei of the cultured cells were actually traversed by 1–16 α‐particles per cell in Chinese hamster V79 cells.( 29 ) In our study, the heavy‐ion microbeam apparatus was used to investigate the impact of the bystander effect on cell killing in a clonogenic assay. In fact, when only 0.0001–0.002% of the cells in the confluent culture were individually irradiated with microbeams of 5–10 carbon ions per cell, a significant reduction in the surviving fraction (9–14% : 104~105‐fold reduction more than estimated) was observed (Fig. 3). These findings support previous study of a radiation‐induced bystander effect for cell survival.
Several reports indicate that the bystander effect can be mediated by soluble factors. Matsumoto et al. reported the bystander response in the survival of cells after irradiation with carbon ions.( 30 ) They showed that the radiosensitivity of A‐172 cells was reduced in the conditioned medium of T98G cells irradiated with carbon‐ion beams compared with conventional fresh growth medium, and the reduction in radiosensitivity was abolished by the addition of an inducible nitric oxide synthase inhibitor to the conditioned medium. On the other hand, evidence has been presented for the involvement of gap junctions in signal transmission between irradiated and non‐irradiated cells.( 9 , 31 ) In the present study, a marked reduction in the surviving fraction was not observed in low‐density culture, although the surviving fraction was remarkably decreased in confluent culture for the condition where 25 cells of the dish were irradiated by 5 ions per cell (Fig. 4). These findings suggest that adjacent cell‐to‐cell communication plays an essential role in bystander killing induced by irradiation of heavy‐ions. In our experiments, conditioned medium was removed just prior to irradiation, and fresh medium was added to the dishes after irradiation. Thus, the signal for the bystander effect which was transmitted through the medium was not observed.
Moreover, in the present study, bystander cell death was enhanced by the addition of cAMP, the stimulator of GJIC, and in contrast, bystander cell death was suppressed by the addition of lindane, the inhibitor of GJIC (Fig. 5). These findings suggest that GJIC plays an important role in the bystander effect, which is consistent with other reports. Azzam et al. have reported direct evidence for the participation of GJIC in the bystander effect in human fibroblasts by α‐particle.( 9 ) They showed the involvement of connexin43 gap junctions with participating to the radiation stress, and changes in p21waf–1 expression correlated well with the induction of DNA damage. On the other hand, Edwards et al. showed downregulation of GJIC as an adaptive, protective response to toxicity, including radiation. They demonstrated that when epithelial cells of rat were irradiated with ultrasoft X‐rays, inhibition of GJIC occurred in non‐irradiated bystander cells, indicating that GJIC may play a role in protecting non‐irradiated bystander cells by closure of the junction, inhibiting the spread of the toxic factor from irradiated cells.( 32 ) The level and nature of the bystander effects via GJIC are likely to be dependent on the cell type.( 33 )
In conclusion, we have demonstrated the bystander effect of heavy‐ion‐influenced clonogenic potential in a cell line of human lung cancer, where GJIC may play an essential role in this bystander response. Understanding the mechanisms of the bystander effect will make it possible to develop techniques for increasing radiation damage in tumors or decreasing radiation damage in normal tissues adjacent to tumors in radiation therapy.
Acknowledgments
We are grateful to Aiko Takeuchi, Hideko Kazama, Masako Shin, Yuka Taguchi, Yukari Yoshida, and Megumi Miyamoto for technical assistance, and the crew at the Takasaki Ion Accelerator for Advanced Radiation Application of the Japan Atomic Energy Agency for help with the heavy‐ion irradiation. This work was supported by a Grant‐in‐Aid for the 21st Century Center of Excellence Program for Biomedical Research Using Accelerator Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Blakely EA, Kronenberg A. Heavy‐ion radiobiology: new approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat Res 1998; 150: S126–45. [PubMed] [Google Scholar]
- 2. Tsujii H, Mizoe J, Kamada T et al . Clinical results of carbon ion radiotherapy at NIRS. J Radiat Res 2007; 48: A1–13. [DOI] [PubMed] [Google Scholar]
- 3. Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha‐particles. Cancer Res 1992; 52: 6394–6. [PubMed] [Google Scholar]
- 4. Matsumoto H, Hayashi S, Hatashita M et al . Induction of radioresistance by a nitric oxide‐mediated bystander effect. Radiat Res 2001; 155: 387–96. [DOI] [PubMed] [Google Scholar]
- 5. Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci USA 2004; 101: 13 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide‐mediated bystander effect induced by heavy‐ions in human salivary gland tumour cells. Int J Radiat Biol 2002; 78: 837–44. [DOI] [PubMed] [Google Scholar]
- 7. Azzam EI, Toledo SM, Little JB. Stress signaling from irradiated to non‐irradiated cells. Curr Cancer Drug Targets 2004; 4: 53–64. [DOI] [PubMed] [Google Scholar]
- 8. Nagasawa H, Cremesti A, Kolesnick R, Fuks Z, Little JB. Involvement of membrane signaling in the bystander effect in irradiated cells. Cancer Res 2002; 62: 2531–4. [PubMed] [Google Scholar]
- 9. Azzam EI, De Toledo SM, Little JB. Direct evidence for the participation of gap junction‐mediated intercellular communication in the transmission of damage signals from alpha‐particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 2001; 98: 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shao C, Furusawa Y, Kobayashi Y, Funayama T, Wada S. Bystander effect induced by counted high‐LET particles in confluent human fibroblasts: a mechanistic study. FASEB J 2003; 17: 1422–7. [DOI] [PubMed] [Google Scholar]
- 11. Shao C, Furusawa Y, Aoki M, Ando K. Role of gap junctional intercellular communication in radiation‐induced bystander effects in human fibroblasts. Radiat Res 2003; 160: 318–23. [DOI] [PubMed] [Google Scholar]
- 12. Hamada N, Matsumoto H, Hara T, Kobayashi Y. Intercellular and intracellular signaling pathways mediating ionizing radiation‐induced bystander effect. J Radiat Res 2007; 48: 87–95. [DOI] [PubMed] [Google Scholar]
- 13. Hamada N. Recent insights into the biological action of heavy‐ion radiation. J Radiat Res 2009; 50: 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Trosko JE. Gap junctional intercellular communication as a biological ‘rosetta stone’ in understanding, in a systems biological manner, stem cell behavior, mechanisms of epigenetic toxicology, chemoprevention and chemotherapy. J Membr Biol 2007; 218: 93–100. [DOI] [PubMed] [Google Scholar]
- 15. McLachlan E, Shao Q, Laird DW. Connexins and gap junctions in mammary gland development and breast cancer progression. J Membr Biol 2007; 218: 107–21. [DOI] [PubMed] [Google Scholar]
- 16. Kojima T, Murata M, Go M, Spray DC, Sawada N. Connexins induce and maintain tight junctions in epithelial cells. J Membr Biol 2007; 217: 13–19. [DOI] [PubMed] [Google Scholar]
- 17. Sato H, Hagiwara H, Ohde Y, Senba H, Virgona N, Yano T. Regulation of renal cell carcinoma cell proliferation, invasion and metastasis by connexin 32 gene. J Membr Biol 2007; 216: 17–21. [DOI] [PubMed] [Google Scholar]
- 18. Funayama T, Wada S, Yokota Y et al . Heavy‐ion microbeam system at JAEA‐Takasaki for microbeam biology. J Radiat Res 2008; 49: 373–9. [DOI] [PubMed] [Google Scholar]
- 19. Hamada N, Kodama S, Suzuki K, Watanabe M. Gap junctional intercellular communication and cellular response to heat stress. Carcinogenesis 2003; 24: 1723–8. [DOI] [PubMed] [Google Scholar]
- 20. Akimoto T, Nonaka T, Ishikawa H et al . Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: possible involvement of inhibition of survival signal transduction pathways. Int J Radiat Oncol Biol Phys 2001; 50: 195–201. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi Y, Funamaya T, Wada S et al . Microbeams of heavy charged particles. Biol Sci Space 2004; 18: 235–40. [DOI] [PubMed] [Google Scholar]
- 22. Funayama T, Hamada N, Sakashita T, Kobayashi Y. Heavy‐ion microbeams – development and biological applications in biological studies. IEEE Trans Plasma Sci 2008; 36: 1432–40. [Google Scholar]
- 23. Sugimoto T, Dazai K, Sakashita T et al . Cell cycle arrest apoptosis in Caenorhabditis elegans germline cells following heavy‐ion microbeam irradiation. Int J Radiat Biol 2006; 82: 31–8. [DOI] [PubMed] [Google Scholar]
- 24. Iwakawa M, Hamada N, Imadome K et al . Expression profiles are different in carbon ion‐irradiated normal human fibroblasts and their bystander cells. Mutat Res 2008; 642: 57–67. [DOI] [PubMed] [Google Scholar]
- 25. Hamada N, Ni M, Funayama T, Sakashita T, Kobayashi Y. Temporally distinct response of irradiated normal human fibroblasts and their bystander cells to energetic heavy ions. Mutat Res 2008; 639: 35–44. [DOI] [PubMed] [Google Scholar]
- 26. Hamada N, Hara T, Funayama T, Sakashita T, Kobayashi Y. Energetic heavy ions accelerate differentiation in the descendants of irradiated normal human diploid fibroblasts. Mutat Res 2008; 637: 190–6. [DOI] [PubMed] [Google Scholar]
- 27. Hamada N, Hara T, Omura‐Minamisawa M et al . Energetic heavy ions overcome tumor radioresistance caused by overexpression of Bcl‐2. Radiother Oncol 2008; 89: 231–6. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K. Relative biological effectiveness for cell‐killing effect on various human cell lines irradiated with heavy‐ion medical accelerator in Chiba (HIMAC) carbon‐ion beams. Int J Radiat Oncol Biol Phys 2000; 48: 241–50. [DOI] [PubMed] [Google Scholar]
- 29. Sawant SG, Zheng W, Hopkins KM, Randers‐Pehrson G, Lieberman HB, Hall EJ. The radiation‐induced bystander effect for clonogenic survival. Radiat Res 2002; 157: 361–4. [DOI] [PubMed] [Google Scholar]
- 30. Matsumoto H, Hayashi S, Hatashita M et al . Induction of radioresistance to accelerated carbon‐ion beams in recipient cells by nitric oxide excreted from irradiated donor cells of human glioblastoma. Int J Radiat Biol 2000; 76: 1649–57. [DOI] [PubMed] [Google Scholar]
- 31. Bishayee A, Hill HZ, Stein D, Rao DV, Howell RW. Free radical‐initiated and gap junction‐mediated bystander effect due to nonuniform distribution of incorporated radioactivity in a three‐dimensional tissue culture model. Radiat Res 2001; 155: 335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edwards GO, Botchway SW, Hirst G, Wharton CW, Chipman JK, Meldrum RA. Gap junction communication dynamics and bystander effects from ultrasoft X‐rays. Br J Cancer 2004; 90: 1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hall EJ. The bystander effect. Health Phys 2003; 85: 31–5. [DOI] [PubMed] [Google Scholar]


