Abstract
The emphasis in anticancer drug discovery has always been on finding a drug with great antitumor potential but few side‐effects. This can be achieved if the drug is specific for a molecular site found only in tumor cells. Here, we find the enhancer of zeste homolog 2 (EZH2) to be highly overexpressed in lung and other cancers, and show that EZH2 is integral to proliferation in cancer cells. Quantitative real‐time PCR analysis revealed higher expression of EZH2 in clinical bladder cancer tissues than in corresponding non‐neoplastic tissues (P < 0.0001), and we confirmed that a wide range of cancers also overexpress EZH2, using cDNA microarray analysis. Immunohistochemical analysis showed positive staining for EZH2 in 14 of 29 cases of bladder cancer, 135 of 292 cases of non‐small‐cell lung cancer (NSCLC), and 214 of 245 cases of colorectal cancer, whereas no significant staining was observed in various normal tissues. We found elevated expression of EZH2 to be associated with poor prognosis for patients with NSCLC (P = 0.0239). In lung and bladder cancer cells overexpressing EZH2, suppression of EZH2 using specific siRNAs inhibited incorporation of BrdU and resulted in significant suppression of cell growth, even though no significant effect was observed in the normal cell strain CCD‐18Co, which has undetectable EZH2. Because EZH2 expression was scarcely detectable in all normal tissues we examined, EZH2 shows promise as a tumor‐specific therapeutic target. Furthermore, as elevated levels of EZH2 are associated with poor prognosis of patients with NSCLC, its overexpression in resected specimens could prove a useful molecular marker, indicating the necessity for a more extensive follow‐up in some lung cancer patients after surgical treatment. (Cancer Sci 2011; 102: 1298–1305)
The emergence of effective cancer chemotherapy is one of the major medical advances of recent years. Adjuvant chemotherapy for lung, breast, or colon cancer can augment the survival benefit afforded by surgical management. Even in patients with advanced solid tumors or recurrences after surgery, chemotherapy can offer lengthened survival of worthwhile quality.( 1 ) In the latter patients, the therapeutic index is narrow: responses are usually partial, and often disappointingly brief.( 2 ) Most antitumor agents have unexpected detrimental side‐effects, therefore, it is critical to discover novel therapeutic targets to extend the capability of cancer chemotherapy and improve patient care.
The N‐terminal tails of histones are subjected to post‐translational modifications, including methylation, acetylation and phosphorylation, which generate an extensive repertoire of chromatin structures. We previously reported that SMYD3, a histone lysine methyltransferase, stimulates proliferation of cells and plays an important role in human carcinogenesis through its methyltransferase activity.( 3 , 4 , 5 , 6 , 7 ) With the exception of Dot1/DOT1L, all histone lysine methyltransferases (HKMTs) contain a SET domain of approximately 130 amino acids.( 8 ) The SET domain was originally identified as a domain shared by three Drosophila proteins involved in epigenetic processes: the suppressor of position‐effect variegation [Su(var)3‐9]; an enhancer of the eye colour mutant zeste which belongs to the polycomb group (PcG) of proteins [E(Z)]; and the homeobox gene regulator trithorax (TRX).( 9 ) Mammalian HKMTs can be classified into several families according to sequence similarities within their SET domain and adjacent sequences, and according to structural features such as the presence of other defined protein domains.( 8 ) Although our knowledge of the physiological functions of histone methyltransferases is growing, their involvement in human disease remains largely unclear.
In order to investigate the roles of HKMTs in human carcinogenesis, we examined expression profiles of human HKMTs in clinical tissues, and found that in various types of cancer, expression levels of the enhancer of zeste homolog 2 (EZH2) were significantly upregulated, compared with their corresponding normal tissues. EZH2 is a PcG protein homologous to Drosophila enhancer of zeste, a histone methyltransferase associated with transcriptional repression. EZH2 has a SET domain and catalyses the addition of methyl groups to histone H3 at lysine 27 (H3K27). This polycomb group protein is conserved during evolution and is required for early mouse development.( 10 ) EZH2 acts as the catalytic subunit of the PRC2 core complex, which also contains the two PcG proteins SUZ12 and EED. The PRC2 complex is responsible for repressing of a large number of genes essential for development and differentiation.( 11 , 12 , 13 ) PcG proteins specify positional information such as antero‐posterior patterning, through activating or repressing the stable state of Hox gene expression.( 14 , 15 ) In addition to these well‐established functions in embryonic development, a series of studies suggest that PcG proteins may influence both Hox‐dependent and independent downstream pathways that control cell proliferation. In this study, we show that deregulation of EZH2 expression is observed in various types of cancers, and correlates with a negative outcome in patients with non‐small‐cell lung cancer (NSCLC) after surgical resection. This work forms the basis for further functional studies, which will explore EZH2 as a potential therapeutic and prognostic target in lung and other cancers.
Materials and Methods
Lung tissue samples for tissue microarray. Primary NSCLC tissue samples, and corresponding normal tissue adjacent to resection margins, were obtained from patients who had no anticancer treatment before tumor resection, with their informed consent.( 16 , 17 , 18 ) All tumors were staged on the basis of the pathologic TNM classification of the International Union Against Cancer. Formalin‐fixed primary lung tumors and adjacent normal lung tissue samples used for immunostaining on tissue microarrays had been obtained from 292 patients undergoing curative surgery at the Saitama Cancer Center (Saitama, Japan).( 19 , 20 ) To be eligible for this study, tumor samples were selected from patients who fulfilled all of the following criteria: (i) patients suffered primary NSCLC with a histologically confirmed stage (only pT1–pT3, pN0–pN2, and pM0); (ii) patients had undergone curative surgery, but did not receive any preoperative treatment; (iii) among them, NSCLC patients with positive lymph node metastasis (pN1, pN2) had been treated with platinum‐based adjuvant chemotherapies after surgical resection, whereas patients with pN0 did not receive adjuvant chemotherapies; and (iv) clinical follow‐up data were available. This study and the use of all clinical materials mentioned were approved by the relevant institutional ethics committees.
Colorectal tissue samples for immunohistochemistry. For Japanese cases, 172 primary human colorectal adenocarcinomas (ADC) were studied by immunohistochemistry. All tumors were obtained from patients who had undergone endoscopic resection or surgery at Nagasaki University Hospital (Nagasaki, Japan) between 2000 and 2004. Of the 172 patients with colorectal carcinoma, there were 91 men and 81 women. The median age was 63.5 years (range, 16–90 years). Each tumor was assigned a histological type according to the World Health Organisation classification: well differentiated ADC; moderately differentiated ADC; poorly differentiated ADC; and mucinous ADC.( 21 )
Immunohistochemical staining and tissue microarray. Immunohistochemical analysis was carried out using a specific mouse‐EZH2 antibody (NCL‐L‐EZH2; Leica Microsystems, Wetzlar, Germany) as described previously.( 18 , 22 , 23 , 24 , 25 ) Tumor tissue microarrays were constructed with 292 primary NSCLCs obtained by a single institutional group (as above) with one protocol for collection, fixation, and preservation of tissues after resection.( 26 , 27 , 28 ) Considering the histologic heterogeneity of individual tumors, a tissue area for sampling was selected based on visual alignment with the corresponding H&E‐stained section on a slide. Three, four, or five tissue cores (diameter, 0.6 mm; depth, 3–4 mm) taken from a donor tumor block were placed into a recipient paraffin block with a tissue microarrayer (Beecher Instruments, Sun Prairie, WI, USA). A core of normal tissue was punched from each case, and 5‐μm sections of the resulting microarray block were used for immunohistochemical analysis. Three independent investigators semiquantitatively assessed EZH2 positivity without prior knowledge of clinicopathologic data. Because the intensity of staining within each tumor tissue core was mostly homogeneous, EZH2 staining was semiquantitatively evaluated using the following criteria: negative (no appreciable staining in tumor cells); and positive (brown staining appreciable in the nucleus of tumor cells). Cases were accepted as positive only if all reviewers independently defined them as such.
Quantitative real‐time PCR. Specific primers for human GAPDH, B2M (housekeeping genes), and EZH2 were designed (primer sequences are given in Table S1). Polymerase chain reactions were carried out using the LightCycler 480 System (Roche Applied Science, Mannheim, Germany) following the manufacturer’s protocol.
siRNA transfection and cell growth assay. siRNA oligonucleotide duplexes were purchased from Sigma‐Aldrich (St. Louis, MO, USA) for targeting the human EZH2 transcripts. siEGFP and siNegative control (siNC, B‐Bridge), which consists of three different oligonucleotide duplexes, were used as control siRNAs. The siRNA sequences are described in Table S2. siRNA duplexes (100 nM final concentration) were transfected into lung and bladder cancer cell lines with Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) for 72 h, and cell growth was examined using the Cell Counting kit‐8 (Dojindo, Kumamoto, Japan).( 3 )
Statistical analysis. Student’s t‐test or Mann–Whitney’s U‐test was used to analyze the difference between two independent subgroups. Survival curves were calculated from the date of surgery to either the time of death relating to only cancer, or to the last follow‐up observation. Kaplan–Meier curves were calculated for each relevant variable and for EZH2 expression; differences in survival times among patient subgroups were analyzed using the log–rank test. Univariate and multivariate analyses were done with the Cox proportional hazard regression model to determine associations between clinicopathological variables and cancer‐related mortality. First, we analyzed associations between death and possible prognostic factors including age, gender, histology, pT classification, and pN classification, taking into consideration one factor at a time. Second, multivariate Cox analysis was applied on backward (stepwise) procedures that always forced strong EZH2 expression into the model, along with any and all variables that satisfied an entry level of P < 0.05. As the model continued to add factors, independent factors did not exceed an exit level of P < 0.05.
Results
EZH2 expression upregulated in clinical bladder cancer tissue but barely detectable in normal tissue. We examined the level of histone lysine methyltransferase gene products in a small subset of British clinical bladder cancer samples by quantitative real‐time PCR, and found significant overexpression of EZH2 in the cancer samples compared with non‐cancerous samples (data not shown). We then analyzed 119 bladder cancer samples and 25 normal control samples (also British), and confirmed a significant elevation of EZH2 expression levels in tumor cells compared with normal cells (Fig. 1A; P < 0.0001, Mann–Whitney U‐test). Importantly, quantitative real‐time PCR analysis showed that expression levels of EZH2 in bladder tumor tissues are notably higher than those in any other normal tissues we examined (Fig. 1B). We then analyzed the expression patterns of EZH2 in a number of clinical samples derived from Japanese bladder cancer subjects examined by cDNA microarray (Fig. 1C), and again identified significant overexpression (P < 0.0001, Mann–Whitney U‐test). To evaluate protein expression levels of EZH2 in bladder tissues, we carried out immunohistochemical analyses using an anti‐EZH2 antibody, and observed strong staining in the nucleus of malignant cells, but weak or absent staining in non‐neoplastic tissues (Fig. 1D). Tissue microarray analysis indicated that EZH2 was positively stained in 14 of 29 bladder cancer cases (48.3%), whereas no staining was observed in normal bladder tissues (Table S3). Subclassification of tumors according to tumor grade identified that Grade III tumors showed significantly higher EZH2 protein expression than Grade I and Grade II tumors, implying that EZH2 expression is more elevated in advanced bladder tumors (Table S4). Importantly, specific EZH2 signals were not detected in normal heart, lung, liver, colon, kidney, or bladder tissues (Fig. 1E), consistent with our real‐time PCR result.
Figure 1.
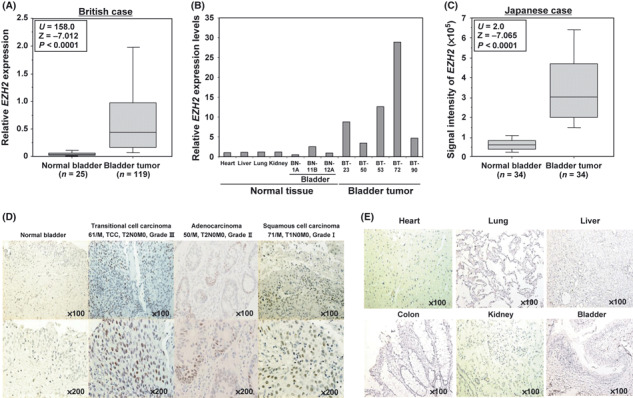
Elevated expression of the histone methyltransferase EZH2 in human bladder cancer. (A) EZH2 gene expression in normal and tumor bladder tissues in British cases. Expression levels of EZH2 were analyzed by quantitative real‐time PCR, and the result is shown by box‐whisker plot (median 50% boxed). Relative mRNA expression shows the value normalized by GAPDH and SDH expressions. The Mann–Whitney U‐test was used for statistical analysis (***P < 0.0001). (B) Quantitative real‐time PCR analysis was carried out in normal heart, liver, lung, and kidney tissues and randomly selected normal and tumor bladder tissues (three cases and five cases, respectively). (C) Comparison of EZH2 expression between normal and tumor bladder tissues in Japanese patients. Signal intensity for each sample was analyzed by cDNA microarray, and the result is shown by a box and whisker plot (median 50% boxed). The Mann–Whitney U‐test was used for statistical analysis. (D) Immunohistochemical staining of EZH2 in bladder tissues. Clinical information for each section is represented above histological pictures. Original magnification, ×100, ×200. (E) Immunohistochemical analysis of EZH2 in various normal tissues. No significant staining was observed. Original magnification, ×100.
EZH2 expression significantly high in lung and colorectal cancer tissues, and correlates with poor prognosis in NSCLC. cDNA microarray experiments showed that EZH2 expression was similarly elevated in lung tumor tissues compared with corresponding non‐neoplastic tissues (Fig. 2A; P < 0.0001, Mann–Whitney U‐test). We then examined EZH2 protein expression levels in lung tissue by immunohistochemistry and observed strong EZH2 staining in cancer tissues but no significant staining in non‐neoplastic tissues (Fig. 2B). To analyze the significance of EZH2 protein expression in lung cancer tissues in more detail, we carried out immunohistochemical analysis on a tissue microarray containing tissue sections from 292 NSCLC patients who had undergone surgical resection (Fig. S1). EZH2 stained positively in 135 out of 292 cases (46.2%) and negatively in 157 cases (53.8%). Subsequently, we analyzed the association of EZH2 expression with clinical outcome, and found that expression of EZH2 in NSCLC patients was significantly associated with male gender (P = 0.0002, Fisher’s exact test; Table 1), non‐ADC histology (P < 0.0001), smoking status (smoker; P < 0.0001), pN factor (N1 + N2; P = 0.0269), and tumor‐specific 5‐year survival after the resection of primary tumors (P = 0.0239 by log–rank test; Fig. 2C). Univariate analysis revealed associations between poor prognosis in NSCLC patients and several factors, including EZH2 expression, age, gender, histologic type (non‐ADC versus ADC), pT stage (tumor size, T1 versus T2 + T3), and pN stage (node status, N0 versus N1 + N2; Table 2). In contrast, multivariate analysis revealed that EZH2 status did not show statistical significance as an independent prognostic factor for surgically treated NSCLC patients enrolled in this study, whereas age and pT and pN factors did (Table 2). This result might be due to the preponderance of EZH2 expression up to the pN factor.
Figure 2.
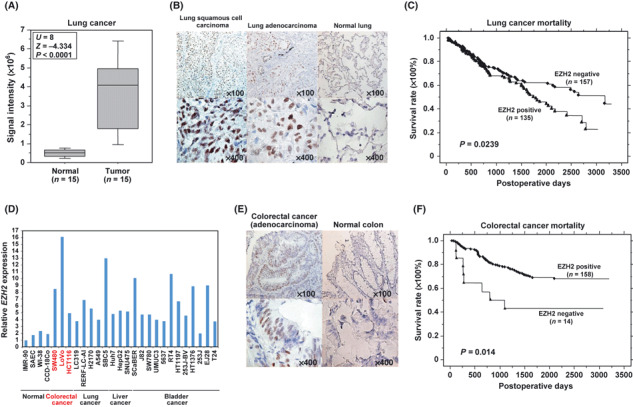
Histone methyltransferase EZH2 was overexpressed in lung and colorectal cancers, and appears to be a prognostic marker of non‐small‐cell lung cancer (NSCLC). (A) Comparison of EZH2 expression between normal and tumor (SCLC) lung tissues in Japanese patients. Signal intensity for each sample was analyzed by cDNA microarray, and the result is shown by a box and whisker plot (median 50% boxed). The Mann–Whitney U‐test was used for statistical analysis. (B) Immunohistochemical staining of EZH2 in lung tissues. Clinical information for each section is represented above histological pictures. Original magnification, ×100, ×400. (C) Kaplan–Meier estimates of overall survival time of patients with NSCLC (P = 0.0239, log–rank test). (D) Expression of EZH2 in four normal cell lines, three colorectal cancer cell lines, five lung cancer cell lines, three liver cancer cell lines, and 12 bladder cancer cell lines. Expression levels were analyzed by quantitative real‐time PCR, and relative mRNA expression shows the value normalized by GAPDH and SDH expressions. (E) Immunohistochemical staining of EZH2 in colorectal tissues. Original magnification, ×100, ×400. (F) Kaplan–Meier estimates of overall survival time of patients with NSCLC (P = 0.014, log–rank test).
Table 1.
Association between EZH2‐positive status in non‐small‐cell lung carcinomas and patients’ characteristics
| Total (n = 292) | EZH2 positive (n = 135) | EZH2 negative (n = 157) | P‐value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 204 | 109 | 95 | 0.0002* |
| Female | 88 | 26 | 62 | |
| Age (years) | ||||
| <65 | 129 | 59 | 70 | NS (0.9063) |
| ≥65 | 163 | 76 | 87 | |
| Histological type | ||||
| ADC | 180 | 63 | 117 | <0.0001*,** |
| SCC | 79 | 50 | 29 | |
| Others | 33 | 22 | 11 | |
| Smoking status | ||||
| Smoker | 208 | 114 | 94 | <0.0001* |
| Never | 84 | 21 | 63 | |
| pT factor | ||||
| T1 | 124 | 49 | 75 | NS (0.0575) |
| T2 + T3 | 168 | 86 | 82 | |
| pN factor | ||||
| N0 | 189 | 78 | 111 | 0.0269* |
| N1 + N2 | 103 | 57 | 46 | |
*P < 0.05 (Fisher’s exact test); **ADC versus non‐ADC. ADC, adenocarcinoma; NS, not significant; Others, large‐cell carcinoma plus adenosquamous cell carcinoma; SCC, squamous cell carcinoma.
Table 2.
Cox’s proportional hazards model analysis of prognostic factors in patients with non‐small‐cell lung carcinoma
| Variables | Hazards ratio | 95% CI | Unfavorable/favorable | P‐value |
|---|---|---|---|---|
| Univariate analysis | ||||
| EZH2 | 1.482 | 1.051–2.091 | Positive/negative | 0.0248* |
| Age (years) | 1.677 | 1.171–2.401 | ≥65/<65 | 0.0048* |
| Gender | 1.620 | 1.089–2.415 | Male/female | 0.0173* |
| Histological type | 1.551 | 1.103–2.181 | Non‐ADC/ADC | 0.0117* |
| Smoking status | 1.308 | 0.882–1.939 | Smoker/never | 0.1821 |
| pT factor | 2.755 | 1.862–4.065 | T2 + T3/T1 | <0.0001* |
| pN factor | 2.427 | 1.724–3.413 | N1 + N2/N0 | <0.0001* |
| Multivariate analysis | ||||
| EZH2 | 1.142 | 0.790–1.652 | Positive/negative | NS(0.4791) |
| Age (years) | 1.821 | 1.266–2.620 | ≥65/<65 | 0.0012* |
| Gender | 1.234 | 0.794–1.919 | Male/female | NS(0.3498) |
| Histological type | 1.056 | 0.718–1.554 | Non‐ADC/ADC | NS(0.6896) |
| pT factor | 2.155 | 1.429–3.257 | T2 + T3/T1 | 0.0003* |
| pN factor | 2.227 | 1.565–3.165 | N1 + N2/N0 | <0.0001* |
*P < 0.05 (Fisher’s exact test). ADC, adenocarcinoma; CI, confidence interval; NS, not significant.
Quantitative real‐time PCR analysis revealed that EZH2 expression levels in cancer cells were significantly higher than those in normal human cells, and intriguingly, expression levels of EZH2 in colorectal cancer cell lines are remarkably high (Fig. 2D). Therefore, we also examined EZH2 expression levels in colorectal clinical tissues by immunohistochemistry and observed that elevated expression of EZH2 was restricted to cancer tissues (Fig. 2E). Tissue microarray analysis showed that overexpression of EZH2 protein could be observed in 55 out of 72 colorectal carcinoma cases (76.4%; Table S5). To clarify the association between EZH2 overexpression and clinical outcomes in colorectal cancer, we carried out additional immunohistochemical analysis using a number of Japanese cases, who had undergone endoscopic resection or surgery at Nagasaki University Hospital (Fig. S2). EZH2 stained positively in 158 out of 174 cases (91.4%) and negatively in 14 cases (8.6%; Table 3). Next, we examined the association of EZH2 expression with clinical outcomes, and found that expression of EZH2 in colorectal cancer patients was significantly associated with P‐factor negative cases (P = 0.019, Fisher’s exact test; Table 3), M‐factor negative (P = 0.011), and also tumor‐specific 5‐year survival after resection of primary tumors (P = 0.014, log–rank test; Fig. 2F). Univariate analysis of association between patient prognosis and several factors, including EZH2 expression, age, gender, serum carcinoembryonic antigen level, serum CA19‐9 level, pT factor, pN factor, and M factor, was examined. All factors except age and gender were associated with poor prognosis (Table 4). In addition, multivariate analysis revealed that EZH2 status and M factor for colorectal cancer did show statistical significance as independent prognostic factors for surgically treated colorectal cancer patients enrolled in this study. These results imply that EZH2 protein is markedly overexpressed at the protein level in colorectal cancer, and EZH2 overexpression indicates a good prognosis, in contrast to the poor prognosis associated with EZH2 overexpression in lung cancer.
Table 3.
Association between EZH2‐positive status in colorectal cancer and patients’ characteristics (n = 172)
| Total (n = 172) | EZH2 positive (n = 158) | EZH2 negative (n = 14) | P‐value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 91 | 82 | 9 | 0.416 |
| Female | 81 | 76 | 5 | |
| Age (years) | ||||
| <65 | 84 | 75 | 9 | 0.272 |
| ≥65 | 88 | 83 | 5 | |
| Serum CEA level | ||||
| <5.0 | 121 | 114 | 7 | 0.123 |
| ≥5.0 | 51 | 44 | 7 | |
| Serum CA19‐9 level | ||||
| <37 | 131 | 123 | 8 | 0.102 |
| ≥37 | 41 | 35 | 6 | |
| pT factor | ||||
| T1≥ | 21 | 19 | 2 | 0.682 |
| T2≤ | 151 | 139 | 12 | |
| pN factor | ||||
| N− | 97 | 87 | 10 | 0.274 |
| N+ | 75 | 71 | 4 | |
| H factor | ||||
| H− | 150 | 140 | 10 | 0.085 |
| H+ | 22 | 18 | 4 | |
| P factor | ||||
| P− | 164 | 153 | 11 | 0.019* |
| P+ | 8 | 5 | 3 | |
| M factor | ||||
| N− | 145 | 137 | 8 | 0.011* |
| N+ | 27 | 21 | 6 | |
*P < 0.05 (Fisher’s exact test). CEA, carcinoembryonic antigen; NS, not significant.
Table 4.
Cox’s proportional hazards model analysis of prognostic factors in patients with colorectal cancer
| Variables | Hazards ratio | 95% CI | Unfavorable/favorable | P‐value |
|---|---|---|---|---|
| Univariate analysis | ||||
| EZH2 | 0.391 | 0.185–0.827 | Positive/negative | 0.014* |
| Age(years) | 0.958 | 0.409–1.157 | ≥65/<65 | 0.158 |
| Gender | 1.022 | 0.611–1.710 | Male/female | 0.934 |
| Serum CEA level | 3.862 | 2.294–6.500 | 5.0>/5.0≤ | <0.001* |
| Serum CA19‐9 level | 2.534 | 1.495–4.293 | 37>/37≤ | 0.001* |
| pT factor | 4.517 | 1.102–18.516 | T1≥/T2≤ | 0.036* |
| pN factor | 2.450 | 1.441–4.167 | N−/N+ | 0.001* |
| M factor | 8.570 | 4.986–14.731 | M0/M1 | <0.001* |
| Multivariate analysis | ||||
| EZH2 | 0.423 | 0.185–0.967 | Positive/negative | 0.041* |
| Age(years) | 0.958 | 0.548–1.675 | ≥65/<65 | 0.881 |
| Gender | 1.196 | 0.688–2.078 | Male/female | 0.526 |
| Serum CEA level | 1.445 | 0.731–2.858 | 5.0>/5.0≤ | 0.29 |
| Serum CA19‐9 level | 1.169 | 0.604–2.262 | 37>/37≤ | 0.643 |
| pT factor | 2.089 | 0.483–9.031 | T1≥/T2≤ | 0.324 |
| pN factor | 1.447 | 0.783–2.673 | N−/N+ | 0.238 |
| M factor | 4.620 | 2.380–8.969 | M0/M1 | <0.001* |
*P < 0.05 (Fisher’s exact test). CEA, carcinoembryonic antigen; CI, confidence interval.
EZH2 required for cancer cell proliferation. In order to examine whether elevated expression of EZH2 plays a critical role in the proliferation of cancer cells, we prepared two independent siRNA oligonucleotide duplexes to specifically suppress the expression of EZH2 (siEZH2#1, #2), and transfected each of them into cancer cells. Knockdown of EZH2 in cancer cells was confirmed by real‐time PCR and Western blotting as shown (Fig. 3A). We carried out cell growth assays on cancer cells transfected with these siRNAs, and observed significant growth suppression in two bladder cancer cell lines (SW780 and RT4), two lung cancer cell lines (A549 and SBC5) and one colorectal cancer cell line (HCT116) (Fig. 3B). When the EZH2 siRNAs were transfected into the normal cell line CCD‐18Co, expressing undetectable levels of EZH2 (Fig. S3), they had no effect on CCD‐18Co cells (Fig. 3B). This implies that suppression of cancer cell growth by treatment with these specific siRNAs does not deploy a mechanism used by normal cells, and thus directly acts on a growth mechanism specific to cancer cells. To further assess the mechanism of growth suppression induced by the siRNA, we carried out BrdU and 7‐amino‐actinomycin D staining and examined cell cycle status (Fig. 3C). The proportion of cancer cells at the G1 phase was significantly higher in the cells treated with siEZH2 than those treated with control siRNAs, whereas the proportion of cancer cells at the S phase was remarkably decreased (Fig. 3C). The data revealed that EZH2 plays a crucial role in the cell cycle progression of cancer cells.
Figure 3.
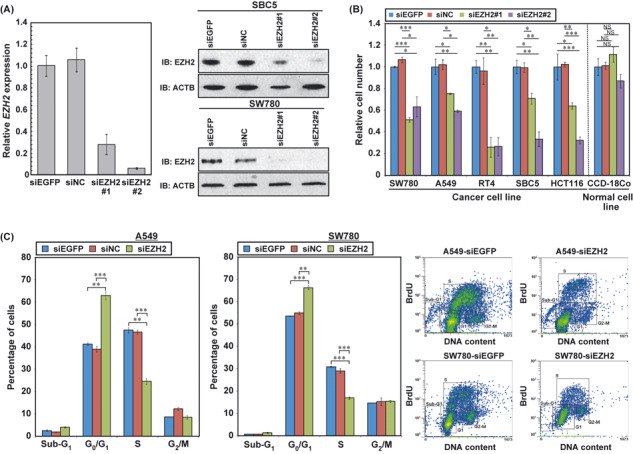
Involvement of histone methyltransferase EZH2 in the cell cycle regulation of cancer cells. (A) Knockdown effects of siRNAs targeting EZH2 on cancer cells. Left, quantitative real‐time PCR showing suppression of endogenous expression of EZH2 by two EZH2‐specific siRNAs (siEZH2#1 and #2) in SBC5 cells. Right, Western blot analysis of EZH2 in SBC5 and SW780 cells after treatment with two EZH2 siRNAs (siEZH2#1 and #2) and two control siRNAs (siEGFP and siNC). β‐actin was used as an internal control. (B) Effect of EZH2 siRNA knockdown on the viability of bladder cancer cell lines (SW780, RT4), lung cancer cell lines (A549, LC319), and a colorectal cancer cell line (HCT116). Relative cell number shows the value normalized to siEGFP‐treated cells. Results are the mean ± SD of three independent experiments. P‐values were calculated using Student’s t‐test (*P < 0.05; **P < 0.01; ***P < 0.001). (C) Effect of EZH2 knockdown on cell cycle kinetics in A549 and SW780 cells. Cells were collected 72 h after treatment with siRNAs, and cell cycle distribution was analyzed by flow cytometry after coupled staining with FITC‐conjugated anti‐BrdU and 7‐amino‐actinomycin D. We show numerical analysis of the FACS results (left) and representative histograms (right) for each experiment. Results are the mean ± SD of three independent experiments. P‐values were calculated using Student’s t‐test (*P < 0.05; **P < 0.01; ***P < 0.001).
Discussion
Cancer‐related death is on the rise in most countries. Although molecular‐targeting agents such as cetuximab and bevacizumab have been developed and proven efficacious, their adverse effects and limited application for some patients makes it important to continue to search for novel molecular‐targeting agents. Recent tumor genomics data will be used to develop rationally selected drugs that target proteins expressed exclusively or at particularly high levels in tumor compared with essential normal adult cells. Specific pharmaceutical targeting of such proteins could result in a new generation of highly active drugs having minimal collateral host toxicity.( 29 ) In this study, we showed that, in various cancer tissues, expression levels of EZH2 were significantly high at both RNA and protein levels whereas EZH2 expression in various normal tissues was barely detected (see also Fig. S4 and Table S6). We also found that EZH2 plays a crucial role in the cell cycle progression of cancer cells. Although knockdown of EZH2 expression by specific siRNAs significantly suppressed the growth of cancer cells, no growth suppression was observed in the normal colon fibroblast CCD‐18Co cells, with undetectable EZH2. These data clearly indicate EZH2 as an ideal molecular target for cancer therapy. Indeed, other researchers have been actively analyzing the involvement of EZH2 in the progression of human cancer.( 30 , 31 , 32 , 33 , 34 ) Development of methyltransferase inhibitors began recently,( 35 , 36 , 37 ) and a specific inhibitor targeting EZH2 could have great potential as an anticancer agent. In addition, as we have been successfully developing therapeutic cancer vaccines,( 38 , 39 ) EZH2 could be a promising target for cancer vaccine therapy.
Our data suggest that EZH2 could also be a good marker, enabling us to predict the prognosis of NSCLC patients, and to carry out a more intensive follow‐up according to EZH2 expression status in resected specimens. In contrast, EZH2 overexpression indicated a good prognosis in colorectal cancer. In this case, EZH2 was overexpressed in the majority of cancer cases (91.4%), and the number of EZH2‐negative cases was small (n = 14). Therefore, although indicative of cancer, it appears difficult to use EZH2 expression as a prognostic marker in colorectal cancer. It is also possible that EZH2 overexpression might affect the prognosis differently, depending on the type of cancer. For instance, it was reported that a high EZH2 index was significantly associated with better relapse‐free survival, and with a trend for better cancer‐specific survival, in colon cancer stages II and III derived from Norwegian subjects,( 31 ) consistent with our results. In contrast, in breast cancer and melanomas, EZH2 expression in tumor cells was linked to an aggressive clinical behavior and worse prognosis.( 40 , 41 ) In addition, in prostate cancer cells, EZH2 was found to promote invasiveness and proliferation.( 42 ) With regard to Japanese colorectal cancer cases in this study, the EZH2 positive ratio is statistically low in P‐factor positive and M‐factor positive cases (P = 0.019 and P = 0.011, respectively), and there is a possibility that EZH2 could repress peritoneal and distant metastases from colorectal cancers. Large‐scale validation of the present results, and further functional analyses of this protein in human carcinogenesis, may assist in comprehending the various modes of EZH2 expression in different cancer types. Furthermore, we need to develop anticancer therapies targeting EZH2 with due attention to cancer type.
Disclosure Statement
The research was funded by OncoTherapy Science, Inc. YI, YY, and KM are employees of OncoTherapy Science, Inc. YD, YN, and RH are scientific advisors of OncoTherapy Science, Inc.
Supporting information
Fig. S1. Representative cases for positive and negative EZH2 staining in lung cancer and normal lung tissues on the tissue microarray.
Fig. S2. Representative cases for positive and negative EZH2 staining in colorectal cancer and normal colorectal tissues.
Fig. S3. Validation of EZH2 protein expression in various cell lines.
Fig. S4. Expression levels of EZH2 in 78 normal tissues.
Table S1. Primer sequences for quantitative real‐time PCR.
Table S2. siRNA sequences used for siRNA transfection and cell growth assay.
Table S3. Clinicopathological characteristics of bladder tissues on tissue microarray.
Table S4. Statistical significance between EZH2 expression and tumor grade.
Table S5. Clinicopathological characteristics of colorectal tissues on tissue microarray.
Table S6. Expression profile of EZH2 analyzed by cDNA microarray.
Data S1. Materials and Methods.
Supporting info item
Acknowledgments
We are grateful to Ms. Haruka Sawada and Ms. Noriko Ikawa for technical assistance. Our bio‐repository is supported by funding from the National Institute for Health Research (UK) and the Cambridge Biomedical Research Centre. This work was supported by a Grant‐in‐Aid for Young Scientists (A) (22681030) from the Japan Society for the Promotion of Science.
References
- 1. Zhao Q, Shentu J, Xu N et al. Phase I study of icotinib hydrochloride (BPI‐2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer 2010: doi: 10.1016/j.lungcan.2010.11.007. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 2. Undevia SD, Gomez‐Abuin G, Ratain MJ. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer 2005; 5: 447–58. [DOI] [PubMed] [Google Scholar]
- 3. Hamamoto R, Furukawa Y, Morita M et al. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol 2004; 6: 731–40. [DOI] [PubMed] [Google Scholar]
- 4. Hamamoto R, Silva FP, Tsuge M et al. Enhanced SMYD3 expression is essential for the growth of breast cancer cells. Cancer Sci 2006; 97: 113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kunizaki M, Hamamoto R, Silva FP et al. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by SMYD3. Cancer Res 2007; 67: 10759–65. [DOI] [PubMed] [Google Scholar]
- 6. Silva FP, Hamamoto R, Kunizaki M, Tsuge M, Nakamura Y, Furukawa Y. Enhanced methyltransferase activity of SMYD3 by the cleavage of its N‐terminal region in human cancer cells. Oncogene 2008; 27: 2686–92. [DOI] [PubMed] [Google Scholar]
- 7. Tsuge M, Hamamoto R, Silva FP et al. A variable number of tandem repeats polymorphism in an E2F‐1 binding element in the 5′ flanking region of SMYD3 is a risk factor for human cancers. Nat Genet 2005; 37: 1104–7. [DOI] [PubMed] [Google Scholar]
- 8. Volkel P, Angrand PO. The control of histone lysine methylation in epigenetic regulation. Biochimie 2007; 89: 1–20. [DOI] [PubMed] [Google Scholar]
- 9. Jenuwein T, Laible G, Dorn R, Reuter G. SET domain proteins modulate chromatin domains in eu‐ and heterochromatin. Cell Mol Life Sci 1998; 54: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb‐group gene Ezh2 is required for early mouse development. Mol Cell Biol 2001; 21: 4330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyer LA, Plath K, Zeitlinger J et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 2006; 441: 349–53. [DOI] [PubMed] [Google Scholar]
- 12. Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome‐wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 2006; 20: 1123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee TI, Jenner RG, Boyer LA et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 2006; 125: 301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gould A. Functions of mammalian Polycomb group and trithorax group related genes. Curr Opin Genet Dev 1997; 7: 488–94. [DOI] [PubMed] [Google Scholar]
- 15. Simon JA, Tamkun JW. Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes. Curr Opin Genet Dev 2002; 12: 210–8. [DOI] [PubMed] [Google Scholar]
- 16. Kato T, Daigo Y, Hayama S et al. A novel human tRNA‐dihydrouridine synthase involved in pulmonary carcinogenesis. Cancer Res 2005; 65: 5638–46. [DOI] [PubMed] [Google Scholar]
- 17. Kikuchi T, Daigo Y, Katagiri T et al. Expression profiles of non‐small cell lung cancers on cDNA microarrays: identification of genes for prediction of lymph‐node metastasis and sensitivity to anti‐cancer drugs. Oncogene 2003; 22: 2192–205. [DOI] [PubMed] [Google Scholar]
- 18. Taniwaki M, Daigo Y, Ishikawa N et al. Gene expression profiles of small‐cell lung cancers: molecular signatures of lung cancer. Int J Oncol 2006; 29: 567–75. [PubMed] [Google Scholar]
- 19. Ishikawa N, Daigo Y, Yasui W et al. ADAM8 as a novel serological and histochemical marker for lung cancer. Clin Cancer Res 2004; 10: 8363–70. [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa N, Takano A, Yasui W et al. Cancer‐testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas. Cancer Res 2007; 67: 11601–11. [DOI] [PubMed] [Google Scholar]
- 21. Kusaba T, Nakayama T, Yamazumi K et al. Expression of p‐STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol 2005; 58: 833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayami S, Kelly JD, Cho HS et al. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer 2011; 128: 574–86. [DOI] [PubMed] [Google Scholar]
- 23. Hayami S, Yoshimatsu M, Veerakumarasivam A et al. Overexpression of the JmjC histone demethylase KDM5B in human carcinogenesis: involvement in the proliferation of cancer cells through the E2F/RB pathway. Mol Cancer 2010; 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Unoki M, Kelly JD, Neal DE, Ponder BA, Nakamura Y, Hamamoto R. UHRF1 is a novel molecular marker for diagnosis and the prognosis of bladder cancer. Br J Cancer 2009; 101: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshimatsu M, Toyokawa G, Hayami S et al. Dysregulation of PRMT1 and PRMT6, Type I arginine methyltransferases, is involved in various types of human cancers. Int J Cancer 2011; 128: 562–73. [DOI] [PubMed] [Google Scholar]
- 26. Callagy G, Cattaneo E, Daigo Y et al. Molecular classification of breast carcinomas using tissue microarrays. Diagn Mol Pathol 2003; 12: 27–34. [DOI] [PubMed] [Google Scholar]
- 27. Callagy G, Pharoah P, Chin SF et al. Identification and validation of prognostic markers in breast cancer with the complementary use of array‐CGH and tissue microarrays. J Pathol 2005; 205: 388–96. [DOI] [PubMed] [Google Scholar]
- 28. Chin SF, Daigo Y, Huang HE et al. A simple and reliable pretreatment protocol facilitates fluorescent in situ hybridisation on tissue microarrays of paraffin wax embedded tumour samples. Mol Pathol 2003; 56: 275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res 2008; 14: 1639–48. [DOI] [PubMed] [Google Scholar]
- 30. Arisan S, Buyuktuncer ED, Palavan‐Unsal N, Caskurlu T, Cakir OO, Ergenekon E. Increased expression of EZH2, a polycomb group protein, in bladder carcinoma. Urol Int 2005; 75: 252–7. [DOI] [PubMed] [Google Scholar]
- 31. Fluge O, Gravdal K, Carlsen E et al. Expression of EZH2 and Ki‐67 in colorectal cancer and associations with treatment response and prognosis. Br J Cancer 2009; 101: 1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kikuchi J, Kinoshita I, Shimizu Y et al. Distinctive expression of the polycomb group proteins Bmi1 polycomb ring finger oncogene and enhancer of zeste homolog 2 in nonsmall cell lung cancers and their clinical and clinicopathologic significance. Cancer 2010; 116: 3015–24. [DOI] [PubMed] [Google Scholar]
- 33. Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 2005; 11: 8570–6. [DOI] [PubMed] [Google Scholar]
- 34. Weikert S, Christoph F, Kollermann J et al. Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med 2005; 16: 349–53. [PubMed] [Google Scholar]
- 35. Ragno R, Simeoni S, Castellano S et al. Small molecule inhibitors of histone arginine methyltransferases: homology modeling, molecular docking, binding mode analysis, and biological evaluations. J Med Chem 2007; 50: 1241–53. [DOI] [PubMed] [Google Scholar]
- 36. Spannhoff A, Heinke R, Bauer I et al. Target‐based approach to inhibitors of histone arginine methyltransferases. J Med Chem 2007; 50: 2319–25. [DOI] [PubMed] [Google Scholar]
- 37. Cheng D, Yadav N, King RW, Swanson MS, Weinstein EJ, Bedford MT. Small molecule regulators of protein arginine methyltransferases. J Biol Chem 2004; 279: 23892–9. [DOI] [PubMed] [Google Scholar]
- 38. Miyazawa M, Ohsawa R, Tsunoda T et al. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 2010; 101: 433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshida K, Nakamura Y. Therapeutic cancer vaccines against gastro‐intestinal cancer. Nippon Shokakibyo Gakkai Zasshi 2010; 107: 1255–61. [PubMed] [Google Scholar]
- 40. Bachmann IM, Halvorsen OJ, Collett K et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 2006; 24: 268–73. [DOI] [PubMed] [Google Scholar]
- 41. Collett K, Eide GE, Arnes J et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res 2006; 12: 1168–74. [DOI] [PubMed] [Google Scholar]
- 42. Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate 2007; 67: 547–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative cases for positive and negative EZH2 staining in lung cancer and normal lung tissues on the tissue microarray.
Fig. S2. Representative cases for positive and negative EZH2 staining in colorectal cancer and normal colorectal tissues.
Fig. S3. Validation of EZH2 protein expression in various cell lines.
Fig. S4. Expression levels of EZH2 in 78 normal tissues.
Table S1. Primer sequences for quantitative real‐time PCR.
Table S2. siRNA sequences used for siRNA transfection and cell growth assay.
Table S3. Clinicopathological characteristics of bladder tissues on tissue microarray.
Table S4. Statistical significance between EZH2 expression and tumor grade.
Table S5. Clinicopathological characteristics of colorectal tissues on tissue microarray.
Table S6. Expression profile of EZH2 analyzed by cDNA microarray.
Data S1. Materials and Methods.
Supporting info item


