Abstract
Discovery of the natural killer (NK) T cell‐specific ligand, α‐galactosylceramide (α‐GalCer) has enabled us to investigate the functional regulation of NKT cells. However, the detailed mechanism of cytokine production by NKT cells remains to be elucidated. Here we evaluated the role of interleukin (IL)‐4 in the production of interferon (IFN)‐γ from NKT cells using IL‐4‐deficient C57BL/6 mice (IL‐4−/– mice). Administration of α‐GalCer into wild‐type C57BL/6 mice caused the production of both IFN‐γ and IL‐4 in serum or cytoplasm within 4 h of the injection. Unexpectedly, however, IL‐4−/– mice‐derived NKT cells did not produce any IFN‐γ at early phase after primary stimulation with α‐GalCer. Because NKT cells from IL‐4−/– mice produced IFN‐γ when they were stimulated secondarily with α‐GalCer in vitro for 72 h, NKT cells from IL‐4−/– mice were not completely genetically deficient for IFN‐γ production. To elucidate which cells, NKT cells or dendritic cells (DC), were responsible for the deficiency in IFN‐γ production in IL‐4−/– mice, we carried out an add‐back experiment using purified NKT cells and DC, which were prepared from either wild‐type mice or IL‐4−/– mice. NKT cells from wild‐type mice produced IFN‐γ when they were cocultured with DC prepared from either wild‐type or IL‐4−/– mice, whereas NKT cells from IL‐4−/– mice did not produce IFN‐γ by coculturing with DC from either wild‐type or IL‐4−/– mice. These results indicate that NKT cells, not DC, were responsible for the deficiency in IFN‐γ production in IL‐4−/– mice. Thus, IL‐4 is required for the activation of NKT cells to produce IFN‐γ in response to α‐GalCer. (Cancer Sci 2007; 98: 721–725)
Natural killer (NK) T cells are characterized by the expression of a single invariant T‐cell receptor α (TCRα) chain encoded by Vα14‐Jα281 and activated by the specific ligand α‐galactosylceramide (α‐GalCer) in a CD1d‐dependent manner.( 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ) They promptly produce a large amount of T‐helper type 1 (Th1) cytokines and T‐helper type 2 (Th2) cytokines simultaneously and are considered to play an important role as immunoregulatory cells in antitumor immunity,( 12 , 13 , 14 , 15 ) autoimmunity,( 16 , 17 ) and in maintaining some forms of tolerance.( 18 , 19 , 20 ) As interferon (IFN)‐γ and interleukin (IL)‐4 are representative cytokines involved in Th1 and Th2 immunity, respectively, the control of cytokine production by NKT cells leads to the regulation of immune diseases, including tumor disease, through the control of immune balance.( 21 , 22 ) For instance, Miyamoto et al. synthesized an α‐GalCer analog named OCH that stimulated NKT cells to produce IL‐4 predominantly rather than IFN‐γ.( 21 ) Thus, OCH administration resulted in the suppression of experimental autoimmune encephalomyelitis induction.( 21 ) Fujii et al. reported that α‐GalCer‐loaded dendritic cells (DC) produced mainly IFN‐γ from NKT cells and inhibited the metastasis of B16 melanoma.( 22 ) Therefore, it is of great importance to investigate the mechanisms underlying the regulation of cytokine production by NKT cells to develop new strategies for the treatment of immune diseases.
Here we demonstrate the role of IL‐4 in the production of IFN‐γ from NKT cells using IL‐4−/– mice during the stimulation of α‐GalCer. As IL‐4 antagonizes IFN‐γ production,( 23 , 24 , 25 ) we first hypothesized that IFN‐γ production from NKT cells might be augmented in IL‐4−/– mice. However, unexpectedly the level of IFN‐γ produced by NKT cells was mostly diminished in the early stage of activation. We also found that NKT cells, not DC, were the cells responsible for the deficiency in IFN‐γ production in IL‐4−/– mice. These results suggest that IL‐4 was a critical cytokine required for NKT cells to produce IFN‐γ in response to α‐GalCer.
Materials and Methods
Mice. C57BL/6 mice were purchased from Charles River Japan (Kanagawa, Japan). IL‐4‐deficient mice on a C57BL/6 background were kindly donated by Dr Y. Iwakura (Tokyo University, Tokyo, Japan).( 26 ) All mice used in the present study were 5–8 weeks old and were maintained in specific pathogen‐free conditions.
Monoclonal antibodies and reagents. Phycoerythrin (PE)‐anti‐IL‐4, fluorescein‐isothiocyanate (FITC)‐anti‐IFN‐γ, allophycocyanin (APC)‐anti‐NK1.1 and peridinin chlorophyll protein (PerCp)‐anti‐CD3 monoclonal antibodies (mAb) were purchased from Pharmingen (San Diego, CA, USA). Mitomycin‐C was purchased from Kyowa (Tokyo, Japan).
α‐GalCer. α‐GalCer, ([2S,3S,4R]‐1‐0‐[α‐d‐galactopyranosyl]‐2‐[N‐hexacosanoyl‐amino]‐1,3,4‐octadecanettriol) used for this study was donated by Kirin Brewery Co. (Tokyo, Japan).( 27 ) The stock solution of α‐GalCer (220 µg/mL) was diluted in 0.5% polysorbate 20 (Nikko Chemical, Tokyo, Japan) in 0.9% NaCl solution. This stock solution was further diluted to an appropriate concentration with saline and used for the experiments. A vehicle control solution was prepared from a solution of 0.5% polysorbate 20 in 0.9% NaCl solution. The vehicle control was used in all experiments.
Intracellular cytokine detection of NKT cells in vivo. Hepatic lymphocytes obtained from mice 1, 2 or 4 h after intravenous (i.v.) injection of α‐GalCer (2 µg/mouse) were stained with PerCp‐anti‐CD3 mAb and APC‐anti‐NK1.1 mAb. After several washes they were fixed with 4% paraformaldehyde and then treated with permeabilizing solution (50 mM NaCl, 5 mM ethylenediaminetetracetic acid, 0.02% NaN3, 0.5% Triton X‐100, pH 7.5). The fixed cells were further stained with FITC‐anti‐IFN‐γ mAb and PE‐anti‐IL‐4 mAb. The percentages of cytoplasmic IL‐4‐ and IFN‐γ‐expressing cells were determined by flour‐color flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA, USA).
Preparation of murine hepatic lymphocytes. Murine livers were removed and passed through a 200‐gauge stainless steel mesh and then suspended in RPMI‐1640 medium (Sigma, Tokyo, Japan) including 0% serum and 10 IU/mL heparin. After one wash, the pellet (containing cells) was resuspended in 33% Percoll solution (Amersham Bioscience, Tokyo, Japan), gently placed on 70% Percoll solution and centrifuged at 1300 g for 20 min at room temperature. Mononuclear cells (MNC) at the interface were harvested and washed twice in RPMI‐1640 medium including 10% serum. MNC were resuspended in RPMI‐1640 medium including 10% serum for subsequent use.
Purification of splenic NKT and DC. Spleen cells were incubated on nylon wool columns for 45 min, and the non‐adherent cells were used for the isolation of NKT cells by cell sorting with a FACSVantage instrument (Becton Dickinson). The purity of the sorted cells was >98%. The details of the staining and sorting have been described previously.( 28 )
DC were prepared according to the method of Steinman et al. with some modifications.( 29 ) In brief, spleen cells were incubated in 10‐cm plastic dishes for 2 h, and the non‐adherent cells were removed from the culture. The adherent cells were further incubated overnight and the non‐adherent cells were harvested. Then, CD11c+B220−CD4−CD8− cells were isolated from the non‐adherent populations by cell sorting and used as the source of DC after mitomycin‐C treatment.
Cytokine production and detection. Splenic DC (2 × 105) from wild‐type (wt) mice or IL‐4−/– mice were cocultured with purified NKT cells (2 × 105) from wt mice or IL‐4−/– mice in the presence of 200 ng/mL α‐GalCer in 96‐well U‐bottomed plates (Corning Costar Co. Ltd, Tokyo, Japan). After incubation for 36 h, the culture supernatants were harvested to detect cytokine levels. IL‐2 and IFN‐γ activity in culture supernatants were determined using the OptEIA mouse IL‐2 or IFN‐γ enzyme‐linked immunosorbent assay (ELISA) systems (PharMingen, San Diego, CA, USA). Serum samples were obtained from wt mice or IL‐4−/– mice after injection of α‐GalCer (2 µg/mouse) and IFN‐γ, or IL‐4 levels were measured using ELISA kits (PharMingen).
Reverse transcription–polymerase chain reaction. Total RNA was extracted using Isogen reagent (Nippon Gene, Tokyo, Japan) and was reverse transcribed to cDNA using Superscript II RNaseH Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Subsequently, cDNA was amplified using Taq DNA polymerase (Promega, Madison, WI, USA) and specific primer pairs for IFN‐γ (forward, 5′‐CGA CTC CTT TTC CGC TTC CTG AG‐3′; and reverse, 5′‐TGA ACG CTA CAA CTG CAT CTT GG‐3′). The expression of IFN‐γ mRNA was investigated 24 h after culture of purified DC and NKT cells with or without α‐GalCer. In the time‐course study of gene expression, polymerase chain reaction products were separated on an ethidium bromide‐containing agarose gel, and were visualized using an ultraviolet transilluminator.
Results
IFN‐γ production by NKT cells was not detected after α‐GalCer administration in IL‐4‐deficient mice in vivo. To investigate the role of IL‐4 in the production of IFN‐γ from NKT cells, we evaluated the serum levels of IFN‐γ and IL‐4 within 4 h of α‐GalCer administration in wt and IL‐4−/– mice (Fig. 1a). In wt mice, the serum levels of IL‐4 peaked 2 h after α‐GalCer administration and the IFN‐γ level was elevated until the 4‐h time point. Interestingly, however, the level of IFN‐γ was not detectable at any point before 4 h in IL‐4−/– mice. Because both wt and IL‐4−/– mice possessed almost the same numbers of NKT cells (Fig. 1b), the defect in IFN‐γ production in IL‐4−/– mice was not due to a decreased number of NKT cells in IL‐4−/– mice.
Figure 1.
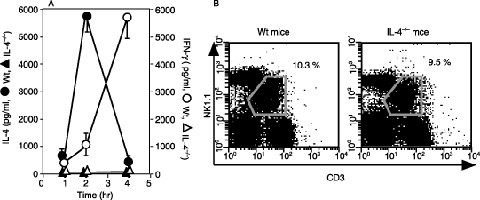
Interferon (IFN)‐γ production was not detected in interleukin (IL)‐4−/– mice by injection of α‐galactosylceramide (α‐GalCer). (a) C57BL/6‐background wild‐type (wt) mice and IL‐4−/– mice were intravenously administrated with 2 µg α‐GalCer. Serum levels of IFN‐γ (○, □) and IL‐4 (•, ▴) in wt mice (○, •) and IL‐4−/– mice (□, ▴) were determined by enzyme‐linked immunosorbent assay 1, 2 and 4 h after the injection. The bars represent mean ± SE of three mice in each group. (b) Hepatic lymphocytes prepared from wt mice and IL‐4−/– mice were stained with APC‐Anti‐NK1.1 and PerCp‐anti‐CD3 monoclonal antibodies (mAb). NK1.1+ CD3+ natural killer (NK) T cells were gated. The numbers mean the percentage of NKT cells among whole hepatic lymphocytes.
To examine the early events in IFN‐γ and IL‐4 production from NKT cells, we next evaluated the kinetics of cytokine production from NKT cells at single‐cell level by intracellular cytokine staining (Fig. 2). Consistent with our previous paper,( 12 ) NKT cells in wt mice differentiated into IL‐4 single‐producing cells or IL‐4 and IFN‐γ double‐producing cells 1 h after α‐GalCer injection (Fig. 2a). Then, NKT cells shifted into IL‐4 and IFN‐γ double‐producing cells from IL‐4 single‐producing cells (Fig. 2b), and finally became IFN‐γ single‐producing cells (Fig. 2c). However, in IL‐4−/– mice IFN‐γ‐producing cells were not detected at any point before 4 h compared with wt mice (Fig. 2d–f). These results indicate that IL‐4 is a critical cytokine for inducing IFN‐γ production from NKT cells during activation with α‐GalCer.
Figure 2.
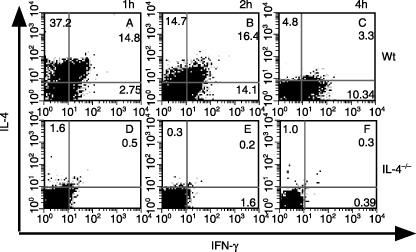
α‐Galactosylceramide (α‐GalCer)‐induced interferon (IFN)‐γ was not detected in natural killer (NK) T cells in interleukin (IL)‐4−/– mice even at single‐cell level. The IFN‐γ‐ and IL‐4‐producing abilities of hepatic NK1.1+ CD3+ NKT cells from wild‐type (wt) or IL‐4−/– mice were examined by flow cytometry (a,d) 1, (b,e) 2 and (c,f) 4 h after intravenous administration of 2 µg α‐GalCer. The experiment shown is representative of three independent experiments.
IL‐4 is prerequested for IFN‐γ production of NKT cells by stimulation with α‐GalCer. As shown in Table 1, the spleen cells from wt mice, which were administrated with α‐GalCer 2 h before sacrifice, spontaneously produced IFN‐γ within 24 h by in vitro primary culture without restimulation with α‐GalCer. In contrast, no spontaneous IFN‐γ production was detected in the primary culture of spleen cells derived from α‐GalCer‐administered IL‐4−/– mice. However, a significant level of IFN‐γ production was demonstrated in secondary in vitro culture of spleen cells from α‐GalCer‐administered IL‐4−/– mice when they were restimulated with α‐GalCer for 72 h. These results indicate that IL‐4 was prerequested for the primary early IFN‐γ production of α‐GalCer‐activated NKT cells but not for the secondary late‐phase IFN‐γ production by α‐GalCer‐activated NKT cells. These results also demonstrate that NKT cells of IL‐4−/– mice are not completely genetically defective in the production of IFN‐γ.
Table 1.
Spleen cells were prepared from wild‐type or interleukin (IL)‐4−/– mice treated with α‐galactosylceramide (α‐GalCer) (2 µg/head) or saline 2 h before death
| Mouse | α‐GalCer | IFN‐γ (pg/mL) | ||||
|---|---|---|---|---|---|---|
| In vivo (2 µg/head) | In vitro (200 ng/mL) | 4 h | 24 h | 48 h | 72 h | |
| Wild type | – | – | – | ND | ND | ND |
| – | + | – | 785 ± 9 | >2000 | >2000 | |
| + | – | 299 ± 15 | >2000 | >2000 | >2000 | |
| + | + | – | >2000 | >2000 | >2000 | |
| IL‐4−/– | – | – | – | ND | ND | ND |
| – | + | – | ND | ND | >2000 | |
| + | – | ND | ND | ND | ND | |
| + | + | – | ND | ND | >2000 | |
The cells were cultured with or without α‐GalCer (200 ng/mL) for 4, 24, 48 or 72 h. Interferon (IFN)‐γ levels in supernatants were decided by enzyme‐linked immunosorbent assay. The values represent mean ± SE of duplicated samples. The experiment shown is representative of three independent experiments ND, not detectable.
NKT cells, not DC, were responsible for the deficiency in IFN‐γ production in IL‐4−/– mice in response to α‐GalCer. Because NKT cells were activated by α‐GalCer present on DC,( 9 , 10 , 11 ) we analyzed which cells, DC or NKT cells, were responsible for the deficiency in IFN‐γ production in IL‐4−/– mice. For this purpose, we tried to reconstitute the defective IFN‐γ production of IL‐4−/– mice in vitro by add‐back experiments using purified NKT cells and DC derived from wt mice or IL‐4−/– mice in the presence of α‐GalCer. As shown in Fig. 3a, IFN‐γ was produced when NKT cells from wt mice were cocultured with DC from either wt or IL‐4−/– mice, whereas no significant level of IFN‐γ was detected when NKT cells from IL‐4−/– mice were cocultured with DC from wt mice or IL‐4−/– mice. Interestingly, IL‐2 was detected even in the culture supernatants of IL‐4−/– NKT cells (Fig. 3a), indicating that IL‐4−/– NKT cells were activated in this add‐back system. This was further confirmed by the finding that α‐GalCer stimulation caused the induction of IFN‐γ mRNA in IL‐4−/– NKT cells, as its elevation level was lower than that of wt NKT cells (Fig. 3b). These results demonstrated that the deficiency in IFN‐γ production observed in IL‐4−/– mice following stimulation with α‐GalCer is due to NKT cells, not DC.
Figure 3.
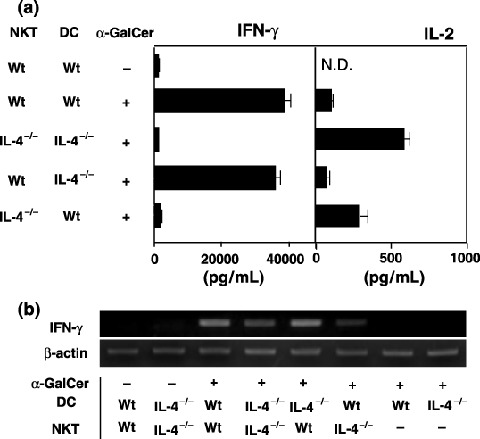
Deficiency in the interferon (IFN)‐γ production of interleukin (IL)‐4−/– mice by the stimulation of α‐galactosylceramide (α‐GalCer) was caused by natural killer (NK) T cells, not dendritic cells (DC). Purified splenic DC (2 × 105) and NKT cells (2 × 105) from wild‐type (wt) mice and IL‐4−/– mice were cocultured with or without α‐GalCer. (a) Supernatants were collected at 36 h and the levels of IFN‐γ and IL‐2 were detected by enzyme‐linked immunosorbent assay. The bars represent mean ± SE of duplicated samples. (b) The mRNA levels of IFN‐γ were examined 24 h after culture by reverse transcription–polymerase chain reaction. The experiment shown is representative of three independent experiments.
Discussion
In the present paper, we demonstrated that IL‐4−/– mice were defective in the early production of IFN‐γ by NKT cells activated with α‐GalCer. It has been reported that NKT cells play a critical role in the regulation of Th1‐dependent and Th2‐dependent immune diseases through production of both IL‐4 and IFN‐γ.( 17 ) Consistent with previous results, NKT cells produced both IFN‐γ and IL‐4 at an early stage after α‐GalCer administration (1, 2). Unexpectedly, however, IL‐4−/– mice produced no significant level of IFN‐γ after α‐GalCer administration. The defect in IFN‐γ production in α‐GalCer‐administered IL‐4−/– mice was confirmed at both the serum and single‐cell levels. These data indicate that the defective IFN‐γ production was derived from a lack of induction of IFN‐γ‐producing NKT cells by α‐GalCer in IL‐4−/– mice. The number of NKT cells in IL‐4−/– mice was the same as that of wt mice, but there appeared to be a functional defect in NKT cells derived from IL‐4−/– mice. We proposed this hypothesis from the following results. NKT cells derived from IL‐4−/– mice produced no significant levels of IFN‐γ when they were cocultured with DC obtained from wt or IL‐4−/– mice in the presence of α‐GalCer. In contrast, NKT cells from wt mice produced IFN‐γ when they were cocultured with either DC from wt or IL‐4−/– mice (Fig. 3a). We confirmed that IL‐4−/– NKT cells were activated in this system because IL‐2 production and the elevation of IFN‐γ mRNA was induced even in IL‐4−/– NKT cells by stimulation with α‐GalCer (Fig. 3a,b). We also concluded that NKT cells from IL‐4−/– mice were not completely genetically defective in terms of IFN‐γ production because NKT cells from α‐GalCer‐administered mice exhibited IFN‐γ production when they were restimulated in vitro with α‐GalCer for 72 h (Table 1), and IFN‐γ mRNA was induced in IL‐4−/– mice by α‐GalCer stimulation at an early time (Fig. 3b). From these results, we concluded that IL‐4 is prerequested for primary early IFN‐γ production by NKT cells, but not for secondary late IFN‐γ production by NKT cells during activation with α‐GalCer. We should further examine whether IFN‐γ production by IL‐4−/– NKT cells could be recovered with stimulation using an alternative substance, such as IL‐12 or combined stimulation with TCR and some other cytokines.
Others groups have reported that under some conditions IL‐4‐primed DC produce IL‐12, which induce NKT cells to produce IFN‐γ.( 30 , 31 , 32 ) Another group reported that IL‐4 enhances the response of NKT cells to IL‐2 and IL‐12, leading to the production of IFN‐γ.( 33 ) Therefore, to examine whether IL‐4 from NKT cells is involved in IFN‐γ production through such mechanisms, we added exogenous recombinant (r)IL‐4 to a cross‐cocultivation system or injected rIL‐4 directly into IL‐4−/– mice. However, IFN‐γ production was not recovered in either system (data not shown), indicating that other unknown mechanisms are involved in the IFN‐γ‐deficient property of IL‐4−/– mice, which could not be recovered by later addition of rIL‐4. We proposed that IL‐4 might play an unknown but critical role in the functional development of NKT cells in vivo for the production of IFN‐γ. We are now planning our next experiment using chimeric IL‐4−/– and IFN‐γ−/– mice to unveil the critical role of IL‐4 in the functional development of NKT cells.
Acknowledgments
We thank Kirin Brewery Co., Japan, for donating α‐GalCer. This work was supported by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science, and Technology, a Grant‐in‐Aid for Scientific Research on priority Areas, a Grant‐in‐Aid for Immunological Surveillance and Its Regulation and a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology Cancer Translational Research Project.
References
- 1. Imai K, Kanno M, Kimoto H, Shigemoto K, Yamamoto S, Taniguchi M. Sequence and expression of transcripts of the T‐cell antigen receptor α‐chain gene in a functional, antigen‐specific suppressor‐T‐cell hybridoma. Proc Natl Acad Sci USA 1986; 83: 8708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I‐specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med 1994; 180: 1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant V alpha 14+ TCR alpha chain in NK1.1+ T cell populations. Int Immunol 1995; 7: 1157–61. [DOI] [PubMed] [Google Scholar]
- 4. Fowlkes BJ, Kruisbeek AM, Ton‐That H et al. A novel population of T‐cell receptor alpha beta‐bearing thymocytes which predominantly expresses a single V beta gene family. Nature 1987; 329: 251–4. [DOI] [PubMed] [Google Scholar]
- 5. Budd RC, Miescher GC, Howe RC, Lees RK, Bron C, MacDonald HR. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes. J Exp Med 1987; 166: 577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballas ZK, Rasmussen W. NK1.1+ thymocytes. Adult murine CD4−, CD8− thymocytes contain an NK1.1+, CD3+, CD5hi, CD44hi, TCR‐V beta 8+ subset. J Immunol 1990; 145: 1039–45. [PubMed] [Google Scholar]
- 7. Sykes M. Unusual T cell populations in adult murine bone marrow: Prevalence of CD3+CD4−CD8− and alpha beta TCR+ NK1.1+ cells. J Immunol 1990; 145: 3209–15. [PubMed] [Google Scholar]
- 8. Levitsky HI, Golumbek PT, Pardoll DM. The fate of CD4−8− T cell receptor‐alpha beta+ thymocytes. J Immunol 1991; 146: 1113–17. [PubMed] [Google Scholar]
- 9. Kawano T, Cui J, Koezuka Y et al. CD1d‐restricted and TCR‐mediated activation of vα14+ NKT cells by glycosylceramides. Science 1997; 278: 1626–9. [DOI] [PubMed] [Google Scholar]
- 10. Burdin N, Brossay L, Koezuka Y et al. Selective ability of mouse CD1 to present glycolipids: α‐galactosylceramide specifically stimulates V α 14+ NK T lymphocytes. J Immunol 1998; 161: 3271–81. [PubMed] [Google Scholar]
- 11. Brossay L, Burdin N, Tangri S, Kronenberg M. Antigen‐presenting function of mouse CD1: one molecule with two different kinds of antigenic ligands. Immunol Rev 1998; 163: 139–50. [DOI] [PubMed] [Google Scholar]
- 12. Chamoto K, Takeshima T, Kosaka A et al. NKT cells act as regulatory cells rather than killer cells during activation of NK cell‐mediated cytotoxicity by α‐galactosylceramide in vivo . Immunol Lett 2004; 95: 5–11. [DOI] [PubMed] [Google Scholar]
- 13. Hayakawa Y, Takeda K, Yagita H et al. Critical contribution of IFN‐γ and NK cells, but not perforin‐mediated cytotoxicity, to anti‐metastatic effect of α‐galactosylceramide. Eur J Immunol 2001; 31: 1720–7. [PubMed] [Google Scholar]
- 14. Smyth MJ, Crowe NY, Pellicci DG et al. Sequential production of IFN‐γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α‐galactosylceramide. Blood 2002; 99: 1259–66. [DOI] [PubMed] [Google Scholar]
- 15. Terabe M, Matsui S, Noben‐Trauth N et al. NKT cell‐mediated repression of tumor immunosurveillance by IL‐13 and the IL‐4R‐STAT6 pathway. Nature Immunol 2000; 1: 515–20. [DOI] [PubMed] [Google Scholar]
- 16. Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. Alpha/beta‐T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin‐dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)‐4 and/or IL‐10. J Exp Med 1998; 187: 1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong S, Wilson MT, Serizawa I et al. The natural killer T‐cell ligand α‐galactosylceramide prevents autoimmune diabetes in non‐obese diabetic mice. Nature Med 2001; 7: 1052–6. [DOI] [PubMed] [Google Scholar]
- 18. Sonoda KH, Exley M, Snapper S, Balk SP, Stein‐Streilein J. CD1‐reactive natural killer T cells are required for development of systemic tolerance through an immune‐privileged site. J Exp Med 1999; 190: 1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seino KI, Fukao K, Muramoto K et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA 2001; 98: 2577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikehara Y, Yasunami Y, Kodama S et al. CD4+ Vα14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest 2000; 105: 1761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 2001; 413: 531–4. [DOI] [PubMed] [Google Scholar]
- 22. Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN‐γ‐producing NKT response induced with α‐galactosylceramide‐loaded DCs. Nat Immunol 2002; 3: 867–74. [DOI] [PubMed] [Google Scholar]
- 23. Zheng W, Flavell RA. The transcription factor GATA‐3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997; 89: 587–96. [DOI] [PubMed] [Google Scholar]
- 24. Ouyang W, Ranganath SH, Weindel K et al. Inhibition of Th1 development mediated by GATA‐3 through an IL‐4‐independent mechanism. Immunity 1998; 9: 745–55. [DOI] [PubMed] [Google Scholar]
- 25. Seki N, Miyazaki M, Suzuki W et al. IL‐4‐induced GATA‐3 expression is a time‐restricted instruction switch for Th2 cell differentiation. J Immunol 2004; 172: 6158–66. [DOI] [PubMed] [Google Scholar]
- 26. Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL‐4 gene blocks Th2 cytokine responses. Nature 1993; 362: 245–8. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 1995; 7: 529–34. [PubMed] [Google Scholar]
- 28. Nishimura T, Takeuchi Y, Ichimura Y et al. Thymic stromal cell clone with nursing activity supports the growth and differentiation of murine CD4+8+ thymocytes in vitro . J Immunol 1990; 145: 4012–17. [PubMed] [Google Scholar]
- 29. Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro . J Exp Med 1979; 149: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hochrein H, O’Keeffe M, Luft T et al. Interleukin (IL)‐4 is a major regulatory cytokine governing bioactive IL‐12 production by mouse and human dendritic cells. J Exp Med 2000; 192: 823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebner S, Ratzinger G, Krosbacher B et al. Production of IL‐12 by human monocyte‐derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL‐4. J Immunol 2001; 166: 633–41. [DOI] [PubMed] [Google Scholar]
- 32. Kitamura H, Iwakabe K, Yahata T et al. The natural killer T (NKT) cell ligand α‐galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)‐12 production by dendritic cells and IL‐12 receptor expression on NKT cells. J Exp Med 1999; 189: 1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bream JH, Curiel RE, Yu CR et al. IL‐4 synergistically enhances both IL‐2‐ and IL‐12‐induced IFN‐γ expression in murine NK cells. Blood 2003; 102: 207–14. [DOI] [PubMed] [Google Scholar]


