Abstract
Malignant mesothelioma is the most common primary pleural neoplasm. Association of simian virus 40 (SV40) with malignant mesothelioma has been reported, suggesting that SV40 plays an important role in the origin of a subset of these tumors. However, significant geographic variation is present as to how often this association occurs. As no study concerning SV40 in malignant mesothelioma has been reported from Japan, we examined the frequency of SV40 infection in Japanese malignant mesothelioma cases. In pleural malignant mesothelioma tissue from 35 patients in Japan, we sought the presence of SV40 large T antigen DNA using real‐time polymerase chain reaction (PCR), as well as expression of the viral protein using immunohistological methods. Real‐time PCR demonstrated that two of 35 mesotheliomas contained DNA sequences encoding portions of SV40 large T antigen. None of the 35 malignant mesothelioma specimens showed immunoreactivity for SV40 large T antigen. SV40 infection does not appear to have a major role in the development of malignant mesothelioma in Japan. (Cancer Sci 2006; 97: 292–295)
Malignant mesothelioma is a highly aggressive tumor arising from serosal membranes, most commonly the pleura.( 1 ) Worldwide incidence is increasing because of widespread exposure to asbestos, the major causal agent. The incidence of this disease also is increasing dramatically in Japan.( 2 ) In general, mesothelioma is resistant to chemotherapy and radiotherapy, and is rarely cured by radical resection.( 3 ) Understanding of the pathogenesis of malignant mesothelioma could be useful to develop targeted molecular therapy.( 1 )
Simian virus 40 (SV40) is a well‐known oncogenic DNA virus of the papovaridae class whose oncogeneic potential is associated with an early gene product, large T antigen.( 4 ) SV40 can cause malignant mesothelioma and other cancers in hamsters.( 5 ) Human mesothelial cells are highly susceptible to SV40 transformation,( 6 , 7 ) via a mechanism related to p53.( 6 ) SV40 infection of human mesothelial cells inactivates p53 and retinoblastoma protein( 8 , 9 ) and induces hepatocyte growth factor/Met receptor activity, which promotes mesothelial cell growth.( 7 ) SV40 infection of human mesothelial cells also results in immediate activation of telomerase,( 10 ) which precedes transformation of the phenotype. Although an association of SV40 and malignant mesothelioma in humans has been reported and appears to be characterized, there has been strong argument against an etiological role for this virus in human mesothelioma as well. Furthermore, there is significant geographical variation in the frequency of SV40 being present in these tumors.( 11 , 12 , 13 , 14 , 15 , 16 ) In addition, no report has addressed the issue of SV40 prevalence in malignant mesothelioma cases in Japan. Whether SV40 is associated with malignant mesothelioma in Japan therefore is an important issue. In the present study we therefore examined the prevalence of SV40 in malignant mesothelioma specimens in Japan to evaluate the virus–tumor relationship in this region.
Materials and Methods
Tissue samples
Thirty‐five malignant mesothelioma tissue specimens were obtained from patients who had undergone biopsy or surgery at Okayama Rosai Hospital, Sanyo National Hospital, or Saga University Hospital between 1982 and 2002. Resected specimens were fixed in formalin and then embedded in paraffin. Table 1 summarizes the characteristics of the patients, who included 32 men and three women with a median age of 61 years (range 34–85 years). Histologically the malignant mesotheliomas studied here were classified into epithelioid type (12 cases), biphasic type (nine cases) and sarcomatoid type (14 cases). Histological diagnosis and clinical stage were determined independently by three pathologists according to the criteria of the World Health Organization classification.( 17 ) Informed consent was obtained from all patients.
Table 1.
Patient characteristics
| Sex (male/female) | 32/3 |
| Median age (years) | 61 |
| Range (years) | 34–85 |
| Histological type | |
| Epithelioid | 12 |
| Biphasic | 9 |
| Sarcomatoid | 14 |
| Stage | |
| I | 6 |
| II | 9 |
| III | 4 |
| IV | 13 |
| Unknown | 3 |
DNA preparation and real‐time polymerase chain reaction analyses
DNA was extracted( 11 ) and analyzed for the presence of SV40 Tag sequences using primers that caused polymerase chain reaction (PCR) to amplify a specific 156‐bp region of the large Tag of SV40.( 18 ) Analysis of SV40 sequences used real‐time PCR assays based on TaqMan technology (Perkin‐Elmer, Foster City, CA, USA).( 19 ) Serial dilutions of DNA from an SV40‐transformed hamster cell line (obtained from Dr M. Carbone, Loyola University Chicago, Maywood, IL, USA) were used to create a standard curve. DNA from lymphocytes of 10 healthy volunteers was used as a negative control in this study.
Sequences of primers and probes used to amplify and specifically detect SV40 sequences have been described previously.( 20 ) For both assays β‐actin was used as an internal control.( 20 ) The amount of SV40 DNA in samples was represented as a ratio of the fluorescence emission intensity values for the SV40 DNA products to that for β‐actin. For convenience in presentation, the ratio was multiplied by 1000.
Immunohistochemical detection of SV40 Large T antigen expression
Two sets of 5 µm‐thick tissue sections (one set from the center of the tumor, and the other from the margin) were subjected to immunohistochemical staining with anti‐SV40 large T antigen antibody (pAb101; Santa Cruz Biotechnology, Santa Cruz, CA, USA) by a standard method, as reported previously.( 21 ) In brief, the sections were deparaffinized in xylene and rehydrated in a graded ethanol series. Endogenous peroxide was blocked with 3% hydrogen peroxide in methanol for 20 min. Sections were then washed three times in phosphate‐buffered saline (PBS). After blocking non‐specific binding with serum (Histofine SAB‐PO kit; Nichirei, Tokyo, Japan) for 30 min, sections were incubated with the primary antibodies in a humid chamber at 4°C overnight. The primary antibody was anti‐SV40 large T antigen antibody diluted at 1:500. After three PBS washes, sections were incubated with biotinylated secondary antibody for 30 min, washed three times in PBS, and incubated with streptavidin‐conjugated peroxidase for 30 min. After three additional washes in PBS, 3,3′‐diaminobenzidine tetrahydrochloride was applied and sections were counterstained with hematoxylin. With the exception of incubation with the primary antibodies, the entire procedure took place at room temperature. Formalin‐fixed, paraffin‐embedded sections of HEK293 cells expressing SV40 large T antigen protein were used as positive controls for SV40 large T antigen. Immunoglobulin M from mice not immunized for SV40 large T antigen was substituted for the primary antibody in negative controls.
Immunohistochemical evaluation
Staining was assessed by three independent observers (KA, TM and AH). Any intensity of brown nuclear staining was accepted as positive, and the percentage of positive nuclei was recorded. For each specimen, five fields were selected at random at the invasive margin of the cancer. On average 500 cells per randomly chosen field were assessed. According to the percentage of the stained cells, we divided the stained specimens into two groups: specimens with no staining cells were classified negative, whereas staining of 1% of cells or more was defined as positive.
Results and Discussion
To clarify whether SV40 is involved in the pathogenesis of malignant mesothelioma in Japan, we studied 35 malignant mesotheliomas using a previously reported TaqMan probe‐based SV40‐specific real‐time PCR method( 20 ) (Fig. 1). As summarized in Table 2, two of 35 mesotheliomas were considered positive for the presence of SV40 large T antigen DNA, showing ratios of 36.0 and 4.9. The ratio in the positive control was 199.0. The two positive cases consisted of one epithelioid tumor and one biphasic tumor. SV40 DNA was reported to be present in epithelial and biphasic types but only very rarely in the sarcomatoid type, and our result was consistent with previous reports despite a low rate of detection. There were no positive cases in lymphocyte DNA from 10 healthy volunteers. In addition, our previous study demonstrated that the real‐time PCR assay for SV40 Tag DNA was highly sensitive and specific.( 20 )
Figure 1.
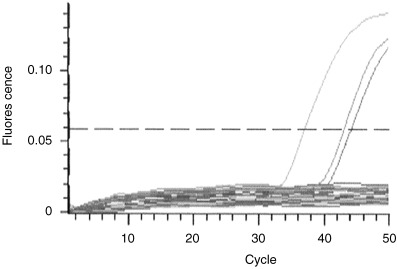
Analysis of samples using real time‐polymerase chain reaction. Cycle numbers required for linear amplification of simian virus 40 (SV40) large T antigen DNA sequence are compared with those needed for equal amplification of the control gene β‐actin.
Table 2.
Patient characteristics in simian virus 40 DNA‐positive cases
| Case | Age (years) | Sex | Histological type | TNM | Stage |
|---|---|---|---|---|---|
| 1 | 85 | Male | Biphasic | T4N2M0 | IV |
| 2 | 55 | Male | Epithelioid | T2N0M0 | II |
TNM, tumor‐node‐metastasis classification.
We next examined expression of SV40 large T antigen by immunohistochemistry. All 35 malignant mesothelioma specimens were negative for staining with SV40 large T antigen antibody; in contrast, diffuse staining for SV40 large T antigen was observed in the cytoplasm and on the cell membranes in the positive control (Fig. 2). These results can be explained by a ‘hit and run’ theory of SV40 involvement in malignant mesothelioma, in which the T antigen of SV40 is necessary to initiate tumorigenesis but not to retain malignant features. Interpreting our results according to this theory, detected viral DNA would be simply a ‘footprint’ of SV40 infection, as active SV40 virus appeared able to produce protein in the course of viral activity. Another possible reason for failure to detect protein could be the relatively low sensitivity of immunohistochemistry compared with real‐time PCR.
Figure 2.
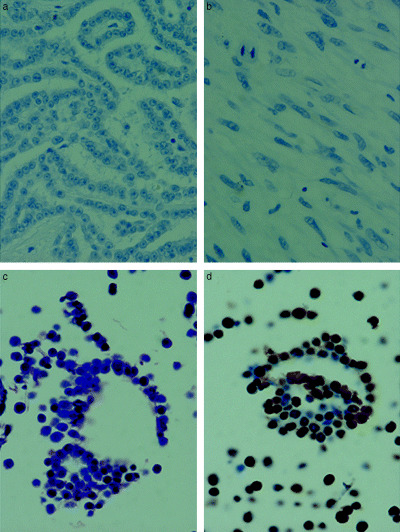
Immunohistochemical staining for simian virus 40 (SV40). Malignant mesothelioma specimens (a) epithelioid and (b) sarcomatoid were negative for SV40 protein expression in malignant mesothelioma, in contrast to the positive controls (c) Hematoxylin‐eosin (HE) and (d) SV40. Original magnification, ×200.
Recent studies from Europe have demonstrated the absence of SV40 in malignant mesothelioma specimens.( 12 , 13 , 14 , 15 ) The authors of those reports interpreted their results as indicating significant geographic variation of frequency of SV40 infection, perhaps related to the distribution of SV40 in contaminated poliovirus vaccine. SV40 is believed to infect certain monkeys, its natural hosts, and ordinarily does not infect humans. Yet it could do so through inoculation of poliovirus vaccines, once prepared using monkey kidneys accidentally contaminated with SV40. Millions of individuals in the USA and many more worldwide were inoculated with potentially contaminated poliovirus vaccines between 1955 and 1963; in Japan, such a vaccine was used from 1961 to 1963.( 22 ) Since 1964, SV40‐free, domestic ally‐produced vaccines have been used in Japan. The low frequency of SV40 infection in Japanese malignant mesothelioma cases is consistent with this policy. Another issue to be investigated is ethnic differences in susceptibility to SV40, which possibly could be lower in the Japanese population than in populations with higher infection rates. This could be examined by comparing SV40 infection rates between racially different populations growing up in the same area.
In conclusion, we found a low rate of SV40 infection in Japanese malignant mesothelioma specimens, suggesting that SV40 may not be involved in the pathogenesis of malignant mesothelioma in Japan.
Acknowledgments
This work was supported by Grants‐in‐Aid for Research for malignant mesothelioma from the Ministry of Health, Labor, and Welfare, Japan.
References
- 1. Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol 2002; 29: 2–17. [DOI] [PubMed] [Google Scholar]
- 2. Morinaga K, Kishimoto T, Sakatani M, Akira M, Yokoyama K, Sera Y. Asbestos‐related lung cancer and mesothelioma in Japan. Indust Health 2001; 39: 65–74. [DOI] [PubMed] [Google Scholar]
- 3. Zellos LS, Sugerbaker DJ. Multimodality treatment of diffuse malignant pleural mesothelioma. Semin Oncol 2002; 29: 41–50. [DOI] [PubMed] [Google Scholar]
- 4. Gazdar AF, Butel JS, Carbone M. SV40 and human tumors: myth, association or causality? Nat Rev Cancer 2002; 2: 957–64. [DOI] [PubMed] [Google Scholar]
- 5. Carbone M, Stach R, DiResata I, Rizzo P. Simian virus 40 oncogenesis in hamsters. Dev Biol Stand 1998; 94: 273–9. [PubMed] [Google Scholar]
- 6. Bocchetta M, Di Resata I, Powers A et al. Human mesothelial cells are unusually susceptible to simian virus 40‐mediated transformation and asbestos cocarcinogenecity. Proc Natl Acad Sci USA 2000; 97: 10 214–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cacciotti P, Libener R, Betta P et al. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral‐related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci USA 2001; 98: 12 032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carbone M, Rizzo P, Grimley PM et al. Simian virus‐40 large‐T antigen binds p53 in human mesotheliomas. Nat Med 1997; 3: 908–12. [DOI] [PubMed] [Google Scholar]
- 9. De Luca A, Baldi A, Esposito V et al. The retinoblastoma gene family pRb/p105, p107, pRb2/p130 and simian virus‐40 large T‐antigen in human mesotheliomas. Nat Med 1997; 3: 913–16. [DOI] [PubMed] [Google Scholar]
- 10. Foddis R, De Reenzo A, Broccoli D et al. SV40 infection induces telomerase activity in human mesothelial cells. Oncogene 2002; 21: 1434–42. [DOI] [PubMed] [Google Scholar]
- 11. Shivapurkar N, Wiethege T, Witsuba I et al. Presence of simian virus 40 sequences in malignant mesothelioma and mesothelial cell proliferations. J Cell Biochem 1999; 76: 181–8. [DOI] [PubMed] [Google Scholar]
- 12. Muratero C, Surentheran T, Rudd RM. Simian virus 40 and human pleural mesothelioma. Thorax 1999; 54: 60–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirvonen A, Mattson K, Karjalainen A et al. Simian virus 40 (SV40)‐link DNA sequences not detectable in Finnish mesothelioma patients not exposed to SV40‐contaminated polio vaccines. Mol Carcin 1999; 26: 93–9. [PubMed] [Google Scholar]
- 14. Emri S, Kocagoz T, Olut A, Gungen Y, Mutti L, Baris Y. Simian virus 40 is not a cofactor in the pathogenesis of environmentally induced malignant mesothelioma in Turkey. Anticancer Res 2000; 20: 891–4. [PubMed] [Google Scholar]
- 15. De Rienzo A, Tor M, Sterman DH, Aksoy F, Albelda SM, Testa JR. Detection of SV40 DNA sequences in malignant mesothelioma specimens from the United States, but not from Turkey. J Cell Biochem 2002; 84: 455–9. [PubMed] [Google Scholar]
- 16. Manfredi JJ, Dong J, Liu WJ et al. Evidence against a role for SV40 in human mesothelioma. Cancer Res 2005; 65: 2602–9. [DOI] [PubMed] [Google Scholar]
- 17. WHO. Histological typing of lung cancer and pleural tumours. In: Travis WD, Colby TV, Corrin C, Shimosato Y, Brambilla E, eds, in collaboration with LH Sobin and pathologists from 14 countries. World Health Organization International Histological Classification of Tumours. Histological Typing of Lung and Pleural Tumours. 3rd edition. Tokyo: Springer, 1999: 7–13. [Google Scholar]
- 18. Huang H, Reis R, Yonekawa Y, Lopes JM, Kleihues P, Ohgaki H. Identification in human brain tumors of DNA sequences specific for SV40 large T antigen. Brain Pathol 1999; 9: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toyooka KO, Toyooka S, Virmani AK et al. Loss of expression and aberrant methylation of the CDH13 (H‐cadherin) gene in breast and lung carcinomas. Cancer Res 2001; 61: 4556–60. [PubMed] [Google Scholar]
- 20. Shivapurkar N, Takahashi T, Reddy J et al. Presence of simian virus 40 DNA sequences in human lymphoid and hematopoietic malignancies and their relationship to aberrant promoter methylation of multiple genes. Cancer Res 2004; 64: 3757–60. [DOI] [PubMed] [Google Scholar]
- 21. Simsar A, Fetsch P, Bedrossian CWM, Ioffe OB, Abati A. Absence of SV‐40 large T antigen (Tag) in malignant mesothelioma effusions: an immunocytochemical study. Diagn Cytopathol 2001; 25: 203–7. [DOI] [PubMed] [Google Scholar]
- 22. Kyoto Meeting on Poliomyelitis Eradication in the Western Pacific Region. The eradication of polio in Japan. In: World Health Organization, eds, Toward a Polio‐Free World. Tokyo: Influx Com, 2000: 7–13. [Google Scholar]


