Abstract
Mitigation of regulatory T cell‐mediated immunosuppression is crucial for optimal in vivo anti‐tumor immune responses. In this study, we examined the anti‐tumor effect induced by oral ingestion of an immunomodulating diet comprised of Lentinula edodes mycelia (L.E.M.) extract. C57BL/6 mice were inoculated subcutaneously in the footpad with B16 melanoma and fed L.E.M. extract. Ingestion of L.E.M. extract significantly inhibited tumor growth, and this in vivo anti‐tumor effect was not observed in nude mice, suggesting a T cell‐dependent mechanism. In addition, ingestion of L.E.M. extract led to significant restoration of H‐2Kb‐restricted and melanoma‐reactive T cells in the spleen and draining lymph nodes of melanoma‐bearing mice. Flow cytometry analysis revealed that the percentage of Foxp3+ CD4+ T cells increased in spleen and draining lymph nodes in melanoma‐bearing mice, but decreased significantly with ingestion of L.E.M. extract. Importantly, selective depletion of CD8+ T cells abolished the L.E.M.‐induced anti‐tumor effect, whereas that of CD4+ T cells or CD25+ cells showed no additive influence on the effect. Real‐time PCR analysis revealed that ingestion of L.E.M. extract by melanoma‐bearing mice decreased the level of Foxp3 mRNA within the tumor tissues, and lowered plasma transforming growth factor (TGF)‐β. Furthermore, an in vitro assay revealed that an immunosuppressive activity of CD4+ T cells from melanoma‐bearing mice was canceled by ingestion of L.E.M. extract. Our results indicate that oral ingestion of L.E.M. extract restores immune responses of class I‐restricted and melanoma‐reactive CD8+ T cells in melanoma‐bearing mice, presumably by a mitigation of regulatory T cells‐mediated immunosuppression. (Cancer Sci 2011; 102: 516–521)
Anticancer immunotherapy has received considerable attention recently as a new treatment modality. The immune system is capable of recognizing tumor antigens, and cancer‐reactive cytotoxic T lymphocytes are the most effective cancer destroying cells.( 1 , 2 , 3 ) Although anticancer immunotherapies, including vaccines and adoptive immunotherapy, have been applied clinically, to date their efficacy has been unsatisfactory.( 4 ) One explanation for the insufficient outcome of anticancer immunotherapy trials is the emergence of immunosuppressive cells, including CD4+ CD25+ regulatory T cells (Tregs) and/or myeloid‐derived suppressor cells, in tumor‐bearing states.( 5 , 6 ) The presence of Tregs correlates strongly with unfavorable prognosis.( 7 , 8 ) Treatment with antibodies or drugs has been proposed as a means of relieving Treg‐mediated immunosuppression.( 9 , 10 , 11 , 12 , 13 , 14 ) Nevertheless, safe and widely applicable treatment modalities to mitigate Treg‐mediated immunosuppression in cancer patients must be therapeutically beneficial.
A dried powder extracted from Lentinula edodes mycelia (L.E.M.) with hot water before germination and after culturing in a medium composed of bagasse and rice bran,( 15 ) has been reported to exhibit anti‐tumor activity and immunomodulatory effects in vitro and in vivo.( 16 , 17 , 18 , 19 , 20 ) Since the L.E.M. extract can be administered orally, patients can be treated as outpatients to reduce their burden.( 21 , 22 ) In this study, we investigated the anti‐tumor effect of L.E.M. extract on subcutaneously (s.c.) established B16 melanomas. Oral ingestion of L.E.M. extract significantly inhibited the growth of B16 melanoma through significant augmentation of class I‐restricted and melanoma‐reactive T cells. Additional analyses suggested that the L.E.M.‐induced anti‐tumor effect involved mitigation of Treg‐mediated immunosuppression. These results provide a scientific basis for oral ingestion of L.E.M. extract for successful relief of Treg‐mediated immunosuppression in cancer patients, and support its use in current cancer immunotherapy.
Materials and Methods
Mice. C57BL/6 (H‐2b: 6 weeks old) and Bagg’s albino (BALB)/c nu/nu (H‐2d: 6 weeks old) female mice were purchased from Japan SLC Inc. (Hamamatsu, Japan). Animals were kept under a specific pathogen‐free condition. Mice were used for experiments at 7 weeks of age. Experiments were performed according to the ethical guidelines for animal experiments of the Kobayashi Pharmaceutical Co. Ltd.
Tumor cell lines. B16BL6 (B16) is a melanoma cell line of C57BL/6 mice origin. B16L is a major histocompatibility complex (MHC) class I loss variant of B16 melanoma, and B16L‐Kb is a subline of B16L that expresses H‐2Kb molecules.( 23 ) All cell lines were maintained in vitro in RPMI 1640 complete medium (Sigma‐Aldrich Japan, Tokyo, Japan) supplemented with 10% heat‐inactivated FBS (Thermo Trace, Melbourne, Vic., Australia) and 1% penicillin‐streptomycin (Wako Japan, Osaka, Japan).
Reagents. A dried powder extract from L.E.M. was prepared with hot water before germination and after culturing the mycelia in medium composed of bagasse and rice bran, as previously described.( 15 )
In vivo anti‐tumor assay. A total of 7.5 × 105 B16 melanoma cells were inoculated s.c. in the footpad of C57BL/6 mice. After 24 h, mice were freely fed food containing L.E.M. extract at a ratio of either 1% or 2%. Tumor size was measured twice weekly thereafter. On day 21, all mice were sacrificed and the tumor weights were determined.
Assay for peptide‐specific or melanoma‐specific T cells. To test T cell responses against a melanoma antigen, an H‐2Kb‐binding tyrosine‐related protein (TRP)‐2181–188 peptide (VYDFFVWLH)( 24 ) was used. OVA257–262 peptide (SIINFEKL) was used as an H‐2Kb‐binding control peptide. Both peptides were purchased from PH Japan (Hiroshima, Japan), and were of >90% purity. Spleen and tumor‐draining lymph node cells were harvested, pooled, and stimulated in vitro with each of the indicated peptides in the presence of 20 U/mL interleukin (IL)‐2 for 3 days at a cell density of 5 × 105 cells/well in 96‐well flat plates. To test for activity against melanoma cells, spleen and tumor‐draining lymph node cells were harvested, pooled, and stimulated in vitro with B16, B16L, or B16L‐Kb in the presence of 20 U/mL IL‐2 for 3 days at a cell density of 5 × 105 cells/well in 96‐well flat plates. Melanoma cells were inactivated by treatment with 100 μg/mL mitomycin C (Kyowa Hakko Kirin, Tokyo, Japan) for 90 min. The level of interferon (IFN)‐γ in the culture supernatants was determined using an ELISA kit (Invitrogen Japan, Tokyo, Japan).
Flow cytometry analysis. Spleen cells and tumor‐draining lymph node cells were stained with FITC‐conjugated antimouse CD4 mAb (eBioscience, Kobe, Japan) and PE‐Cy5 conjugated antimouse/rat Foxp3 mAb (eBioscience), and were analyzed by EPICS (Beckman Coulter, Tokyo, Japan).
In vivo administration of antibodies. To selectively deplete CD4+ T cells or CD8+ T cells in vivo, mice were intraperitoneally (i.p.) administered with 200 μg of anti‐CD4 mAb (GK1.5: rat IgG2b) or anti‐CD8 mAb (53‐6.72: rat IgG2a) on days −1 and +7 after tumor inoculation. To selectively deplete CD25+ cells in vivo, mice were i.p. administered with 250 μg of anti‐CD25 mAb (PC61 5.3: rat IgG1) on days −1 and +3 after tumor inoculation. All of these antibodies were purchased from eBioscience.
Real‐time PCR. Total RNA was isolated using RNeasy Plant Mini kits (Qiagen, Tokyo, Japan) according to the manufacturer’s instructions. First‐strand cDNA was generated using the Superscript First‐Strand Synthesis System (Invitrogen) and random primers. The synthesized first‐strand cDNA was amplified using Platinum Taq DNA polymerase (Invitrogen) with EXPRESS SYBER GreenER qPCR SuperMixes (Invitrogen). Real‐time PCR was carried out in duplicate using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster, CA, USA). Thermal cycling included an initial denaturation step of 2 min at 95°C, followed by 40 cycles of 95°C for 15 s, and 60°C 1 min. Relative mRNA levels were calculated compared to β‐actin. The following primers (sense and antisense, respectively) were used for Foxp3: 5′‐TGCAGGGCAGCTAGGTACTTGTA‐3′ and 5′‐TCTCGGAGATCCCCTTTGTCT‐3′; transforming growth factor (TGF)β1: 5′‐AAACGGAAGCGCATCGAA‐3′ and 5′‐GGGACTGGCGAGCCTTAGTT‐3′; β‐actin: 5′‐AGAGGGAAATCGTGCGTGAC‐3′ and 5′‐CAATAGTGATGACCTGGCCGT‐3′.
Measurement of TGF‐β1. To avoid platelet‐derived TGF‐β1, peripheral blood was taken with heparin, and the level of plasma TGF‐β1 was determined by ELISA.
Assay of IFN‐γ production from melanoma‐bearing and/or L.E.M. extract‐fed mice. To examine the effect of L.E.M. ingestion on an immunosuppressive activity of CD4+ T cells from melanoma‐bearing mice, naïve whole spleen cells (1 × 105 cells) were cultured with CD4+ T cells (1 × 105 cells) purified from either naïve mice, melanoma‐bearing mice, or melanoma‐bearing and L.E.M. extract‐fed mice in anti‐CD3 antibody‐coated 96‐well plates (BD Biosciences, Tokyo, Japan) for 3 days. Purified CD4+ T cells were negatively isolated using a CD4 T cell Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The level of IFN‐γ in the culture supernatants was determined by ELISA.
Results
Ingestion of the L.E.M. extract significantly inhibits established B16 melanomas. We first determined whether or not oral ingestion of L.E.M. extract could inhibit the growth of B16 melanoma cells. C57BL/6 mice were inoculated s.c. in the right foot pad with B16 melanoma cells, and oral ingestion of L.E.M. extract began 24 h after the tumor inoculation. As shown in Figure 1a, tumor growth in mice that were freely fed either 1% or 2% L.E.M. extract was significantly inhibited compared to the untreated group. Tumor growth in mice that were freely fed 2% L.E.M. extract was significantly suppressed compared to mice fed 1% L.E.M. extract. The means and SD of tumor size and weight are shown in Figure 1b. On average, each mouse was fed approximately 4 g of 2% L.E.M. food, equivalent to 0.08 g L.E.M. extract (data not shown). There was no difference in tumor growth between mice that were freely fed 2% L.E.M. extract or 10% L.E.M. extract (data not shown). Therefore, subsequent experiments were performed using 2% L.E.M. extract.
Figure 1.
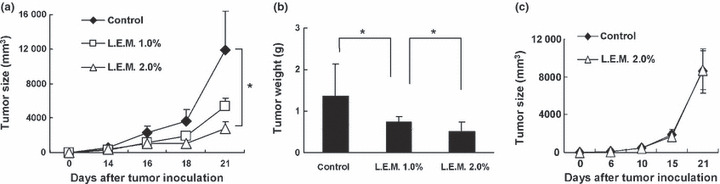
Anti‐tumor effect induced by oral ingestion of Lentinula edodes mycelia (L.E.M.) extract. C57BL/6 mice were injected s.c. in the right foot‐pad with 7.5 × 105 B16 cells. The next day, L.E.M. extract feeding was begun. (a) Fourteen days after tumor inoculation, the tumor size (mm3) was started to measure. Each group consisted of eight mice. Similar results were obtained in four independent experiments. *Indicates statistical significance (P < 0.05). (b) Twenty‐one days after tumor inoculation, mice were sacrificed and tumor weight was determined. The data represent tumor weight of eight mice. *Statistical significance (P < 0.05). (c) B16 melanoma cells (7.5 × 105) were injected s.c. into BALB/c nude mice. On the next day, L.E.M. extract feeding was begun. Six days after tumor inoculation, the tumor size (mm3) was started to measure. Each group consisted of five mice.
We also determined whether the L.E.M.‐induced anti‐tumor effect is T cell‐dependent (Fig. 1c). Ingestion of L.E.M. extract had no effect on the growth of s.c. established B16 melanomas in BALB/c nu/nu mice. These results indicate that oral ingestion of L.E.M. extract induces anti‐tumor effects on s.c. established B16 melanomas in a T cell‐dependent manner.
Ingestion of L.E.M. extract increases H‐2Kb‐restricted and melanoma‐reactive T cells in melanoma‐bearing mice. Next, we determined whether the number of melanoma antigen‐derived peptide‐reactive or melanoma‐reactive T cells increased in B16 melanoma‐bearing and L.E.M. extract‐fed mice. We used the TRP‐2181–188 peptide as a melanoma antigen‐derived and H‐2Kb binding peptide.( 24 ) Spleen or tumor‐draining lymph node cells from mice in each group were harvested, pooled, and cultured in vitro for 3 days with each of the indicated peptides in the presence of 20 U/mL IL‐2. Ingestion of L.E.M. extract significantly increased TRP‐2181–188 peptide‐specific IFN‐γ production by spleen cells and draining lymph node cells from melanoma‐bearing mice (Fig. 2).
Figure 2.
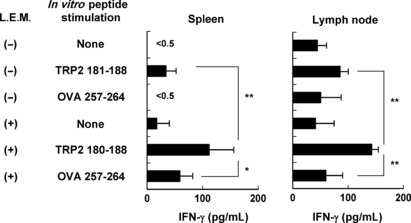
Induction of tyrosine‐related protein (TRP)‐2 peptide‐specific T cells in melanoma‐bearing and Lentinula edodes mycelia (L.E.M.)‐fed mice. C57BL/6 mice were injected s.c. in the right foot pad with 7.5 × 105 B16 cells. The next day, L.E.M. extract feeding was begun. On day 21, the spleen and tumor‐draining lymph nodes were harvested, and pooled cells were stimulated in vitro with each of the indicated peptides in the presence of 20 U/mL interleukin‐2. After 3 days of culture, the level of IFN‐γ in the supernatant was determined by ELISA. Each data consists of three wells. Similar results were obtained in three independent experiments. *Statistical significance at P < 0.05. **Statistical significance at P < 0.01.
We also tested whether ingestion of L.E.M. extract increases H‐2Kb‐restricted and melanoma‐reactive T cells in melanoma‐bearing mice. Spleen cells and tumor‐draining lymph node cells were stimulated in vitro with either B16, B16L (a class I loss variant of B16), or B16L‐Kb (H‐2Kb‐expressing B16L), and we determined the level of IFN‐γ in the supernatants. Ingestion of L.E.M. extract significantly increased IFN‐γ production in the presence of B16 melanoma cells (Fig. 3). Importantly, spleen and tumor‐draining lymph node cells produced a higher level of IFN‐γ in the presence of B16‐Kb compared to B16L, clearly indicating an H‐2Kb‐restricted and melanoma‐reactive T cell response. Altogether, these results indicate that ingestion of L.E.M. extract effectively increases MHC class I‐restricted and melanoma antigen‐reactive T cells in B16 melanoma‐bearing mice.
Figure 3.
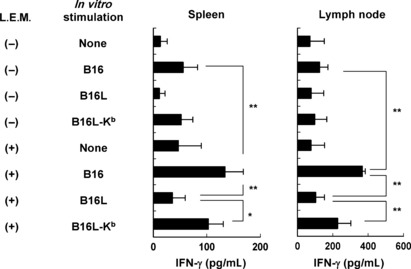
Induction of H‐2Kb‐restricted and melanoma‐reactive T cells in melanoma‐bearing and Lentinula edodes mycelia (L.E.M.)‐fed mice. C57BL/6 mice were injected s.c. in the right foot pad with 7.5 × 105 B16 cells. The next day, L.E.M. extract feeding was begun. On day 21, the spleen and tumor‐draining lymph nodes were harvested, and pooled cells were stimulated in vitro with the indicated tumor cell line in the presence of 20 U/mL interleukin‐2. After 3 days of culture, the level of IFN‐γ in the supernatant was determined by ELISA. Each data consists of three wells. Similar results were obtained in three independent experiments. *Statistical significance at P < 0.05. **Statistical significance at P < 0.01.
Ingestion of L.E.M. extract decreases Tregs and TGF‐β in melanoma‐bearing mice. We attempted to reveal the underlying mechanism by which ingestion of L.E.M. extract inhibits melanoma growth and subsequently induces H‐2Kb‐restricted and melanoma‐reactive T cells in melanoma‐bearing mice. We focused on Tregs because accumulating evidence suggests they play a crucial role in anti‐tumor immune responses. We examined the percentage of CD4+ Foxp3+ cells in the spleen and draining lymph nodes of melanoma‐bearing mice. Our results showed that the B16 melanoma‐bearing state significantly increases the percentage of CD4+ Foxp3+ cells in the spleen and draining lymph nodes. Intriguingly, ingestion of L.E.M. extract significantly decreases the percentage of these cells (Fig. 4a,b). When naïve C57BL/6 mice were fed L.E.M. extract, there was no change in the percentage of CD4+ Foxp3+ cells in the spleen and lymph nodes (Fig. 4a,b) or in bodyweight (data not shown). We next attempted to determine whether CD4+ T cells or CD8+ T cells were involved in the L.E.M. extract‐induced anti‐tumor effect and whether the anti‐tumor effect was mediated through a mitigation of Treg‐mediated immunosuppression in melanoma‐bearing mice (Fig. 4c,d). The positive percentages of splenic CD4+ T cells and CD8+ T cells in naïve C57BL/6 mice, 10.6% and 16.7%, were decreased to be 0.3% and 1.0% after in vivo administration of anti‐CD4 and anti‐CD8 depleting antibodies, respectively. To deplete Treg in vivo, we administered anti‐CD25 antibody, as previously reported.( 12 ) The positive percentage of CD25+ cells in splenic CD4+ T cells of naïve C57BL/6 mice was 6.7%, and decreased to be 0.5% after in vivo administration of anti‐CD25 depleting antibody. The results showed that the L.E.M. extract‐induced anti‐tumor effect was completely cancelled by selective depletion of CD8+ T cells, but that no change was observed when CD4+ T cells were depleted. If the underlying mechanisms are different between the selective depletion of CD25+ cells and ingestion of L.E.M. extract, an additive anti‐tumor effect could be observed in CD25+ cell‐depleted and L.E.M. extract‐fed mice. The result was that selective depletion of CD25+ cells showed no additive effect on the L.E.M. extract‐induced anti‐tumor effect, either.
Figure 4.
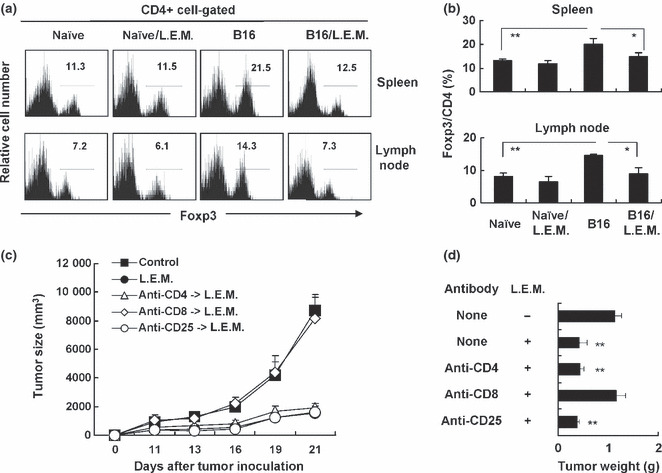
Participation of regulatory T cells (Tregs) in antitumor effect induced in melanoma‐bearing and Lentinula edodes mycelia (L.E.M.) extract‐fed mice. C57BL/6 mice were injected s.c. in the right foot pad with 7.5 × 105 B16 cells. The next day, L.E.M. extract feeding was begun. Naïve C57BL/6 mice were also fed with L.E.M. extract. On day 21, the spleen and tumor‐draining lymph nodes were harvested, and the percentage of Tregs (CD4+ Foxp3+ cells) was determined by flow cytometry. (a) Representative results that were gated on CD4+ T cells are shown. Numbers represents the percent of Foxp3+ cells among CD4+ T cells. (b) The means and SD of each group are shown. Each group consisted of eight mice. Similar results were obtained in three independent experiments. (c) In some groups, mice were i.p. injected with either anti‐CD4 (200 μg on days −1 and +7), anti‐CD8 (200 μg on days −1 and +7), or anti‐CD25 (250 μg on days −1 and +3) antibody, respectively. Eleven days after tumor inoculation, the tumor size (mm3) was started to measure. Each group consisted of five mice. (d) Twenty‐one days after tumor inoculation, mice were sacrificed and tumor weight was determined. The data represent tumor weight of five mice. **Statistical significance (P < 0.01) compared to the other groups.
We also analyzed the tumor tissues. Twenty‐one days after tumor inoculation, B16 melanoma tissues were removed individually, and expression of Foxp3 and TGF‐β mRNA in each sample was examined by real‐time PCR. Ingestion of L.E.M. extract significantly decreased expression of Foxp3 mRNA. On the other hand, expression of TGF‐β mRNA in tumor tissues showed a tendency to decrease, but the difference was not significant (Fig. 5a). We also measured the level of plasma TGF‐β and found that the melanoma‐bearing state was associated with increased levels of plasma TGF‐β, and that the level of TGF‐β significantly decreased with ingestion of L.E.M. extract (Fig. 5b).
Figure 5.
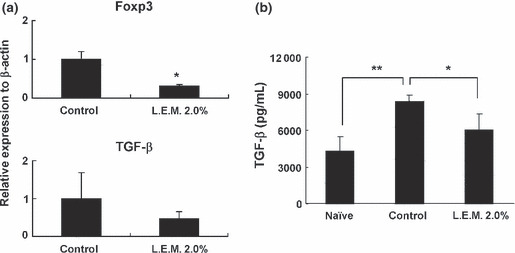
Real‐time PCR analysis of melanoma tissues and the level of plasma TGF‐β in melanoma‐bearing and Lentinula edodes mycelia (L.E.M.)‐fed mice. (a) Twenty‐one days after tumor inoculation, B16 melanoma tissues were removed individually, and the expression of Foxp3 and TGF‐β mRNA in each sample was determined by real‐time PCR. Each group consisted of eight mice. *Statistical significance at P < 0.01. (b) Twenty‐one days after tumor inoculation, plasma was collected from each mouse and the level of plasma TGF‐β was determined by ELISA. Each group consisted of five mice. *Statistical significance at P < 0.05. **Statistical significance at P < 0.01.
Ingestion of L.E.M. extract cancels Treg‐mediated immunosuppression induced in melanoma‐bearing mice. Finally, we confirmed that ingestion of L.E.M. extract relieves Treg‐mediated immunosuppression in melanoma‐bearing mice. Naïve whole spleen cells were cultured with CD4+ T cells purified from either naïve mice, melanoma‐bearing mice, or melanoma‐bearing and L.E.M. extract‐fed mice in wells that were precoated with anti‐CD3 monoclonal antibody (Fig. 6). The addition of CD4+ T cells from melanoma‐bearing mice significantly inhibited the ability of naïve whole cells to produce IFN‐γ, compared to that of CD4+ T cells from naïve mice. In contrast, ingestion of L.E.M. extract significantly restored the IFN‐γ production. These results indicate that CD4+ T cells in melanoma‐bearing mice contain functional Tregs, but that ingestion of L.E.M. extract can efficiently relieve the Treg‐mediated immunosuppression.
Figure 6.
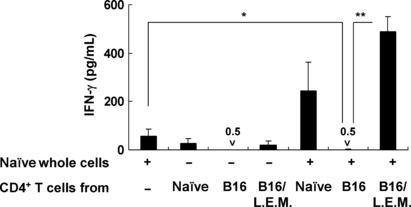
Mitigation of regulatory T cell (Treg)‐mediated immunosuppression by ingestion of Lentinula edodes mycelia (L.E.M.) extract. C57BL/6 mice were injected s.c. in the right foot pad with 7.5 × 105 B16 cells. The next day, L.E.M. extract feeding was begun. On day 21, the spleen was harvested, and purified CD4+ T cells from each group were cultured for 3 days with whole naïve spleen cells in anti‐CD3 antibody‐coated wells. The level of IFN‐γ in the supernatant was determined by ELISA. Each data consists of three wells. *Statistical significance at P < 0.05. **Statistical significance at P < 0.01.
Discussion
Anticancer immunotherapies (e.g., vaccines and adoptive immunotherapy) have been applied clinically, but the clinical efficacy of these treatments thus far has been unsatisfactory.( 4 ) One major obstacle to effective anticancer immunotherapy is Treg‐mediated immunosuppression in tumor‐bearing hosts.( 7 , 8 ) Developing safe and widely applicable treatment modalities that can mitigate Treg‐mediated immunosuppression in cancer patients and be therapeutically beneficial when combined with current cancer immunotherapies is therefore a high priority. In this study, we investigated the anti‐tumor effects of oral ingestion of L.E.M. extract against s.c. established B16 melanoma, and attempted to elucidate the underlying mechanism. Our results showed that ingestion of L.E.M. extract significantly suppresses the growth of B16 melanomas (Fig. 1a,b). In addition, the L.E.M. extract‐induced anti‐tumor effect was not observed in T cell‐deficient nude mice (Fig. 1c) and in CD8+ T cell‐depleted mice (Fig. 4c,d), suggesting that the tumor retardation was exerted through CD8+ T cells, but not through innate immune cells such as macrophages and natural killer cells.
It is important to elucidate the effect of L.E.M. extract on melanoma‐reactive T cells in melanoma‐bearing mice. We initially used TRP2181–188 peptide as an H‐2Kb‐restricted melanoma antigen‐derived peptide that could be recognized by B16 melanoma‐reactive cytotoxic T lymphocytes.( 24 ) We found that spleen cells and draining lymph node cells from melanoma‐bearing mice that had ingested L.E.M. extract produced a higher level of IFN‐γ in response to the TRP2181–188 peptide compared to mice that had not ingested L.E.M. extract (Fig. 2). We also tested whether oral ingestion of L.E.M. extract helps in priming melanoma‐reactive T cells in melanoma‐bearing mice by in vitro stimulation of B16L, an H‐2 loss variant of B16 melanoma, or B16L‐Kb, an H‐2Kb‐expressing B16L variant. Ingestion of L.E.M. extract significantly increased H‐2Kb‐restricted and B16 melanoma‐reactive T cells in spleen cells and draining lymph node cells from melanoma‐bearing mice (Fig. 3). Considering that an MHC class I‐restricted T cell response is usually responsible for CD8+ T cells, these lines of evidence indicate that oral ingestion of L.E.M. extract effectively restores in vivo priming of H‐2Kb‐restricted and melanoma antigen‐reactive CD8+ T cells in B16 melanoma‐bearing mice. In support of this, selective depletion of CD8+ T cells completely abolished the L.E.M. extract‐induced anti‐tumor effect in vivo (Fig. 4c), suggesting that MHC class I‐restricted CD8+ T cells are main effector cells in melanoma‐bearing and L.E.M. extract‐fed mice.
We also attempted to elucidate the underlying mechanism by which oral ingestion of L.E.M. extract restores in vivo priming of melanoma‐reactive T cells. We focused on the effect of ingestion of L.E.M. extract on Tregs because Tregs play a crucial role in immunosuppression in B16 melanoma‐bearing hosts.( 25 , 26 ) Indeed, the B16 melanoma‐bearing state was accompanied by an increase of Tregs in the spleen and lymph nodes, whereas oral ingestion of L.E.M. extract significantly decreased the percentage of Tregs (Fig. 4a,b). In addition, we examined the expression of Foxp3 (a Treg‐associated transcription factor)( 27 ) and TGF‐β (a Treg‐inducing cytokine)( 28 ) mRNA. Oral ingestion of L.E.M. extract resulted in a significant decrease in expression of Foxp3 mRNA. As for expression of TGF‐β mRNA, there was a tendency to decrease with L.E.M. extract ingestion, but the difference was not significant (Fig. 5a). However, the level of plasma TGF‐β, which increased in the B16 melanoma‐bearing state, decreased significantly with oral ingestion of L.E.M. extract (Fig. 5b). We also attempted to confirm that ingestion of L.E.M. extract could relieve Treg‐mediated immunosuppression in melanoma‐bearing mice using an in vitro assay (Fig. 6). The melanoma‐bearing state was accompanied by a severe impairment in the ability of splenic CD4+ T cells to produce IFN‐γ. Such CD4+ T cells, containing Tregs, exerted a significant immunosuppressive effect on the IFN‐γ production by naïve whole spleen cells in response to anti‐CD3 antibody. Importantly, this immunosuppression was completely turned off when melanoma‐bearing mice were fed the L.E.M. extract.
There remains one important question how oral ingestion of L.E.M. extract affects Tregs in melanoma‐bearing mice. Several explanations could be proposed. The first possibility is that L.E.M.‐induced reduction of Tregs in melanoma‐bearing mice was just a result of tumor retardation. To test this, we examined an influence of selective depletion of CD25+ cells in the melanoma‐bearing and L.E.M. extract‐fed mice (Fig. 4c,d). If the underlying mechanisms are different between the selective depletion of CD25+ cells and ingestion of L.E.M. extract, an additive anti‐tumor effect could be observed in CD25+ cell‐depleted and L.E.M. extract‐fed mice. However, the result was that selective depletion of CD25+ cells showed no augmenting effect on the L.E.M.‐induced anti‐tumor activity. In addition, a similar result was obtained when CD4+ T cells were selectively depleted. It is probable that anti‐CD4 depleting antibody destructed CD4+ Tregs in melanoma‐bearing mice. These results could be explained that both ingestion of L.E.M. extract and selective depletion of either CD25+ cells or CD4+ T cells can exert an anti‐tumor effect through the overlapped mechanism, that is, a mitigation of Treg‐mediated immunosuppression. These lines of evidence suggest that the L.E.M.‐induced anti‐tumor effect was mediated primarily through reduction of Tregs in melanoma‐bearing mice, but not a result of tumor retardation. In addition, we suppose that oral ingestion of L.E.M. extract primarily decreased the level of TGF‐β, and secondarily resulted in reduction of Tregs. It is well known that inducible Tregs are induced in the presence of TGF‐β( 29 ), and oral ingestion of L.E.M. extract significantly decreased the level of plasma TGF‐β in melanoma‐bearing mice (Fig. 5b). We suppose that this result is correlated with the induced Tregs in B16‐melanoma bearing mice. We are planning to test this hypothesis experimentally. The second possibility is that oral ingestion of L.E.M. affected the status of inflammation in melanoma‐bearing mice. It is well known that the cancer‐bearing state is usually associated with chronic inflammation,( 30 ) and that inflammation increases indoleamine 2,3 dioxygenase‐producing plasmacytoid dendritic cells in draining lymph nodes, resulting in an increase of Tregs.( 31 , 32 , 33 ) In addition, pro‐inflammatory Th17 cells and immunosuppressive Tregs are reciprocally controlled by pro‐inflammatory cytokine IL‐6 and retinoic acid, the derivative of vitamin A. Retinoic acid promotes and maintains TGF‐β‐induced Treg differentiation, while inhibiting IL‐6‐driven induction of Th17 cells.( 34 , 35 ) This regulation is particularly relevant for the intestine, where L.E.M. extract affects first after oral ingestion. However, at present, we have no answer on how immune responses in the gut‐associated lymphoid tissues affects on systemic and local anti‐tumor T cell responses in tumor‐bearing mice. Nevertheless, ingestion of L.E.M. extract can mitigate hepatitis,( 36 ) and we observed that ingestion of L.E.M. extract relieved splenomegaly in melanoma‐bearing mice (unpublished observation). These findings lead us to hypothesize that ingestion of L.E.M. extract attenuates Tregs in the tumor‐bearing state through a mitigation of cancer‐associated inflammation. We are planning to test this hypothesis experimentally.
In conclusion, our findings revealed that oral ingestion of L.E.M. extract restores melanoma‐reactive T cells in melanoma‐bearing mice, and that this effect may be a result of mitigation of Treg‐mediated immunosuppression. With regard to cancer treatment, relief of Treg‐mediated immunosuppression in cancer patients is a major hurdle that must be overcome for successful treatment outcomes. Notably, the L.E.M. extract can be administered orally, and patients can be treated as outpatients, thus minimizing the burden associated with inpatient therapy.( 21 , 22 ) We believe that this type of immunotherapy is safe, reliable, and widely applicable to the treatment of many cancers.
References
- 1. Rosenberg SA, Yang JC, Schwartzentruber DJ et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4: 321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nestle FO, Alijagic S, Gilliet M et al. Vaccination of melanoma patients with peptide‐or tumor lysatepulsed dendritic cells. Nat Med 1998; 4: 328–32. [DOI] [PubMed] [Google Scholar]
- 3. Jager E, Gnjatic S, Nagata Y et al. Induction of primary NY‐ESO‐1 immunity: CD8+ T lymphocyte and antibody responses in peptide‐vaccinated patients with NY‐ESO‐1+ cancers. Proc Natl Acad Sci USA 2000; 97: 12198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol 2006; 16: 115–23. [DOI] [PubMed] [Google Scholar]
- 6. Ostrand‐Rosenberg S, Sinha P. Myeloid‐derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182: 4499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–9. [DOI] [PubMed] [Google Scholar]
- 8. Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102: 18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loeffler M, Kruger JA, Reisfeld RA. Immunostimulatory effects of low‐dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res 2005; 65: 5027–30. [DOI] [PubMed] [Google Scholar]
- 10. Wada S, Yoshimura K, Hipkiss EL et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res 2009; 69: 4309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghiringhelli F, Larmonier N, Schmitt E et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004; 34: 336–44. [DOI] [PubMed] [Google Scholar]
- 12. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti‐CD25(interleukin‐2 receptor alpha) monoclonal antibody. Cancer Res 1999; 59: 3128–33. [PubMed] [Google Scholar]
- 13. Tawara I, Take Y, Uenaka A, Noguchi Y, Nakayama E. Sequential involvement of two distinct CD4+ regulatory T cells during the course of transplantable tumor growth and protection from 3‐methylcholanthrene‐induced tumorigenesis by CD25‐depletion. Jpn J Cancer Res 2002; 93: 911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamaguchi T, Hirota K, Nagahama K et al. Control of immune responses by antigen‐specific regulatory T cells expressing the folate receptor. Immunity 2007; 27: 145–59. [DOI] [PubMed] [Google Scholar]
- 15. Kojima H, Akaki J, Nakajima S, Kamei K, Tamesada M. Structural analysis of glycogen‐like polysaccharides having macrophage‐activating activity in extracts of Lentinula edodes mycelia. J Nat Med 2010; 64: 16–23. [DOI] [PubMed] [Google Scholar]
- 16. Tajima S, Saito Y, Suzuki I, Tokuda Y. Antibody treatment of breast cancer. Biotherapy (Tokyo) 2003; 17: 437–46 (in Japanese). [Google Scholar]
- 17. Sugano N, Choji Y, Hibino Y, Yasumura S, Maeda H. Anticarcinogenic antion of an alcohol‐insoluble fraction (LAP1) from culture medium of Lentinus edodes mycelia. Cancer Lett 1985; 27: 1–6. [DOI] [PubMed] [Google Scholar]
- 18. Sugano N, Hibino Y, Choji Y, Maeda H. Anticarcinogenic actions of water‐soluble and alcohol‐insoluble fractions from culture medium of Lentinus edodes mycelia. Cancer Lett 1982; 17: 109–14. [DOI] [PubMed] [Google Scholar]
- 19. Ichikawa Y, Mizoguchi Y, Kobayashi K, Morisawa S. Effect of extract from culture medium of Lentinus edodes mycelia on intracellular calcium ion concentration in the peritoneal exudate exudate macrophages. J Tradit Med 1991; 8: 162–6 (in Japanese). [Google Scholar]
- 20. Suzuki H, Iiyama K, Yoshida O, Yamazaki S, Yamamoto N, Toda S. Structural characterization of the immunoactive and antiviral water‐solubilized lignin in an extract of the culture medium of Lentinus edodes mycelia (L.E.M.). Agric Biol Chem 1990; 54: 479–87. [PubMed] [Google Scholar]
- 21. Yoshioka Y, Tamesada M, Nagayama A. The safety of excessive intake of the food containing extract of cultured Lentinula edodes mycelia (L.E.M.) in healthy adult volunteers. JCAM 2009; 6: 9–15 (in Japanese). [Google Scholar]
- 22. Yoshioka Y, Matsui Y, Kobayashi M et al. Safety evaluation of extract from cultured Lentinula edodes mycelia: study of acute toxicity, genotoxicity and inhibiting effect of drug‐metabolizing enzme, cytochrome P‐450 3A4. JCAM 2010; 7: 51–7 (in Japanese). [Google Scholar]
- 23. Harada M, Tamada K, Abe K et al. Characterization of B16 melanoma‐specific cytotoxic T lymphocytes. Cancer Immunol Immunother 1998; 47: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bloom MB, Perry‐Lalley D, Robbins PF et al. Identification of tyrosinase‐related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med 1997; 185: 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kline J, Brown IE, Zha YY et al. Homeostatic proliferation plus reglatory T‐cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res 2008; 14: 3156–67. [DOI] [PubMed] [Google Scholar]
- 26. Matsushita N, Pilon‐Thomas SA, Martin LM, Riker Al. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods 2008; 333: 167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wing K, Onishi Y, Prieto‐Martin P et al. CTLA‐4 control over Foxp3+ regulatory T cell function. Science 2008; 322: 271–5. [DOI] [PubMed] [Google Scholar]
- 28. Lu L, Ma J, Wang X et al. Synergistic effect of TGF‐beta superfamily members on the induction of Foxp3+ Treg. Eur J Immunol 2010; 40: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL‐2 is essential for TGF‐beta to convert naive CD4+ CD25‐cells to CD25+ Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007; 178: 2018–27. [DOI] [PubMed] [Google Scholar]
- 30. Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer 2010; 127: 768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muller AJ, Sharma MD, Chandler PR et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A 2008; 105: 17073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munn DH, Sharma MD, Hou D et al. Expression of indoleamine 2,3‐dioxygenase by plasmacytoid dendritic cells in tumor‐draining lymph nodes. J Clin Invest 2004; 114: 280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma MD, Baban B, Chandler P et al. Plasmacytoid dendritic cells from mouse tumor‐draining lymph nodes directly activate mature Tregs via indoleamine 2,3‐dioxygenase. J Clin Invest 2007; 117: 2570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mucida D, Park Y, Kim G et al. Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007; 317: 56–260. [DOI] [PubMed] [Google Scholar]
- 35. Maynard C, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all‐trans retinoic acid in TGF‐b‐meidated induction of Foxp3 and IL‐10 genes in developing regulatory T cells. J Exp Med 2009; 206: 2131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Itoh A, Isoda K, Kondoh M et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a‐induced liver injury. Biol Pharm Bull 2009; 32: 1215–9. [DOI] [PubMed] [Google Scholar]


