Abstract
Immunotherapy with photodynamic therapy (PDT) offers great promise as a new alternative for cancer treatment; however, its use remains experimental. In this study, we examined the immunotherapeutic significance of human papillomavirus (HPV)‐immortalized tumor cell lysates induced by PDT with CpG‐oligodeoxynucleotide (ODN). PDT‐cell lysates were generated by irradiating Radachlorin (5 µg/mL) preloaded TC‐1 cells carrying HPV 16 E7. PDT‐cell lysates plus ODN coinjection for protection against E7‐expressing tumors as well as specific immune responses were evaluated with the following tests: heat shock protein 70 (HSP70) enzyme‐linked immunosorbent assay, in vitro and in vivo tumor growth inhibition, interferon‐γ (IFN‐γ) and tumor necrosis factor‐α (TNF‐α) assay, cytotoxic T‐lymphocyte assay, and fluorescence activated cell sorting (FACS) analysis. PDT‐cell lysates plus ODN coinjection showed a significant suppression of tumor growth at both prophylactic and therapeutic levels, compared to PDT (or F/T)‐cell lysates or ODN alone. In addition, we evaluated the level of the immune response with the coinjection. HSP70, an important regulator of inflammatory and immune response, was observed in abundance in the PDT‐cell lysates. IFN‐γ production and cytotoxic T lymphocytes (CTL) responses were induced by PDT‐cell lysates plus ODN injection. The coinjection resulted in PDT‐cell lysate‐specific antibodies (IgG1, IgG2a, IgG2b, and IgG3) and T‐helper cell responses significantly higher than PDT‐cell lysates alone. Moreover, IFN‐γ production and CTL responses were significantly induced in the PDT‐cell lysate plus ODN immunized groups. These enhanced immune responses appeared to be mediated by CD8+ T cells only. These data suggest that PDT‐cell lysates plus ODN injection may be an effective approach to induce CTL immune responses as a possible immunotherapeutic strategy for cancer therapy. (Cancer Sci 2007; 98: 747–752)
Photodynamic therapy (PDT) is a new method used for treating malignant tumors; it is based on the effects of photodynamic damage to tumor cells as a result of a photochemical reaction.( 1 , 2 , 3 ) Recent studies have suggested that photo‐oxidative lesions produced by photodynamic therapy (PDT)‐treated tumors are recognized by the host as altered self,( 4 ) prompting a strong inflammatory and immune response.( 5 ) In addition, the use of PDT vaccines has been studied to generate antitumor immunity and control tumor growth, suggesting that further improvements can be achieved in the optimization of the protocols for the generation of PDT‐generated cancer vaccines.( 6 , 7 ) Because of the inflammatory/immune response triggered by PDT, this therapy has been shown to be particularly suitable for combination with a variety of immunotherapy based treatments, including angiogenic growth factors, matrix metalloproteinases, cytokines and adoptive transfer of immune cells.( 8 , 9 , 10 , 11 , 12 ) Overexpression of these molecules within PDT‐targeted tissue can adversely affect tumor response. Therefore, experimental protocols combining PDT with procedures targeting these molecules are being examined in an effort to improve treatment efficacy.
Oligodeoxynucleotide (ODN) containing unmethylated cytosine–phosphate–guanosine (CpG) motifs was originally isolated from components of bacterial DNA.( 13 ) CpG‐ODN immunotherapy has been studied as a strategy for tumor prevention as well as for treatment of immune disorders.( 14 , 15 ) A variety of studies have shown that CpG‐ODN can activate B cells, monocytes and natural killer cells, and induce a Th1‐like pattern of cytokine production.( 16 , 17 , 18 ) The CpG sequences drive macrophages to secrete interleukin‐12, a potent inducer of interferon‐γ (IFN‐γ) production in vivo from natural killer cells. IFN‐γ production drives Th1‐type immune responses by inducing differentiation of type‐1 Th cells, which see antigen in the presence of IFN‐γ from the uncommitted T‐cell pool.( 19 , 20 ) Moreover, ODN enhances humoral responses and induces the development of enhanced cytotoxic T lymphocytes (CTL) activity.( 17 , 21 ) ODN has been studied extensively as a strong immunomodulatory agent.( 22 , 23 , 24 )
The aim of the present study was to further characterize the immunotherapeutic significance of the combination of CpG‐oligodeoxynucleotide and TC‐1 tumor cell lysates induced by PDT. We examined the in vivo antitumor effect of PDT (or freezing/thawing)‐generated cell lysates plus ODN injection and the immune response in a mouse model. Our results showed that PDT‐cell lysates plus ODN showed a significant suppression of tumor growth at both prophylactic and therapeutic levels, compared to PDT (or F/T)‐cell lysates or ODN alone.
Materials and Methods
Preparation of PDT‐generated tumor cell lysates. Radachlorin( 25 ) was purchased from RADA‐PHARMA group (RADA‐PHARMA, Moscow, Russia). The light source was a diode laser with a 662 ± 3 nm wavelength (Won‐PDT D662, Won Technology, Daejeon, Korea). The irradiation power in vitro was fixed to be 6.25 J/cm2 at 20 mW/cm2 for 5 min irradiation measured at a distance of 3 cm from the exit slit.
To generate cell lysates by Radachlorin/PDT, TC‐1 cells carrying human papillomavirus (HPV) 16 E7 (ATCC CRL 2785) (5 × 106 in a 100 mm flask) were incubated for a total of 12 h at 37°C with Radachlorin (5 µg/mL) in Roswell Park Memorial Institute (RPMI) 1640 containing 10% fetal bovine serum (FBS). Following incubation, the cells were washed with serum‐free medium and exposed to light (with a dose equivalent to the LD99: 6.25 J/cm2). Freezing/thawing (F/T) lysates were generated by subjecting cells (5 × 106 cells in a 100 mm dish) to three F/T cycles, followed by centrifugation at 800 g to remove the cell debris.
Oligodeoxynucleotides. The immunostimulatory CpG ODN (Biobasic, Toronto, Canada) designated as 1826 (5′‐TCCATGACGTTCCTGACGTT‐3′) was used as an immuno‐adjuvant in this study.
Animals and tumor model. Pathogen‐free female C57BL/6 mice, 6 or 7 weeks of age, were maintained in a specific pathogen free (SPF) animal facility at the department of Laboratory of Animals, the Catholic University of Korea. TC‐1 cells (1 × 106) were insoculated subcutaneously on the depilated lower dorsum of the mice. Tumors were used for experimentation 10–12 days after inoculation when reaching a surface diameter of 6–8 mm and a thickness of 3–4 mm for tumor growth suppression assay.
Hsp70 enzyme‐linked immunosorbent assay. The amount of HSP70 released from PDT‐treated TC‐1 cells was determined by HSP70 enzyme‐linked immunosorbent assay (ELISA) using a commercially available kit from StressGen Biotechnologies (Ann Arbor, MI, USA). The absorbance of the samples was measured at 450 nm using a microplate spectrophotometer (Spectra Max 340/Molecular Devices, Sunnyrale, CA, USA).
In vivo treatment of PDT‐cell lysates plus ODN injection. Tumor‐bearing mice were given a peritumor subcutaneous injection (at 0, 2, 4, 7 and 14 days) of PDT‐cell lysates (5 × 105 cells/mouse) and/or ODN (1 mg/kg), F/T lysates or medium alone. The tumor diameter was determined by orthogonal caliper measurement (1/2[length + width]). In addition, the survival of the treated tumor‐bearing mice was observed for approximately 40 days.
Cytotoxic T‐lymphocyte assay. A 51Cr release assay was carried out. Briefly, splenocytes were stimulated for 5 days in the presence of 20 units/mL of interleukin‐2 (R & D Systems, Minneapolis, MN, USA) with TC‐1 cells treated previously for 3 h with mitomycin C (30 µg/mL). TC‐1 target cells were labeled with 100 µCi/mL Na251 CrO4 for 2 h and used to incubate the stimulated splenocytes for 5 h at 37°C. One‐hundred microliters of supernatants were harvested and counted on a gamma counter (Perkin‐Elmer, Waltham, MA).
Interferon‐γ TNF‐α assay. IFN‐γ and TNF‐α were measured in splenocytes of the PDT‐cell lysates plus ODN treated mice. Briefly, a 1‐mL aliquot containing 6 × 106 splenocytes was added to wells of 24‐well plates. Then, 50 µL PDT‐cell lysates (5 × 105 cells) or 4 × 105 TC‐1 cells treated previously with mitomycin C (30 µg/mL for 3 h) was added to each well. After 3 days of incubation at 37°C in 5% CO2, cell supernatants were secured and then used for detecting levels of IFN‐γ using commercial cytokine kits (Biosource, Camarillo, CA, USA) by adding the extracellular fluids to the IFN‐γ‐specific ELISA plates.
Intracellular IFN‐γ staining and fluorescence activated cell sorting (FACS) analysis. Mice were sacrificed to obtain cells from the spleen at 3 weeks after the last injection with PDT‐cell lysates and/or ODN. Cells from the spleen (2 × 106) were stimulated with 20 µL of PDT‐cell lysates for 6 h. The cells were incubated with antimouse Fc receptor (BD Biosciences, San Jose, CA, USA) for 1 h on ice, and then reacted with phycoerythrin (PE) conjugated antimouse CD8 (Pharmingen, San Diego, CA) for 30 min on ice. The subsequently fixed/permeabilized cells in Perm/Wash solution were stained with anti‐IFN‐γ‐fluorescein isothiocyanate (FITC; Pharmingen) for 30 min at 4°C. After washing three times with FACS buffer, cells were analyzed for the percentage of IFN‐γ or CD8+ cells on a flow cytometer (BD Biosciences). To identify dead cells and store INF‐γ in cytoplasm, PI and Brefeldin A (BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit, Cat. no. 555028) were used as described in the manuals, respectively.
Statistics. Results are presented as means ± the standard errors of the means (SEMs). The data were analyzed using one‐way analysis of variance (anova) and the Student's t‐test, and differences were considered significant at P‐values of <0.0001 and <0.01, respectively.
Results
Photodynamic therapy greatly induces HSP70 release from TC‐1 cells. Photodynamic therapy has been shown to enhance the host antitumor immune response. To determine whether this enhancement was at least a consequence of the effects of PDT on tumor cells, we measured the amount of HSP70 released from PDT treated‐ or F/T‐cell lysates (LD99). HSP70 has been shown to have potent adjuvant capability to induce immune responses, and be abundantly released from stress‐induced cells, compared to other HSPs. As shown in Fig. 1(a), PDT significantly induced HSP70 release from TC‐1 tumor cells, compared to F/T‐cell lysates. These results show that PDT further induces immunogenic or immunotherapeutic HSP70 release from tumor cells, compared to the cell‐stress route by F/T cycles.
Figure 1.
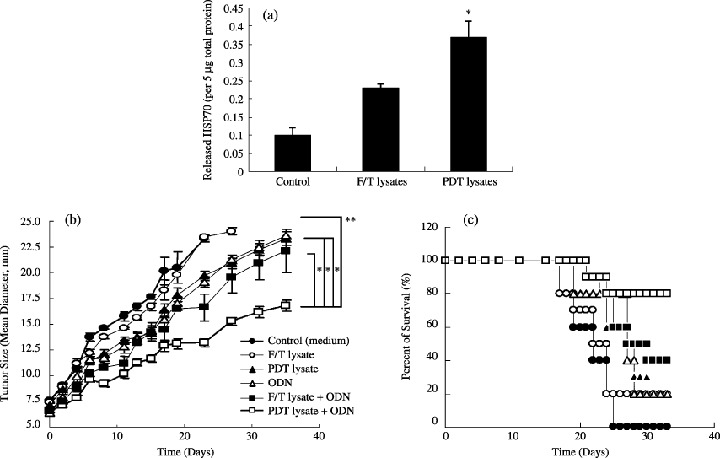
(a) Photodynamic therapy (PDT)‐induced heat shock protein 70 (HSP70) release from TC‐1 tumor cells. (b) Growth and (c) survival curves for TC‐1 tumor of mice treated with PDT (or freezing/thawing[F/T])‐cell lysates and/or oligodeoxynucleotide (ODN). *Statistically significant at P < 0.0001; bars ± SD.
Photodynamic therapy‐cell lysates + ODN therapeutically protected animals from tumor growth. To further enhance the immune response with PDT‐generated cell lysates, including HSP70, we investigated the effects of the combination of PDT‐cell lysates and ODN in vivo. TC‐1 tumor‐bearing mice were given a subcutaneous injection of PDT‐cell lysates and/or ODN, as described above. The tumor growth was significantly suppressed in mice given injections of PDT‐cell lysates plus ODN injection when compared with each separate group (Fig. 1b). These mice displayed a more slowly growing tumor, compared with the other groups. The survival rate of tumor‐bearing mice was closely related to the antitumor effect (Fig. 1c). Despite therapy, tumors kept growing in most mice. Evaluation of the survival of mice in the coinjection group and the other groups after 40 days showed that the survival of mice in the coinjection group was prolonged compared to the other groups. In addition, there was no effect in animals immunized with tumor cell lysates obtained by the freeze‐thaw (F/T) technique compared to lysates produced by PDT. Thus, the generation of tumor lysate by PDT is more effective than simple killing of the cells, for example, freeze‐thaw. These results demonstrate that the antitumor effect of PDT‐cell lysates was significantly enhanced, particularly, when combined with ODN.
Photodynamic therapy‐cell lysates plus ODN enhanced systemic IgG subclass production. We investigated the in vivo effects of PDT‐cell lysates and PDT‐cell lysates plus ODN on the modulation of the immune response by favoring the development of Th1 versus Th2 cells. As shown in Fig. 2, we analyzed the levels of IgG subclasses, as they provide an indication of the Th1 versus Th2 nature of the immune response. We analyzed the IgG subclasses induced by the PDT‐cell lysates and/or ODN. The results showed that PDT‐cell lysates plus ODN injection enhanced all four types of IgG isotype production significantly higher than PDT (or F/T)‐cell lysates or ODN alone. In particular, IgG2b production was predominant after PDT‐cell lysates plus ODN injection, as compared with PDT‐cell lysates alone. IgG3 was found at low levels. IgG2b/IgG1 was calculated as 1.5 (PDT‐cell lysates) and 1.80 (PDT‐cell lysates plus ODN). These results may indicate an ongoing immune response associated with Th1 activity in these mice.
Figure 2.
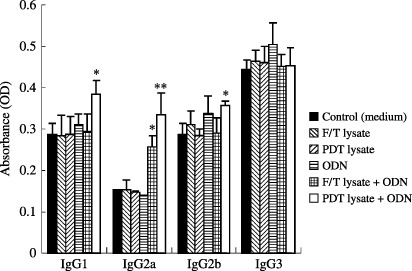
Induction of photodynamic therapy (PDT)‐generated tumor cell lysate‐specific IgG isotypes by injection with PDT (or freezing/thawing[F/T]) generated tumor cell lysate and/or oligodeoxynucleotide (ODN). *Statistically significant at P < 0.05; bars ± SD.
Photodynamic therapy‐cell lysates plus ODN injection induces significant secretion of IFN‐γ. We were next interested in evaluating whether IFN‐γ was over‐secreted by PDT (or F/T)‐cell lysates plus ODN. IFN‐γ has been known to play an important role in driving Th1‐type immune responses as well as inducing cytotoxic T‐cell responses. As shown in Fig. 3(a), IFN‐γ production from splenocytes was dramatically induced by injection with PDT‐cell lysates plus ODN. However, little induction of IFN‐γ production was observed after injecting PDT (or F/T)‐cell lysates or ODN alone. This is consistent with our previous observation that the antitumor effects were greatly enhanced only by PDT‐cell lysates plus ODN injection. By contrast, splenocytes of animals injected with PDT‐cell lysates and/or ODN were subsequently stimulated in vitro with TC‐1 cells. As shown in Fig. 3(b), IFN‐γ production was significantly induced by injection with PDT‐cell lysates plus ODN. However, little induction of IFN‐γ production was observed by injecting PDT (or F/T)‐cell lysates or ODN alone. This illustrates that PDT‐cell lysates plus ODN could induce IFN‐γ production to a more significant level from splenocytes. In particular, TNF‐α production was significantly induced by injection with PDT‐cell lysates plus ODN, compared to the injection with PDT‐cell lysates or ODN alone, as shown in Fig. 4.
Figure 3.
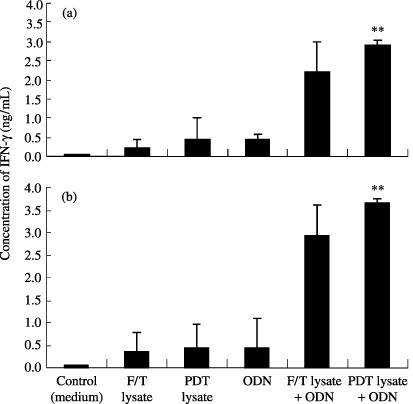
Production levels of interferon (IFN)‐γ from splenocytes in mice injected with photodynamic therapy (PDT) (or freezing/thawing[F/T])‐cell lysates and/or oligodeoxynucleotide (ODN). A 1‐mL aliquot containing 6 × 106 splenocytes was added to wells of 24‐well plates. Then, 50 µL of PDT lysates (5 × 105 cells). (a) or 4 × 105 TC‐1 cells treated previously with mitomycin C (30 µg/mL for 3 h). (b) was added to each well. Samples were assayed in triplicate. Values represent mean of released IFN‐γ concentrations. *Statistically significant at P < 0.01; bars ± SD.
Figure 4.
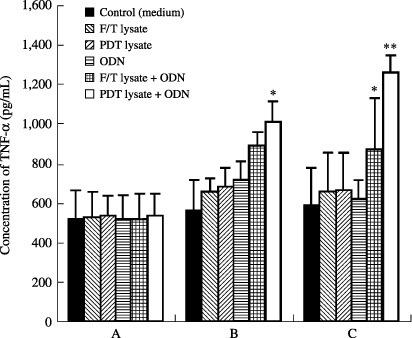
Production levels of tumor necrosis factor (TNF)‐α from splenocytes in mice injected with photodynamic therapy (PDT) (or freezing/thawing[F/T])‐cell lysates and/or oligodeoxynucleotide (ODN). Splenocytes were not stimulated (a) and stimulated with PDT lysates (5 × 105 cells) (b) or mitomycin C‐treated TC‐1 cells for 3 days (c). Samples were assayed in triplicate. Values represent mean of released TNF‐α concentrations; *Statistically significant at P < 0.05; bars ± SD.
CTL was induced by immunization of PDT‐cell lysates plus ODN. To determine whether PDT‐cell lysates plus ODN could induce PDT‐cell lysate‐specific CTL activity in vivo, we immunized animals with PDT (or F/T)‐cell lysates and/or ODN. As shown in Fig. 5, injection with PDT‐cell lysates plus ODN induced CTL activity to a significant level. However, animals immunized with PDT (or F/T)‐cell lysates or ODN alone showed only a slight induction of CTL, compared to the control. This finding suggests that PDT‐cell lysates in the presence of ODN can induce PDT‐cell lysates‐specific CTL responses in vivo.
Figure 5.
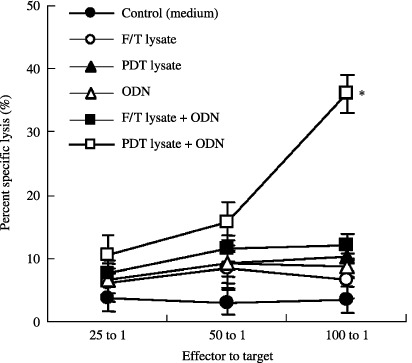
Cytotoxic T lymphocytes responses by injection with photodynamic therapy (PDT) or (or freezing/thawing[F/T])‐cell lysates and/or oligodeoxynucleotide (ODN). The experiments were repeated two more times with similar results. *Statistically significant at P < 0.01; bars ± SD.
Photodynamic therapy‐cell lysates plus ODN vaccination resulted in significant protection from tumor formation. It is important for antigen‐specific immune modulation to influence tumor formation. We analyzed the protective efficacy of ODN coinjection in a murine tumor formation model. Mice were immunized with PDT‐cell lysates and/or ODN, and then challenged with TC‐1 cells (data not shown). PDT‐cell lysates plus ODN injection alone resulted in remarkable protection from tumor formation, whereas PDT‐cell lysates or ODN vaccine alone showed little tumor protection in animals similar to the negative control. These results showed that when animals immunized previously with PDT‐cell lysates plus ODN were challenged with tumor cells, ODN as a vaccine adjuvant induced significant tumor growth inhibition.
Tumor protection was mediated by IFN‐γ from CD8+ T Cells. We were next interested in evaluating the CD8+ T cell dependent production level of IFN‐γ. Splenocytes from animals immunized with PDT (or F/T)‐cell lysates and/or ODN were subsequently stimulated in vitro with TC‐1 cells. IFN‐γ and TNF‐α production were dramatically induced by injection with PDT‐cell lysates plus ODN. However, little induction of IFN‐γ production was observed in the groups injected with PDT (or F/T)‐cell lysates or ODN alone. This was also consistent with intracellular IFN‐γ staining levels of CD8+ T cells, as shown in Fig. 6. PDT‐cell lysates plus ODN‐immunized animals alone showed a significant level of IFN‐γ‐releasing CD8+ T cells. However, animals individually injected showed little induction of IFN‐γ‐releasing CD8+ T‐cell populations. By contrast, splenocytes did not respond to intracellular IFN‐γ and CD4+ T‐cell staining at all (data not shown), suggesting that PDT‐cell lysates plus ODN injection can elicit IFN‐γ production responses to a more significant level from CD8+ T cells.
Figure 6.
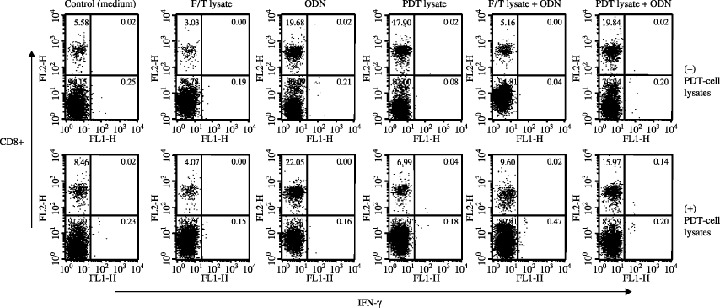
Intracellular interferon (IFN)‐γ and CD8+ T‐cell staining of splenocytes after stimulation in vitro with photodynamic therapy (PDT) (or freezing/thawing[F/T])‐cell lysates.
Discussion
It has been reported that the PDT‐generated tumor cell lysates were potent vaccines and PDT‐generated vaccines are more effective than other modes of creating whole tumor vaccines, (i.e. UV or ionizing irradiation).( 7 ) It has also shown that although both UV and PDT‐generated tumor cell lysates are able to induce phenotypic dendritic cell (DC) maturation, only PDT‐generated lysates are able to activate DCs to express IL‐12, which is critical to the development of a cellular immune response. The limitation of genetically engineered cancer vaccines have revitalized interest in the use of ‘crude’ tumor lysates as vaccines, which are generally created by UV or IR of tumor cells and are coinjected with an adjuvant.( 26 ) Therefore, PDT‐generated tumor cell lysates as vaccines were superior at eliciting cytolytic activity and providing protection, compared to those prepared by UV or IR.
Recent studies have demonstrated that synthetic ODN containing CpG motifs induce strong Th1‐like innate and acquired immune responses. In particular, NK and dendritic cells are induced to produce pro‐inflammatory cytokines such as IFN‐γ and IL‐12, and mediate cellular cytotoxicity.( 26 , 27 ) In addition, B cells are costimulated to secrete antibody.( 28 ) Depending on the ODN backbone and the bases flanking the CpG, ODN can induce predominant NK cell activation or B‐cell proliferation. Even though ODN has already been used as an adjuvant for tumor therapy, we show for the first time that the protective and immunotherapeutic significance of the combination of ODN and TC‐1 tumor cell lysates induced by PDT, compared to other ODN combination therapies. These findings suggest that both PDT‐cell lysates and ODN are required for longer‐lasting and more robust prophylactic and therapeutic vaccine efficacies in this tumor model system. It has been reported that the efficacy of CpG‐ODN, as adjuvant, in some models appears to be limited in the induction of a protective immune response, and that single administration of ODN does not provide a long‐term protective effect.( 29 ) Even in cases where protection for longer than a week is desired, repeated administration of CpG ODNs has been recommended.( 30 ) However, the potential side‐effects associated with administration of a non‐specific proinflammatory stimulus requires consideration.
Most previous reports have shown the possible effects of ODN on the immune response. For example, coinjection with immune response modifiers plus ODN was reported to be capable of initiating an immune response in the absence of additional adjuvant by enhancing IgG2a levels and CTL responses.( 31 ) In the present study, however, ODN alone showed no beneficial effects on tumor regression. This difference might be because of ODN types tested, the doses of ODN, or injection routes tested.( 14 ) Instead, coinjection with PDT‐cell lysates plus ODN significantly enhanced systemic IgG production higher than the PDT‐cell lysate vaccination alone. This is compatible with the prior findings of other groups.( 32 ) Coinjection of CpG DNA together with tumor antigen reveals a powerful effect of CpG ODNs as adjuvant that includes antigen‐specific B cell and T cell responses.( 33 , 34 , 35 ) Furthermore, a significantly enhanced production of all four IgG isotypes, IgG1, IgG2a, IgG2b, and IgG3 by ODN coinjection was also observed. This is consistent with the previous findings that an increase in both IgG1 and IgG2a has been observed following intranasal coadministration of hepatitis B surface antigen with ODN.( 36 ) The isotype profile elicited by coinjection with PDT‐cell lysates plus ODN suggests that the immune response could be described as Th1‐cell proliferative responses. In addition, the immunization with a combination of CpG ODN and PDT‐cell lysate in mice resulted in a Th1‐cell response and improved vaccine efficacy.
Generation of CTL activity was also evaluated when antigen was delivered along with ODN. CTL was significantly induced after injection with PDT‐cell lysates plus ODN alone. This is consistent with the previous findings that antigen‐specific CTL activity is induced by codelivery with the antigens of hepatitis B surface Ag or HPV 16 E7 plus ODN;( 14 , 37 ) therefore, ODN codelivery could be useful for the induction of antigen‐specific CTL activities. It has been reported that the interaction of ODNs with toll‐like receptor‐9 leads to the secretion of proinflammatory cytokines such as IL‐1, IL‐6, TNF‐α and IL‐12.( 38 ) IL‐12 acts on T and NK cells for the production of cytokines, primarily IFN‐γ.( 39 ) Consequently, coinjection with PDT‐cell lysates plus ODN induces a Th1‐like pattern of cytokine production that is dominated by TNF‐α and IFN‐γ. In addition, ODN may be useful as an adjuvant for cellular and humoral immunity. The induction of such innate immune responses and production of Th1‐related cytokines is very important for controlling the type of PDT‐cell lysate‐specific immune response.
CD4+ T cells are also considered to be the predominant source of IFN‐γ, and one study has suggested that the percentage of CD4+ cells producing IFN‐γ was reduced in patients with allergic disorders.( 40 ) It has been reported that the proportions of cells producing IFN‐γ and the amounts of IFN‐γ produced per cell were similar for CD8+ and CD4+ cells, suggesting that they both contribute significantly to IFN‐γ production.( 41 ) In this study, CD8+ T cells were shown to contribute to immune defenses against this antigen stimulation. Thus, PDT‐cell lysate‐specific CD8 + cells may contribute to the immune response by lysing infected cells or by producing IFN‐γ or molecules with antitumor activity. The results showed that both PDT‐cell lysate and ODN are required for induction of IFN‐γ from CD8+ T cells against the tumor challenge. In addition, the ability of PDT‐cell lysates plus ODN to prime Th1 cell responses, as well as CD8+ cytotoxic T‐lymphocyte responses, could hold important therapeutic implications for modulating the immune response in infectious diseases.
In addition, PDT significantly induced HSP70 release from TC‐1 tumor cells, compared to F/T cell lysates, suggesting that PDT had a profound effect on HSP70 release, which could have a significant effect on the therapeutic outcome. HSP70 has been shown to interact with antigen‐presenting cells and has a potent adjuvant capability to induce an antigen‐specific CD8+ CTL response.( 42 ) Although we have not investigated induction of various heat shock proteins from PDT‐generated cell lysates, our observation was consistent with previous findings that HSP70 is abundantly released from PDT‐generated tumor cell lysates, compared to other heat shock proteins.( 32 )
In conclusion, the synergistic effects of PDT‐tumor cell lysates plus ODN may be useful for the induction of a significant protective immune response in the TC‐1 cancer model through IFN‐γ secretion and CTL activation. This study provides information that may be beneficial for immune therapy in vaccine research.
References
- 1. Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg 2002; 20: 3–7. [DOI] [PubMed] [Google Scholar]
- 2. Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol 2001; 74: 656–69. [DOI] [PubMed] [Google Scholar]
- 3. Yuan J, Mahama‐Relue PA, Fournier RL, Hampton JA. Predictions of mathematical models of tissue oxygenation and generation of singlet oxygen during photodynamic therapy. Radiat Res 1997; 148: 386–94. [PubMed] [Google Scholar]
- 4. Korbelik M. Role of Toll‐like receptors in photodynamic therapy‐elicited host response. Proc SPIE 2004; 5319: 87–95. [Google Scholar]
- 5. Dougherty TJ, Gomer CJ, Henderson BW et al. Photodynamic therapy. J Natl Cancer Inst 1998; 90: 889–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Korbelik M, Sun J. Photodynamic therapy‐generated vaccine for cancer therapy. Cancer Immunol Immunother 2006; 55: 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res 2002; 62: 1604–8. [PubMed] [Google Scholar]
- 8. Ferrario A, Von Tiehl KF, Rucker N, Schwarz MA, Gill PS, Gomer CJ. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res 2000; 60: 4066–9. [PubMed] [Google Scholar]
- 9. Ferrario A, Chantrain CF, Von Tiehl K et al. The matrix metalloproteinase inhibitor prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res 2004; 64: 2328–32. [DOI] [PubMed] [Google Scholar]
- 10. Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy‐elicited antitumor response by localized treatment with granulocyte‐macrophage colony‐stimulating factor. Cancer Res 1996; 56: 3281–6. [PubMed] [Google Scholar]
- 11. Jalili A, Makowski M, Switaj T et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin Cancer Res 2004; 10: 4498–08. [DOI] [PubMed] [Google Scholar]
- 12. Korbelik M, Sun J, Naraparaju VR, Yamamoto N. Eradication of solid cancers by photodynamic therapy combined with dendritic cell‐based adoptive immunotherapy. Cancer Res Proc 1999; 40: 86. [Google Scholar]
- 13. Kline JN, Krieg AM. CpG oligodeoxynucleotides. Prog Respir Res 2001; 31: 229–32. [Google Scholar]
- 14. Kim TY, Myoung HJ, Kim JH et al. Both E7 and CpG‐oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8+ T cells in protection. Cancer Res 2002; 62: 7234–40. [PubMed] [Google Scholar]
- 15. Horner AA, Raz E. Immunostimulatory sequence oligodeoxynucleotide‐based vaccination and immunomodulation: two unique but complementary strategies for the treatment of allergic diseases. J Allergy Clin Immunol 2002; 110: 706–12. [DOI] [PubMed] [Google Scholar]
- 16. Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon γ. Proc Natl Acad Sci USA 1996; 93: 2879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krieg AM, Yi AK, Matson S et al. CpG motifs in bacterial DNA trigger direct B cell activation. Nature 1995; 374: 546–9. [DOI] [PubMed] [Google Scholar]
- 18. Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol 1996; 157: 1840–5. [PubMed] [Google Scholar]
- 19. Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligonucleotides act as adjuvants that switch on T helper (Th1) immunity. J Exp Med 1997; 186: 1623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roman M, Martin‐Orozco E, Goodman JS et al. Immunostimylatory DNA sequences function as T helper‐1‐promoting adjuvants. Nat Med 1997; 3: 849–54. [DOI] [PubMed] [Google Scholar]
- 21. Warren TL, Bhatia SK, Acosta AM et al. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I‐restricted T cells. J Immunol 2000; 165: 6244–51. [DOI] [PubMed] [Google Scholar]
- 22. Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol 1998; 160: 870–6. [PubMed] [Google Scholar]
- 23. Kline JN, Waldschmidt TJ, Businga TR et al. Modulation of airway inflammation against asthma by CpG oligonucleotides in a murine model of asthma. J Immunol 1998; 160: 2555–9. [PubMed] [Google Scholar]
- 24. Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus‐2 in the genital tract. J Immunol 2001; 166: 3451–7. [DOI] [PubMed] [Google Scholar]
- 25. Uzdensky AB, Dergacheva OY, Zhavoronkova AA, Reshetnikov AV, Ponomarev GV. Photodynamic effect of novel chlorine e6 derivatives on a single nerve cell. Life Sci 2004; 74: 2185–97. [DOI] [PubMed] [Google Scholar]
- 26. Bodey B, Bodey B Jr, Siegel SE, Kaiser HE. Failure of cancer vaccines: the significant limitations of their approach to immunotherapy. Anticancer Res 2000; 20: 2665–76. [PubMed] [Google Scholar]
- 27. Suzuki Y, Wakita D, Chamoto K et al. Liposome‐encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res 2004; 64: 8754–60. [DOI] [PubMed] [Google Scholar]
- 28. Brummel R, Lenert P. Activation of marginal zone B cells from lupus mice with type A (D) CpG‐oligodeoxynucleotides. J Immunol 2005; 174: 2429–34. [DOI] [PubMed] [Google Scholar]
- 29. Gramzinski RA, Doolan DL, Sedegah M, Davis HL, Krieg AM, Hoffman SL. Interleukin‐12‐ and gamma interferon‐dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect Immun 2001; 69: 1643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krieg AM. Antitumor applications of stimulating toll‐like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Re 2004; 6: 88–95. [DOI] [PubMed] [Google Scholar]
- 31. Vasilakos JP, Smith RM, Gibson SJ et al. Adjuvant activities of immune response modifier R‐848: comparison with CpG ODN. Cell Immunol 2000; 204: 64–74. [DOI] [PubMed] [Google Scholar]
- 32. Korbelik M, Sun J, Cecic I. Photodynamic therapy‐induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res 2005; 65: 1018–26. [PubMed] [Google Scholar]
- 33. Kovarik J, Bozzotti P, Tougne C et al. Adjuvant effects of CpG oligodeoxynucleotides on responses against T‐independent type 2 antigens. Immunology 2001; 102: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang XS, Sheng Z, Ruan YB, Guang Y, Yang ML. CpG oligodeoxynucleotides inhibit tumor growth and reverse the immunosuppression caused by the therapy with 5‐fluorouracil in murine hepatoma. World J Gastroenterol 2005; 11: 1220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tewary P, Sukumaran B, Saxena S, Madhubala R. Immunostimulatory oligodeoxynucleotides are potent enhancers of protective immunity in mice immunized with recombinant ORFF leishmanial antigen. Vaccine 2004; 22: 3053–60. [DOI] [PubMed] [Google Scholar]
- 36. McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol 1998; 161: 4463–6. [PubMed] [Google Scholar]
- 37. Wild J, Grusby MJ, Schirmbeck R, Reimann J. Priming MHC‐I‐restricted cytotoxic T lymphocyte responses to exogenous hepatitis B surface antigen is CD4+ T cell dependent. J Immunol 1999; 163: 1880–7. [PubMed] [Google Scholar]
- 38. Spies B, Hocrhrein H, Vabulas M et al. Vaccination with plasmid DNA activates dendritic cells via Toll‐like receptor 9 (TLR9) but functions in TLR9‐deficient mice. J Immunol 2003; 171: 5908–12. [DOI] [PubMed] [Google Scholar]
- 39. Stern AS, Magram J, Presky DH. Interleukin‐12 an integral cytokine in the immune response. Life Sci 1996; 58: 639–54. [DOI] [PubMed] [Google Scholar]
- 40. Bhattacharyya S, Singla R, Dey AB, Prasad HK. Dichotomy of cytokine profiles in patients and high‐risk healthy subjects exposed to tuberculosis. Infect Immun 1999; 67: 5597–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shams H, Wizel B, Weis SE, Samten B, Barnes PF. Contribution of CD8(+) T cells to gamma interferon production in human tuberculosis. Infect Immun 2001; 69: 3497–01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu Y, Wan T, Zhou X et al. Hsp70‐like protein 1 fusion protein enhances induction of carcinoembryonic antigen‐specific CD8+ CTL response by dendritic cell vaccine. Cancer Res 2005; 65: 4947–54. [DOI] [PubMed] [Google Scholar]


