Abstract
(Cancer Sci 2010; 101: 759–766)
Matrix metalloproteinase (MMP)‐9, the 92‐kDa type IV collagenase, contributes to tumor invasion and metastases, and strategies to down‐regulate its expression could ultimately be of clinical utility. A pyrrole‐imidazole (PI) polyamide that targets the activator protein‐1 (AP‐1)‐binding site of the MMP‐9 promoter was designed and synthesized as a gene‐silencing agent for tumor metastases. The synthesized product showed selective DNA binding ability. The MMP‐9 PI polyamide significantly inhibited MMP‐9’s mRNA expression, protein level, and enzymatic activity in human breast adenocarcinoma cells (MDA‐MB‐231). Furthermore, the MMP‐9 PI polyamide inhibited migration and invasion by in vitro wound‐healing and matrigel‐invasion assay. The FITC‐labeled PI polyamide was localized in nuclei in 45 min of incubation with an MDA‐MB‐231 cell and remained in the nuclei for up to 96 h after incubation in vitro. It was also quickly localized in the mouse cellular nuclei of many tissues, including liver, kidney, and spleen, after intravenous injection without using any drug‐delivery system. Moreover, the polyamide treatment significantly decreased metastasis in a mouse model of liver metastasis. Our results suggest that this PI polyamide, which targets the MMP‐9 gene promoter, can be a novel MMP‐9 down‐regulating molecule for antimetastasis.
Metastasis is a complex multi‐step process and, in the cascade of metastasis, the invasion of the basement membrane and extracellular matrix by tumor cells is thought to be one of the most critical steps.( 1 ) This degradative process is mediated largely by matrix metalloproteinases (MMPs), such as 92‐kDa type IV collagenase MMP‐9 (gelatinase B, MMP‐9) and MMP‐2 (gelatinase A, MMP‐2), which contain fibronectin‐like domains for collagen binding, and are capable of degrading type I, IV, V, VII, and XI collagens and laminin.( 2 , 3 ) There is increasing evidence that MMP‐9 expression and MMP‐2 activity are increased in malignant cancers compared with benign tumors and noninvasive ones, and there is compelling evidence for the role of type IV collagenases in tumor invasion in vitro and in vivo,( 4 , 5 ) which is known to be associated with disruption of the basement membrane.
The MMP‐9 gene is encoded on chromosome 20, and its expression is under the control of a 2.2‐kb upstream regulatory region containing several functional regulatory motifs that interact with well‐characterized transcription factors, including nuclear factor‐kappa B (NF‐κB), activator protein‐1 (AP‐1), and stimulatory protein‐1 (Sp1).( 6 , 7 ) Mutations in these binding sites could reduce or abolish the induction of MMP‐9 by TPA (12‐O‐tetradecanoyl‐phorbol‐13‐acetate),( 7 ) and the suppression of MMP‐9 can reduce the invasive and metastatic ability of tumor cells.( 7 , 8 ) Suppression of the transcription factor function is, therefore, a major target for the therapeutic purpose of inhibiting MMP‐9 expression and activity.
The “transcription therapy” concept, proposed by Dervan to explain the control of gene‐expression by blocking eukaryotic transcription factors with pyrrole‐imidazole (PI) hairpin polyamides, has attracted attention.( 9 ) Hairpin polyamides are derived from distamycin A, which binds to A:T‐rich sequences of DNA.( 10 ) Polyamides containing the aromatic amino acids N‐methylpyrrole (Py) and N‐methylimidazole (Im) bind DNA with affinities and specificities comparable to naturally occurring DNA‐binding proteins.( 9 , 11 ) A set of pairing rules describes the interactions between pairs of these heterocyclic rings and Watson‐Crick base pairs within the minor groove of double‐stranded DNA in a sequence‐specific manner: Im/Py is specific for G·C, and Py/Py binds both A·T and T·A.
Here we have used the PI polyamide that targets the AP‐1 binding site, which has been reported to be crucial for MMP‐9 expression.( 3 , 4 ) Our results demonstrated that this PI polyamide inhibited MMP‐9 expression and activity, subsequently decreasing cell migration and the invasive activity of the MDA‐MB‐231 (human breast adenocarcinoma cell line) and HeLa cells using in vitro wound‐healing and invasion assays. We have also demonstrated that the PI polyamide decreased liver metastasis in in vivo experiments. Thus, we provide the first compelling evidence that MMP‐9 down‐regulation by PI polyamide has an inhibitory effect on tumor cell migration, invasion, and metastasis.
Materials and Methods
Synthesis of PI polyamide targeting human MMP‐9. A PI polyamide was designed to span the boundary of the AP‐1 binding site (−70 to −77) of the human MMP‐9 promoter (Fig. 1a), where a conserved region among most mammals, including rodents, is located. The numbering refers to the start of the open reading frame as +1.( 12 ) Polyamide was synthesized( 13 , 14 ) and the dissolved polyamide was measured according to previously described methods.( 15 ) The stock solution of PI polyamide was prepared by dissolving the compound in DMSO at 10 mm. The working solution was serially diluted with distilled water to obtain the desired concentration.
Figure 1.
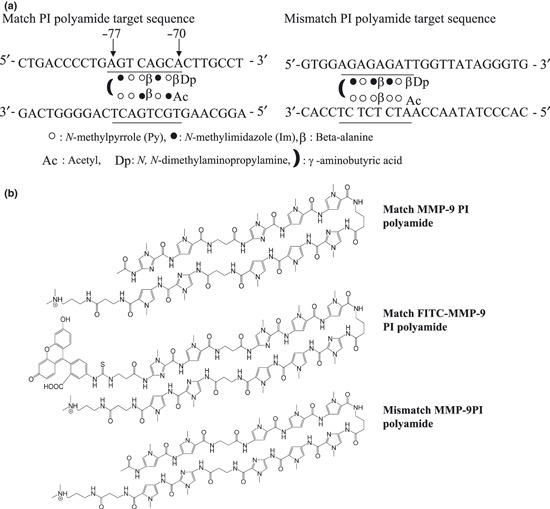
Target sequence and structure of synthetic PI polyamide that targets the human matrix metalloproteinase (MMP‐9) promoter. (a) The polyamide was designed to bind to the human MMP‐9 promoter adjacent to the activator protein 1 (AP‐1) binding site (−70 to −77) (left). Mismatch polyamide was designed not to bind the transcription‐binding sites of the promoter (right). (b) Structure of the human MMP‐9‐specific pyrrole‐imidazole (PI) polyamide (upper), the FITC‐labeled human MMP‐9‐specific PI polyamide (middle), and mismatch PI polyamide (lower). Polyamide was synthesized by a solid‐phase method and purified by HPLC (0.1% AcOH/CH3CN 0 to 66% linear gradient, 0 to 20 min, 254 nm, through a Chemcobond 5‐ODS‐H column).
DNA binding assay. FITC‐labeled match oligonucleotides corresponding to −64 and −85 (5′‐GACCCCTGAGTCAGCACTTGCC), including the AP‐1 binding site and 2‐bp mutated oligonucleotides (5′‐GACCCCTGAGTAGGCACTTGCC), were synthesized for a gel mobility shift assay. 0.6 μM of the FITC‐labeled match or mutated mismatch oligonucleotides were incubated with 16 mM of MMP‐9 PI polyamide for 1 h at 37°C, respectively. The resulting complexes were separated by electrophoresis and visualized with the luminescent image analyzer LAS‐4000 (Fujifilm, Tokyo, Japan).
Cell‐type and culture conditions. The MDA‐MB‐231, HeLa, and HT‐29 (human colon adenocarcinoma cell line) cells used in this study (obtained from ATCC, Manassas, VA, USA) were cultured in DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 100 μg/mL streptomycin, 100 units/mL penicillin, and 10% FBS in a humidified atmosphere containing 5% CO2 at 37°C. Experimental control cells were cultured in 1% DMSO.
Animals. Six‐week‐old male athymic nude mice (BALB/c nu/nu: CAnN.Cg‐Foxn1nu/CrlCrlj) were used (Charles River Japan, Yokohama, Japan) and housed in specific pathogen‐free conditions. The room was maintained at 23 ± 1°C under a 12‐h light/12‐h dark cycle. Food and drinking water were provided ad libitum. All of the experiments were carried out in accordance with guidelines approved by the Laboratory Animal Care Committee at Nihon University School of Medicine, Tokyo, Japan.
Distribution of FITC‐labeled polyamide in vitro and in vivo. MDA‐MB‐231 cells were seeded on six‐well plates at 3.0 × 104 cells per well and grown in 2 mL of DMEM supplemented with 10% FBS. Cells were maintained at 37°C in 5% CO2. After 24 h, the MDA‐MB‐231 cells were incubated with a final 3 μm of FITC‐labeled PI polyamide compounds in a growth medium for 2 h. The cells were then washed with PBS and viewed according to the method described previously.( 16 )
0.15 mg of FITC‐labeled polyamide was injected into BALB/c nu/nu mice via the tail vein. At days 1 and 6 after injection, the liver, kidneys, spleen, and other organs were removed, and frozen specimens were made and observed by fluorescence microscope.
In vitro cell proliferation. MDA‐MB‐231 and HeLa cells were seeded on 96‐well microplates to 2.5 × 103 cells per well and cultured at 37°C in 5% CO2. The tested polyamide compounds were added at appropriate concentrations and incubated for 72 h. The cell proliferation assay was carried out by WST‐8 assay (Nacalai Tesque, Tokyo, Japan). The absorbance (A 450) of each well was measured by a Wallac 1420 multilabel counter (Amersham Bioscience, Piscataway, NJ, USA).
Wound‐healing migration assay. To measure cell migration during wound‐healing, MDA‐MB‐231 and HeLa cells (3 × 105) were seeded in individual wells in an eight‐well chamber slide. When the cells reached a confluent state, cell layers were wounded with a plastic micropipette tip that had a large orifice. The medium and debris were aspirated away and replaced by 400 μl of fresh medium with diverse concentrations of PI polyamide. The cells were stained with Diff‐Quik solution (Kokusaishiyaku, Kobe, Japan) and photographed at 48 h after wounding by phase‐contrast microscopy.
Matrigel invasion assay. A cell‐invasion assay was carried out using BioCoat Matrigel Invasion Chambers (Becton Dickinson Labware, Bedford, MA, USA) consisting of Transwell membrane filter inserts in a 24‐well tissue‐culture plate. The Transwell filter has 8‐μm pore‐sized membranes coated with Matrigel. MDA‐MB‐231, HeLa, and HT‐29 cells (0.25 × 104 cells/well) suspended in DMEM medium containing 0.1% FBS with diverse concentrations of PI polyamide were added to the upper chambers, and DMEM medium containing 5% FBS was placed in the lower well and then incubated for 48 h at 37°C in 5% CO2. Noninvading cells on the upper surface of the membrane were removed by wiping them out with a cotton swab, and invaded cells were fixed and stained with a Diff‐Quik solution. The number of invaded cells per membrane was counted under a light microscope at ×200 magnification in each of the 10 fields of triplicate membranes.
Real‐time RT‐PCR. MDA‐MB‐231 cells were treated with diverse concentrations of PI polyamide. After 48 h, the cell fraction was processed for RNA isolation with Trizol reagent (Invitrogen Life Technologies) according to the manufacturer’s instructions, and RNA was treated with DNase before cDNA synthesis. First‐strand cDNA was synthesized and real‐time RT‐PCR was carried out using SYBR Premix Ex Taq, Perfect Real Time (Takara Bio, Otsu, Japan). The primers for MMP‐9 (forward, 5′‐GAGACCGGTGAGCTGGATAG‐3′; reverse, 5′‐TACACGCGAGTGAAGGTGAG‐3′) and the internal control human endogenous GAPDH were used (forward, 5′‐GCACCGTCAAGGCTGAGAAC‐3′; reverse, 5′‐TGG TGAAGACGCCAGTGGA‐3′). The relative quantity for the MMP‐9 gene was normalized for GAPDH‐expression.
Western blot analysis. MDA‐MB‐231 cells were treated with diverse concentrations of PI polyamide. After 48 h, the cells were collected and total cell lysates were prepared in an extraction buffer containing 50 mM Tris‐HCl, 150 mM NaCl, 10 mM EDTA, and 1% Triton‐X containing a protease‐inhibitor cocktail (Boehringer Mannheim, Germany). Homogenates were centrifuged at 15 000 g for 10 min at 4°C. Supernatants were collected and protein concentrations were determined. Protein samples (10 μg) were electrophoresed on NuPAGE+ 10% Bis‐Tris gels (Invitrogen Life Technologies) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were incubated with a rabbit anti‐MMP‐9 polyclonal antibody (1:500) (Calbiochem Biosciences, La Jolla, CA, USA) overnight. The membranes were washed three times with a Tris‐buffered saline containing 0.2% Tween 20. The immunocomplexed proteins were identified by reaction with a peroxidase‐conjugated goat antibody to rabbit IgG (Calbiochem Biosciences), followed by enhanced chemiluminescent detection (Amersham, Piscataway, NJ, USA).
Zymography analysis. A substrate gel zymography of the activity of the MMP‐9 of crude proteins from MDA‐MB‐231 and HT‐29 cell culture supernatants was performed with Novex zymogram gels using the XCell SureLock Mini‐cell (Invitrogen Life Technologies). Briefly, 500 μL aliquots containing 2.5 × 105 of MDA‐MB‐231 and HT‐29 cells were added to each of the triplicate wells containing DMEM with 10% FBS. After incubation for 24 h, several concentrations of PI polyamide were added to the cells in a culture medium without FBS. The supernatant was collected after 48 h’ incubation. The crude proteins from each sample were mixed with Novex Tris‐Glycine SDS Sample Buffer (2X) and resolved on a 10% Zymogram (gelatin) gel. The gels were run and washed in zymogram renaturing buffer and developing buffer, according to the manufacturer’s instructions (Invitrogen Life Technologies). Finally, the gels were digitized using a method described previously.( 17 )
Liver metastasis model of human colon cancer. Six‐week‐old male nude mice were bred and maintained in specific pathogen‐free conditions. HT‐29 cells were harvested from 100‐cm2 culture flasks by overlaying subconfluent monolayer cultures with 0.05% trypsin and 0.02% EDTA. The resulting suspension was centrifuged for 3 min at 120g and washed twice with PBS without Ca2+ and Mg2+. For in vivo implantation, 3 × 106 HT‐29 cells in 100 μL of PBS were injected into the spleen of nude mice as previously described.( 18 , 19 ) Each group contained two or three nude mice.
Treatment schedule. The MMP‐9 PI polyamide treatment group and control group were divided into three groups, according the following schedule. Group 1: The cells were treated with or without 3 μm MMP‐9 polyamide for 1 h before tumor cell inoculation. Group 2: 0.15 mg of MMP‐9 PI polyamide was injected into BALB/c nu/nu mice via the tail vein at day 7 after implantation and then the same concentration of polyamide was injected once a week for a period of 35 days. Group 3: 1 h after implantation, 0.15 mg of PI polyamide was injected into BALB/c nu/nu mice via the tail vein and then the same concentration of polyamide was injected at days 7 and 14 (Fig. 5b). The mice were sacrificed at days 42 or 35 after injection, and the spleen and liver were collected and fixed in 10% neutral buffered formalin and embedded in paraffin for histological observation. Paraffin sections (3 μm thick) were made and stained with hematoxylin–eosin (H&E). The area of tumor metastasis in the liver was determined using the Lumina Vision image analysis system (Mitani, Tokyo, Japan). In order to identify the vessel density in tumors in the spleen and liver, immunofluorescence staining was performed with biotinylated GSL I isolectin B4 (B‐1205; Vector Laboratories, Burlingame, CA, USA). In order to detect biotinylated lectin, slides were incubated with Cy3‐conjugated streptavidin (S6402; Sigma‐Aldrich, St. Louis, MO, USA) diluted 1:500 in PBS at room temperature for 1 h. Sections were counterstained with 5 μg/mL Hoechst 33342 (B2261; Sigma‐Aldrich). Five to eight random fields were selected from each slide, and positive areas (red) were measured and quantitated by using the Lumina Vision image analysis system (Mitani). The results were expressed as the mean vessel density in the liver tumor.
Figure 5.
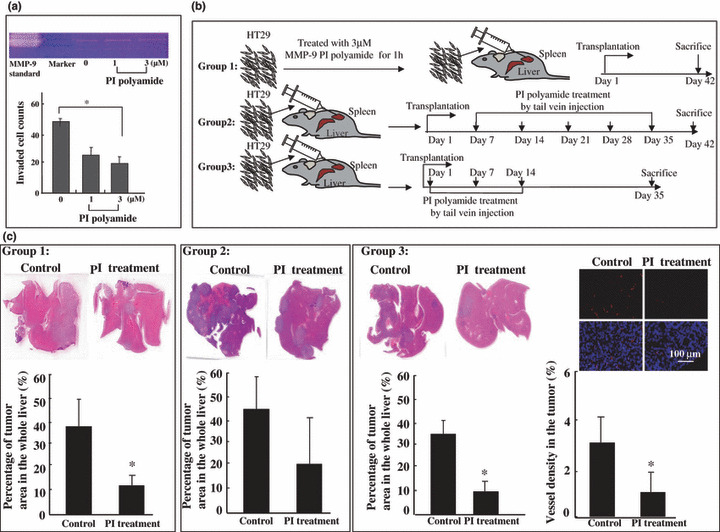
Suppression of liver metastasis by matrix metalloproteinase (MMP)‐9 pyrrole‐imidazole (PI) polyamide treatment. (a, upper) Decreased MMP‐9 activity by gelatin‐based zymography of culture medium of HT‐29 (human colon adenocarcinoma cell line) cells treated with PI polyamide. (lower) In vitro invasiveness of HT‐29 cells. The 2500 cells were seeded on Matrigel‐coated membrane with or without PI polyamide. After 48 h, the invaded cells were quantified, as described in the Materials and Methods. Values represent mean ± SD from biological triplicate experiments. *P < 0.05. (b) Group 1: After treatment with PI polyamides for 1 h, HT‐29 cells were injected into the spleens of nude mice (3 × 106/per mouse), and the mice were sacrificed 42 days later. Group 2: At day 7 after implantation, 0.15 mg of PI polyamide was injected into BALB/c nu/nu mice via the tail vein and then the same concentration of PI polyamide was injected via the tail vein once a week for a period of 35 days. The mice were sacrificed at day 42. Group 3: 1 h after implantation, 0.15 mg of PI polyamide was injected into BALB/c nu/nu mice via the tail vein and then the same concentration of PI polyamide was injected via the tail vein at days 7 and 14. The mice were sacrificed at day 35 (n = 3 or n = 2 for each group). (c) Groups 1, 2, and 3 (left, upper): Representative micrograph of liver sections from control and PI polyamide treatment groups. Liver metastasis was reduced in the MMP‐9 PI polyamide‐treated group. Bar graphs indicate results expressed as the average percentage of the metastatic liver tumor area of the whole liver lesion. Four randomly selected whole liver sections per mouse were examined. The area sizes were quantitated by the Lumina Vision Image analysis system (lower). Quantitative analysis of isolectin B4‐positive vessels in the liver tumors (Group 3, right). Immunofluorescent stained area of GSL I isolectin B4 was quantitated by the Lumina Vision Image analysis system. Results are presented as the mean ± SD of six and eight randomly selected areas for control and PI polyamide‐treated group liver tumors, respectively. Data are expressed as the mean ± SD (n = 3 for each group). *P < 0.05.
Statistical analysis. All values were expressed as the mean ± SE, and the statistical significance was analyzed using the Student’s t‐test. A P‐value of <0.05 was considered significant.
Results
Binding of the polyamide to double‐stranded DNA. To determinate the binding affinity and specificity of polyamide to target DNA, gel mobility shift assays were performed. The polyamide bound the appropriate 22‐bp double‐stranded DNA containing a match AP‐1 site but did not bind DNA with the 2‐bp mutated sequence from the AP‐1 consensus (Fig. 2a).
Figure 2.
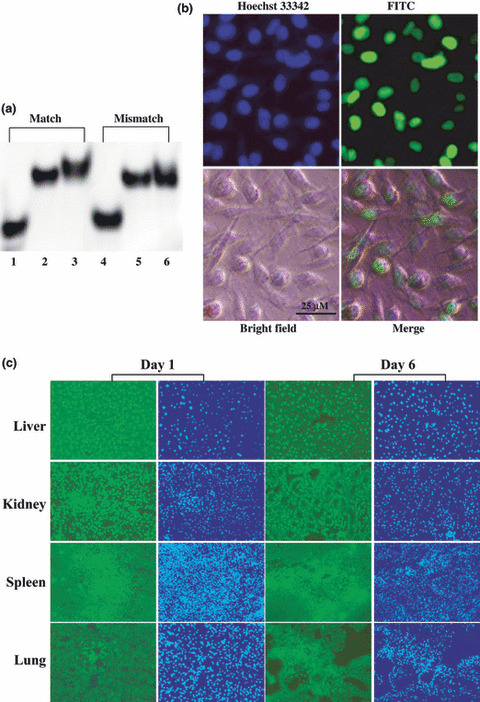
Gel mobility shift assay and distribution of FITC‐labeled pyrrole‐imidazole (PI) polyamide in vitro and in vivo. (a) FITC‐labeled DNA corresponding to the activator protein 1 (AP‐1) binding site and 2‐bp mutated DNA were synthesized and incubated with PI polyamides for 1 h at 37°C and loaded onto a 20% polyacrylamide gel. Lane 1, single‐stranded DNA; lane 2, double‐stranded DNA; lane 3, match double‐stranded DNA with polyamide targeting matrix metalloproteinase (MMP)‐9; lane 4, 2‐bp mutated mismatch single‐stranded DNA; lane 5, 2‐bp mutated mismatch double‐stranded DNA; lane 6, 2‐bp mutated mismatch double‐stranded DNA with polyamide. (b) The distribution of FITC‐labeled MMP‐9 PI polyamide in MDA‐MB‐231 cells after 45 min’ incubation was demonstrated. MDA‐MB‐231 cells were incubated with FITC‐labeled polyamide and examined by fluorescence microscopy under living cell conditions, then fixed and viewed again. Nuclei were stained with Hoechst 33342 (blue). (c) Distribution of FITC‐labeled PI polyamide in vivo. 0.15 mg of FITC‐labeled polyamide was injected into nude mice intravenously. The liver, kidney, spleen, and lungs were removed at days 1 and 6 after injection, and frozen specimens were made. This polyamide can quickly localize, mostly to the nucleus in mice livers, kidneys, and spleens, and remain stable in these tissues after a single intravenous injection. It was weak in the lung tissues.
Distribution of FITC‐labeled polyamide in vitro and in vivo. The distribution of FITC‐labeled MMP‐9 PI polyamide (Fig. 1b, middle) in MDA‐MB‐231 cells after 45 min is shown in Figure 2(b). FITC‐labeled polyamide compounds in growth medium immediately localized in the nuclei after 45 min’ incubation with the MDA‐MB‐231 cells. The FITC‐labeled polyamide compounds remained in the nuclei for up to 96 h (data not shown). In vivo distribution of the FITC‐labeled polyamide in the liver, kidney, spleen, and lung after intravenous injection is shown in Figure 2(c). FITC‐labeled polyamide quickly localized in these tissues and still remained there after 6 days. Intriguingly, despite the very stable and lengthy localization of PI polyamide in the major organs, the mice were healthy. There was no obvious difference between the treated and non‐treated groups.
MMP‐9 PI polyamide treatment decreased the mobility of human MDA‐MB‐231 and HeLa cells. Increased expression of MMP‐9 is associated with the invasion and metastases of tumor cells such as HeLa( 20 , 21 ) and MDA‐MB‐231 cells.( 20 , 21 , 22 , 23 ) Various treatment strategies have been targeted to prevent occurrences of metastasis using these two cell lines by migration and invasion assay,( 20 , 21 , 22 , 23 ) so we first incubated MDA‐MB‐231 and HeLa cells with various concentrations of PI polyamide to evaluate the role of MMP‐9 PI polyamide in the migration ability of tumor cells. As shown in Figure 3(a), treatment with a DMSO solution induced these tumor cells to close the wound area in 48 h. In contrast, treatment with MMP‐9 PI polyamide inhibited the migration of tumor cells in a dose‐dependent manner, indicating that MMP‐9 PI polyamide appears to inhibit the migrating ability of tumor cells.
Figure 3.
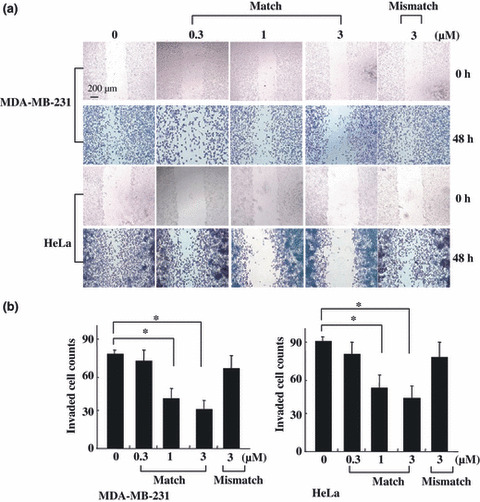
Matrix metalloproteinase (MMP)‐9 pyrrole‐imidazole (PI) polyamide treatment reduces the migration and invasion activity of MDA‐MB‐231 and HeLa cells in vitro. (a) In vitro cell motility of the polyamide‐treated MDA‐MB‐231 and HeLa cells. Confluent cell cultures were wounded with plastic micropipette tips (top). Cells were photographed at 48 h after wounding by phase contrast microscopy (bottom). (b) In vitro invasiveness of MDA‐MB‐231 and HeLa cells. The indicated cells (2500 cells) were seeded on a Matrigel‐coated membrane with or without PI polyamide. After 48 h, cells were removed from the top aspect of the filter. The cells that had passed through the membranes were counted. The invasion was quantified, as described in Materials and Methods. Values represent mean ± SD from biological triplicate experiments. *P < 0.05.
Inhibitory effect of MMP‐9 PI polyamide on the invasion of tumor cells. Proteolytic degradation of ECM components is crucial for tumor‐cell invasion. To evaluate the impact of MMP‐9 PI polyamide on tumor‐cell invasiveness, an invasion assay was performed by incubating various concentrations of PI polyamide with MDA‐MB‐231 and HeLa cells for 48 h. We compared the invasive ability of those cells treated with diverse concentrations of PI polyamide to those treated with a corresponding DMSO solution without PI polyamide. Concentration‐dependent reduced levels of cell invasion across the matrigel were observed in both tumor cells. In contrast, 3‐μM mismatch polyamide did not affect either the migration or invasion of the two tumor cells within the effective concentration range of the match polyamide. To verify that these two observed effects were not attributed to general cytotoxicity, we performed a cell‐viability assay, and we found that this MMP‐9 PI polyamide treatment demonstrated no significant reduction in viability or increase in cell death. (Quantification confirmed that treatment with 300 nM, 1 μM, and 3 μM affected the growth of MDA‐MB‐231 cells by 97%, 91%, and 85%, and HeLa cells by 99%, 96%, and 88%, respectively, compared with DMSO solution‐treated controls.)
Decreased production of MMP‐9 by MMP‐9 PI polyamide treatment. To further examine whether the inhibition of migration and invasion activity by MMP‐9 PI polyamide was correlated with decreased MMP‐9 expression, we compared the MMP‐9 level in MDA‐MB‐231 cells between the MMP‐9 PI polyamide‐treated group and DMSO solution‐treated control group by using real‐time RT‐PCR, Western blotting, and zymography analysis. Real‐time RT‐PCR analysis demonstrated that there was a decrease in the level of MMP‐9 mRNA by polyamide treatment (Fig. 4a). Thus treatment with MMP‐9 PI polyamide might affect transcription of the MMP‐9 gene. Then we analyzed the level of MMP‐9 protein in the MDA‐MB‐231 cells. The result also showed a concentration‐dependent effect (Fig. 4b). In order to explore whether the diminished amount of MMP‐9 was related to down‐regulation of MMP‐9 enzymatic activity, the conditioned media was collected and measured for zymography analysis 48 h after treatment of 2.5 × 105 cells with PI polyamide. In accordance with the MMP‐9 protein level, the same level of activity was observed (Fig. 4c). Our results revealed that the MMP‐9 level was suppressed by treatment with MMP‐9 PI polyamide but not by treatment with mismatch polyamide.
Figure 4.
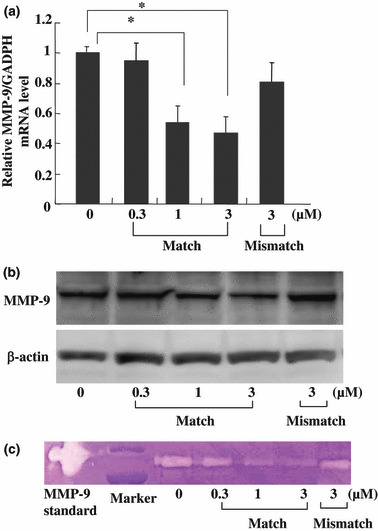
Matrix metalloproteinase (MMP)‐9 RNA and protein‐level expressions as well as enzymatic activity decreased in the MDA‐MB‐231 cells after MMP‐9 pyrrole‐imidazole (PI) polyamide treatment. (a) Real‐time RT‐PCR analysis of MMP‐9 expression. Cells were subjected to different concentrations of polyamide for 48 h incubation, and expression of MMP‐9 was determined. Expression level of the MMP‐9 gene is presented as a percentage compared to the control. Data are expressed as the mean ± SE (n = 3 for each group). *P < 0.05. (b) Proteins isolated from MDA‐MB‐231cells were subjected to NuPAGE+ 10% Bis‐Tris gels under reducing conditions and probed with antibodies (Ab) against MMP‐9 (top). The same membrane was re‐probed with the antibody for β‐actin to verify that equal amounts of protein were loaded (bottom). (c) Concentration‐dependent decreased MMP‐9 activity by gelatin‐based zymography of culture medium of MDA‐MB‐231cells treated with PI polyamide. After treatment with diverse doses of PI polyamide for 48 h, the culture media was examined. Equal amounts of conditioned media were electrophoresed on 10% Zymogram (gelatin) gel. The clear bands on the gel represent the activity of MMP.
Suppression of liver metastasis by MMP‐9 PI polyamide treatment in vivo. The liver is a common site of systemic metastases from colorectal cancer.( 24 ) To analyze the effects of MMP‐9 PI polyamide treatment on tumor metastasis, we used a well‐established liver metastasis model that involves injecting colorectal cancer cells into the spleens of athymic mice.( 18 , 19 ) Prior to the in vivo analysis, HT‐29 cells were confirmed in enzymatic reduction of MMP‐9 and cell invasiveness in vitro after MMP‐9 PI polyamide administration (Fig. 5a). With Group 1, results suggested that 1‐h MMP‐9 PI polyamide treatment before tumor cell inoculation significantly decreased metastases compared with control groups, and results were further confirmed by the percentage of the tumor area in the entire liver (Fig. 5c, Group 1). With Group 2, it was found that PI treatment by tail vein injection from day 7 after HT‐29 cell implantation decreased tumor development in the liver, but there was no significant difference (Fig. 5c, Group 2). With Group 3, PI treatment by tail vein injection from 1 h after the HT‐29 cell implantation significantly decreased tumor metastasis in the liver, in contrast to the control group (Fig. 5c, Group 3 left). There were no significant differences in body weight and spleen tumor area in any of the three groups (data not shown).
MMP‐9 PI polyamide treatment inhibited tumor vessel density by tail vein injection. There were no significant differences in tumor vessel density between mice in Groups 1 and 2 (data not shown). With Group 3, significant suppression of vessel count was observed in the MMP‐9 PI polyamide treatment group (Fig. 5c, Group 3 right).
Discussion
Nucleic acid medicines, such as antisense DNA, ribozymes, and decoy, have been developed as gene‐silencing agents. However, these agents are degraded easily by nucleases and require drug‐delivery systems for sufficient distribution into the organs. Also, several small molecule inhibitors of MMPs were involved in clinical trials in a variety of cancers.( 25 ) The mixed results obtained with synthetic MMP inhibitors have led researchers to decline strategies for treatment designs directed against the action of MMP molecules( 26 , 27 ). Therefore, a specific inhibitor for an MMP without any delivery system is needed.
For this purpose, we designed a PI polyamide compound for targeting the MMP‐9 AP‐1 promoter because data obtained from different experimental models in vitro and in vivo indicate that the AP‐1 protein functions as an important regulator of cell proliferation, differentiation, apoptosis, and transformation by means of cell growth signal transactivation.( 28 ) In addition, the AP‐1 binding region of MMP‐9 is conserved among most mammalian species. This PI polyamide showed selective binding to the target DNA in the gel mobility shift. In our in vitro and in vivo experiments, this PI polyamide was localized in the cell nuclei immediately and maintained a steady presence. It was also noted that a relatively high dose of a single injection of FITC‐labeled PI polyamide did not cause any health issues in the injected mice (data not shown).
The present study shows that this PI polyamide has an inhibitory effect on the invasive activity of MDA‐MB‐231 and HeLa cells, suggesting the possibility that this PI polyamide has a promising effect on inhibiting in vitro tumor cell invasion in MMP‐9 over‐expressed cancers. We also assessed the effect of the MMP‐9 PI polyamide on migration and proliferation because migration is one of the first steps in invasion. Our results showed that tumor cells treated with this PI polyamide have significantly reduced migration, but there is almost no effect on proliferation. All these findings suggest that this PI polyamide may inhibit metastasis. To identify the anti‐invasion mechanisms of MMP‐9 PI polyamide, we have investigated MMP‐9 expression and activity level. Our results here showed that both MMP‐9 expression and enzymatic activity were reduced by the PI polyamide in a dose‐dependent manner. These results suggest that the inhibitory effect of this polyamide on tumor‐cell invasion can be partially attributed to the down‐regulation of MMP‐9 expression, which is required for degradation of the extracellular matrix.( 2 , 3 )
To further determine the potential efficacy of this PI polyamide approach on the treatment of tumor metastasis, we used a more clinically relevant in vivo model of liver metastasis, injecting colon cancer cells (HT‐29) into the spleens of athymic nude mice, as has been described previously.( 18 , 19 ) We showed that a 1‐h treatment with MMP‐9 PI polyamide before cell implantation and MMP‐9 PI polyamide intravenous treatment 1 h after cell implantation significantly suppressed liver metastasis. MMP‐9 PI polyamide was retained for more than 96 h in the nuclei of in vitro cultured cells, and its stable presence in the liver in vivo might repress MMP‐9 expression of HT‐29 cells and host cells for a while. In MMP‐9‐deficient mice, which do not have MMP‐9 expression in host mesenchymal cells, reduction in the number of metastatic colonies was seen after intravenous implantation of B16‐BL6 melanoma cells and Lewis lung carcinoma cells compared with wild‐type mice.( 29 ) In this study it was also anticipated MMP‐9 expression inhibition may not only occur in human xenografted cells but also in mouse mesenchymal cells because MMP‐9 PI polyamide also recognized the AP‐1 site of conserved rodent MMP‐9 promoters. Inhibition of vascularization by intravenous administration of MMP‐9 PI polyamide may also support its effect to the host mouse cells. On the other hand, MMP‐9 polyamide treatment of Groups 1 and 3, but not Group 2, significantly reduced tumor area in the liver, suggesting that MMP‐9 polyamide may play an important role against migration and invasion of primary tumor. These findings suggest that therapies targeting MMP‐9 by using PI polyamide may be useful for inhibiting tumor metastasis.
In summary, we developed a novel small‐molecule PI polyamide compound targeting the MMP‐9 promoter. The PI polyamide inhibited MMP‐9 expression and decreased liver metastasis. Because this PI polyamide can penetrate into nuclei without any delivery system and remain in the cell nuclei for a long time, and may bind to the target sequence more efficiently without having obvious adverse effects in mice, it will be useful for application in in vivo therapy and be more clinically applicable for treating disease states that require a long‐acting effect. It has been reported that radiation therapy and the chemotherapeutic agents doxorubicin (Adriamycin) and docetaxel (Taxotere) increased transforming growth factor (TGF)‐β levels and accelerated metastasis, and that blocking TGF‐β, a well‐known factor in inducing MMP‐9 expression, prevented tumor metastases in mouse models.( 30 ) MMP‐9 inhibition may also be clinically useful in combination with primary therapies in reducing secondary metastatic induction.
Acknowledgments
We thank Mr Motoaki Kataba, Mr Shigeki Nakai, and Ms Yuki Yamada for their technical support. This work was supported by the Nihon University Multidisciplinary Research Grant for 2007; the Academic Frontier Project for 2006 for Private Universities: Matching fund subsidy from MEXT to H.N.; and a grant from the National Institute of Environmental Health Services to H.N. (no. ES012249‐01).
References
- 1. Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature 1980; 283: 139–46. [DOI] [PubMed] [Google Scholar]
- 2. Moses MA. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells 1997; 15: 180–9. [DOI] [PubMed] [Google Scholar]
- 3. McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 2000; 6: 149–56. [DOI] [PubMed] [Google Scholar]
- 4. Katori H, Nozawa A, Tsukuda M. Increased expression of matrix metalloproteinase‐2 and 9 and human papilloma virus infection are associated with malignant transformation of sinonasal inverted papilloma. J Surg Oncol 2006; 93: 80–5. [DOI] [PubMed] [Google Scholar]
- 5. Lakka SS, Gondi CS, Dinh DH et al. Specific interference of urokinase‐type plasminogen activator receptor and matrix metalloproteinase‐9 gene expression induced by double‐stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem 2005; 280: 21882–92. [DOI] [PubMed] [Google Scholar]
- 6. Takahra T, Smart DE, Oakley F, Mann DA. Induction of myofibroblast MMP‐9 transcription in three‐dimensional collagen I gel cultures: regulation by NF‐kappaB, AP‐1 and Sp1. Int J Biochem Cell Biol 2004; 36: 353–63. [DOI] [PubMed] [Google Scholar]
- 7. Vu TH, Werb Z. Gelatinase B: Structure, Regulation, and Function, Matrix Metalloproteinases. San Diego ed.: Academic Press, 1998. [Google Scholar]
- 8. Himelstein BP, Canete‐Soler R, Bernhard EJ, Muschel RJ. Induction of fibroblast 92 kDa gelatinase/type IV collagenase expression by direct contact with metastatic tumor cells. J Cell Sci 1994; 107: 477–86. [DOI] [PubMed] [Google Scholar]
- 9. Dervan PB. Molecular recognition of DNA by small molecules. Bioorg Med Chem 2001; 9: 2215–35. [DOI] [PubMed] [Google Scholar]
- 10. Arcamone F, Penco S, Orezzi P, Nicolella V, Pirelli A. Structure and synthesis of distamycin A. Nature 1964; 203: 1064–5. [DOI] [PubMed] [Google Scholar]
- 11. Dervan PB, Edelson BS. Recognition of the DNA minor groove by pyrrole‐imidazole polyamides. Curr Opin Struct Biol 2003; 13: 284–99. [DOI] [PubMed] [Google Scholar]
- 12. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 2003; 253: 269–85. [DOI] [PubMed] [Google Scholar]
- 13. Wurtz NR, Turner JM, Baird EE, Dervan PB. Fmoc solid phase synthesis of polyamides containing pyrrole and imidazole amino acids. Org Lett 2001; 3: 1201–3. [DOI] [PubMed] [Google Scholar]
- 14. Bando T, Sugiyama H. Synthesis and biological properties of sequence‐specific DNA‐alkylating pyrrole‐imidazole polyamides. Acc Chem Res 2006; 39: 935–44. [DOI] [PubMed] [Google Scholar]
- 15. Rucker VC, Foister S, Melander C, Dervan PB. Sequence specific fluorescence detection of double strand DNA. J Am Chem Soc 2003; 125: 1195–202. [DOI] [PubMed] [Google Scholar]
- 16. Best TP, Edelson BS, Nickols NG, Dervan PB. Nuclear localization of pyrrole‐imidazole polyamide‐fluorescein conjugates in cell culture. Proc Natl Acad Sci U S A 2003; 100: 12063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Liang J, Koike T et al. Overexpression of human matrix metalloproteinase‐12 enhances the development of inflammatory arthritis in transgenic rabbits. Am J Pathol 2004; 165: 1375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bertrand V, Couturier‐Turpin MH, Louvel A, Panis Y, Couturier D. Relation between cytogenetic characteristics of two human colonic adenocarcinoma cell lines and their ability to grow locally or metastasize or both: an experimental study in the nude mouse. Cancer Genet Cytogenet 1999; 113: 36–44. [DOI] [PubMed] [Google Scholar]
- 19. Yoshida Y, Kishimoto T, Ishiguro H et al. Dexamethasone modifies the susceptibility to serum cytotoxicity and increases the metastatic efficiency of a colon carcinoma cell line. Exp Mol Pathol 2006; 81: 77–84. [DOI] [PubMed] [Google Scholar]
- 20. Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Suppression of human cervical cancer cell lines Hela and DoTc2 4510 by a mixture of lysine, proline, ascorbic acid, and green tea extract. Int J Gynecol Cancer 2006; 16: 1241–7. [DOI] [PubMed] [Google Scholar]
- 21. Chen PN, Kuo WH, Chiang CL, Chiou HL, Hsieh YS, Chu SC. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u‐PA expression. Chem Biol Interact 2006; 163: 218–29. [DOI] [PubMed] [Google Scholar]
- 22. Morini M, Mottolese M, Ferrari N et al. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP‐9) activity. Int J Cancer 2000; 87: 336–42. [PubMed] [Google Scholar]
- 23. Huang Q, Shen HM, Ong CN. Inhibitory effect of emodin on tumor invasion through suppression of activator protein‐1 and nuclear factor‐kappaB. Biochem Pharmacol 2004; 68: 361–71. [DOI] [PubMed] [Google Scholar]
- 24. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003; 53: 5–26. [DOI] [PubMed] [Google Scholar]
- 25. Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs 2000; 9: 2167–77. [DOI] [PubMed] [Google Scholar]
- 26. Baxter AD, Bhogal R, Bird J et al. Arylsulphonyl hydroxamic acids: potent and selective matrix metalloproteinase inhibitors. Bioorg Med Chem Lett 2001; 11: 1465–8. [DOI] [PubMed] [Google Scholar]
- 27. Drummond AH, Beckett P, Brown PD et al. Preclinical and clinical studies of MMP inhibitors in cancer. Ann N Y Acad Sci 1999; 878: 228–35. [DOI] [PubMed] [Google Scholar]
- 28. Milde‐Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 2005; 41: 2449–61. [DOI] [PubMed] [Google Scholar]
- 29. Itoh T, Tanioka M, Matsuda H et al. Experimental metastasis is suppressed in MMP‐9‐deficient mice. Clin Exp Metastasis 1999; 17: 177–81. [DOI] [PubMed] [Google Scholar]
- 30. Biswas S, Guix M, Rinehart C et al. Inhibition of TGF‐beta with neutralizing antibodies prevents radiation‐induced acceleration of metastatic cancer progression. J Clin Invest 2007; 117: 1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


