Abstract
Goblet cell depletion and down‐regulation of MUC2 expression are observed in a significant percentage of human non‐mucinous colorectal adenocarcinomas. Direct evidence for the role of MUC2 in gastrointestinal tumor formation was demonstrated by a knockout of Muc2 in mice that resulted in the development of adenocarcinomas in the small and large intestine. The secretory phospholipase Pla2g2a is a protein that confers resistance to Apc Min/+‐induced intestinal tumorigenesis. Like Muc2, in the large intestine Pla2g2a is exclusively expressed by the goblet cells and Pla2g2a's tumor resistance is also strongest in the large intestine. Possible genetic interactions between Muc2 and Pla2g2a were examined by creating C57BL/6‐Muc2 −/– Pla2g2a transgenic mice. Expression of a Pla2g2a transgene reduced tumorigenesis in the large intestine by 90% in male Muc2 −/– mice and by nearly 100% in female Muc2 −/– mice. Expression of Pla2g2a also inhibited tumor progression. Microarray gene expression studies revealed Pla2g2a target genes that modulate intestinal energy metabolism, differentiation, inflammation, immune responses and proliferation. Overall, results of the present study demonstrate an Apc‐independent role for Pla2g2a in tumor resistance and indicate that Pla2g2a plays an important role, along with Muc2, in protection of the intestinal mucosa. (Cancer Sci 2008; 99: 2113–2119)
Abbreviations:
- APC
adenomatous polyposis coli
- B6
C57BL/6
- MUC2
Mucin 2
Mucins are the principal components of intestinal mucus, a viscous substance that provides protection and lubrication to the epithelial mucosa.( 1 , 2 ) In the intestinal epithelium of mice and humans, the major secretory mucin is MUC2, which is expressed in the goblet cell population.( 3 , 4 ) MUC2 is a large, heavily glycosylated, protein (5200 amino acids) that constitutes the major structural component of mucus, thus contributing to barrier functions. The role of MUC2 in intestinal tumorigenesis appears to be complex. Goblet cell depletion and down‐regulation of MUC2 expression( 5 , 6 , 7 ) or MUC2 protein alteration( 8 ) occur in a significant percentage of human non‐mucinous colorectal adenocarcinomas.( 9 ) MUC2 is robustly expressed in normal colon tissue( 3 ) and its expression is observed in early stages of adenomagenesis but progressive loss of MUC2 function occurs in the transition to carcinogenesis.( 5 , 9 , 10 , 11 , 12 ) In contrast, up‐regulation of MUC2 is occasionally observed in mucinous adenocarcinomas that are also characterized by microsatellite instability.( 10 , 12 , 13 , 14 , 15 , 16 , 17 ) In addition, mucins secreted by colon cancer cells may contribute to the metastatic process,( 12 ) and are reported to increase the production of prostaglandin E2 (PGE2) and cyclooxygenase‐2 (COX‐2) in macrophages.( 18 )
Velcich and colleagues generated a germline knockout of Muc2 ( 19 ) in mice on a C57BL/6J‐129/SvOla mixed genetic background that resulted in the absence of recognizable goblet cells along the entire length of the intestine, although the expression of some goblet cell differentiation markers was retained. Muc2 knockout mice developed small intestinal tumors by 6 months of age and eventually adenocarcinomas in the duodenum and rectum. Muc2−/– tumors showed no alterations in the β‐catenin pathway; thus Muc2 deficiency likely represents an Apc‐independent tumor pathway. Despite the different pathways of tumorigenesis, inactivation of p21 WAF1/CIP1 similarly affects tumor formation in both the Muc2 and mutant Apc mouse models of intestinal carcinogenesis.( 20 )
The secretory phospholipase Pla2g2a is a modulator of tumorigenesis that has been shown to confer resistance to intestinal cancer in the mouse. The Pla2g2a gene is part of the Mom1 (Modifier of Min‐1) complex of genes on distal mouse chromosome four that confers resistance to tumorigenesis in the Apc Min/+ mouse.( 21 , 22 ) Pla2g2a is naturally mutant in three strains of mice (including C57BL/6 J and 129/SvOla) that develop a severe Apc Min/+ phenotype and is wildtype in six strains that demonstrate resistance to intestinal tumorigenesis.( 23 , 24 ) Genetic evidence for the function of Pla2g2a as a tumor suppressor was demonstrated by the reduction in tumors observed in Apc Min/+ mice carrying a wildtype Pla2g2a transgene derived from the AKR strain.( 25 ) The Pla2g2a transgene resistance phenotype is strongest in the large intestine and like Muc2, Pla2g2a is exclusively expressed by the goblet cell population.( 22 )
Pla2g2a and Muc2 share several common genetic and biochemical pathways. Cytokines such as interleukin‐1β (IL‐1β),( 26 ) growth factors such as epidermal growth factor (EGF)( 27 ) and prostanoids such as PGE2 that lie upstream (IL‐1β) and downstream (EGF, COX‐2, prostaglandins) of Pla2g2a are potent activators of MUC2. MUC2 and Pla2g2a also share a common signaling pathway that involves protein kinase C (PKC), mitogen‐activated protein kinase (MAPK), cyclic adenosine monophosphate (cAMP), tumor necrosis factor alpha (TNF‐α), extracellular signal regulated kinase 1 and 2 (ERK 1, ERK 2) and nuclear factor B (NF‐κB). For example, phorbol myristate acetate (PMA) induces expression of both MUC2 and Pla2g2a and Muc2 is induced by 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) through PKC and by cAMP through protein kinase A (PKA) in HT29 colon cancer cells.( 28 , 29 ) Further, arachidonic acid, a long‐chain fatty acid that results from Pla2g2a hydrolysis of membrane glycerophospolipids, has been shown to induce secretion of mucins.( 30 )
Because of the commonalities shared by Pla2g2a and Muc2 and as the genetic background used by Velcich and coworkers in the original Muc2 knockout study was naturally mutant for Pla2g2a (both C57BL/6 J and 129/SvOla), we sought to directly test for genetic interactions between MUC2 and Pla2g2a by generating C57BL/6J‐MUC2−/– mice that also expressed a wildtype Pla2g2a transgene.
Materials and Methods
Mice. C57BL/6 J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Muc2 knockout mice( 19 ) were obtained from Dr Anna Velcich, Albert Einstein School of Medicine (Bronx, NY, USA). Pla2g2a transgenic mice( 22 ) were obtained from a colony maintained at the University of Minnesota Medical School (Duluth, MN, USA). All mice were housed and bred at the University of Minnesota Medical School Duluth Animal Services Facility (AALAC accredited) and all mouse experiments followed a protocol approved by the University of Minnesota Institutional Animal Care and Use Committee. Mice were housed in microisolator cages that were changed in a laminar flow hood following a customary protocol to prevent pathogen transfer. Sentinel mice caged on the same racks as experimental mice tested negative for a panel of common pathogens during the entire period of the study. All mice were fed food (Purina 5001 Rodent Chow) and tap water ad libitum. All mice were monitored daily for signs of distress and sacrificed when either distressed or moribund or at greater than 180 days of age up to 15 months. Test mice were clustered at mean ages of 30 weeks or 60 weeks of age.
Experimental crosses. Muc2 mutant mice that were predominantly congenic on the C57BL/6 genetic background were backcrossed to C57BL/6 J for an additional six generations to ensure that both the Muc2 mutation and the Pla2g2a transgene were on an isogenic C57BL/6 J background. A three‐generation cross established breeding pairs of Muc2−/– mice that were either positive or negative for the Pla2g2a transgene. For gene expression analyses, C57BL/6J‐Muc2 −/– mice both positive and negative for the Pla2g2a transgene and of both sexes were sacrificed at 100 days of age and the distal colons were removed and processed in Qiagen RNA Later (Qiagen, Valencia, CA, USA).
Genotyping. DNA was isolated from tail snips using a Qiagen DNA Easy kit (Qiagen, Valencia, CA, USA). The genotype of the Pla2g2a locus was determined by PCR assay as previously described.( 22 ) The Muc2 genotype was determined by a published PCR protocol.( 19 ) All PCR was performed on an Applied Biosystems GeneAmp 9700 Thermal Cycler (Applied Biosystems, Foster City, CA, USA).
Tumor statistical analysis. Two‐sided P‐values for tumor counts were determined by use of the non‐parametric Wilcoxon Rank Sum Test comparing sex‐ and age‐matched classes produced in the same genetic crosses.
Microarray and Data Analysis; RNA Extraction; Quantitative Real Time PCR; Tumor Analysis; Immunohistochemistry; Histopathology. See Supplemental Materials.
Results
Expression of Pla2g2a significantly reduced tumor multiplicity and incidence in the large intestine of Muc2 mutant mice. To test for interactions between Pla2g2a and Muc2 in Muc2‐induced intestinal cancer, we introgressed a Pla2g2a transgene into the B6‐Muc2−/– strain, eventually creating Muc2−/– mice that were either positive or negative for the Pla2g2a transgene. We found that expression of Pla2g2a strongly inhibited tumorigenesis in the Muc2−/– mouse (Table 1). In male Muc2−/– mice, Pla2g2a reduced tumor number in the large intestine by 88% in mice at 30 weeks of age (P = 0.03) and by 91% in mice at 60 weeks of age (P = 0.0001). Expression of Pla2g2a also produced a corresponding reduction (by ~ 2/3) in tumor incidence in the large intestine of male Muc2−/– mice. Almost all of the large intestinal tumors of both genotypes were found clustered midway between the cecum and distal rectum, generally in the distal colon. Colonic tumors that did appear in the Pla2g2a transgenic mice also tended to be much smaller (generally less than 3 mm in diameter, even at 60 weeks) than tumors from Muc2−/– mice (which were often as large as 6 mm or more in diameter) but there were too few samples to conduct a meaningful size comparison. In female Muc2−/– mice expression of Pla2g2a nearly completely abolished tumorigenesis in the large intestine.
Table 1.
Expression of Pla2g2a prevents tumorigenesis in Muc2−/– mice
| Genotype | Sex | N | Large intestine tumor multiplicity | Large intestine tumor incidence | Duodenum tumor multiplicity | Duodenum tumor incidence | Ileum tumor multiplicity | Ileum tumor incidence |
|---|---|---|---|---|---|---|---|---|
| Muc2−/– | M | |||||||
| Combined | 29 | 3.6 ± 2.9 | 90% | 0.9 ± 1.2 | 45% | 2.3 ± 3.4 | 55% | |
| 30 weeks | 15 | 2.6 ± 1.8 | 87% | 0.3 ± 0.6 | 20% | 2.5 ± 3.5 | 60% | |
| 60 weeks | 14 | 5.4 ± 3.1 | 100% | 1.6 ± 1.1 | 70% | 1.9 ± 3.4 | 50% | |
| Muc2−/– Pla2+ | M | |||||||
| Combined | 18 | 0.4 ± 0.6 | 33% | 0.5 ± 0.5 | 33% | 0.2 ± 1.2 | 5% | |
| 30 weeks | 10 | 0.3 ± 0.4* | 30% | 0.3 ± 0.5 | 30% | 0 | 0 | |
| 60 weeks | 8 | 0.5 ± 0.8** | 38% | 0.8 ± 1.0*** | 38% | 0.5 ± 1.7 | 13% | |
| Muc2−/– | F | |||||||
| Combined | 21 | 1.5 ± 1.7 | 62% | 0.3 ± 0.6 | 19% | 1.8 ± 2.3 | 52% | |
| 30 weeks | 8 | 2.6 ± 1.6 | 100% | 0.4 ± 1.7 | 25% | 0.9 ± 2.8 | 25% | |
| 60 weeks | 13 | 0.8 ± 1.4 | 33% | 0.2 ± 0.7 | 15% | 2.3 ± 2.3 | 70% | |
| Muc2−/– Pla2+ | F | |||||||
| Combined | 21 | 0.1 ± 0.6 | 5% | 0 | 0 | 1.2 ± 2.3 | 33% | |
| 30 weeks | 14 | 0.2 ± 0.8 | 7% | 0 | 0 | 0.3 ± 0.6 | 21% | |
| 60 weeks | 7 | 0 | 0 | 0 | 3.0 ± 1.5 | 57% | ||
P = 0.03;
P = 0.0001;
P = 0.21.
P‐values compare age and sex matched classes. N = number of mice.
Duodenal and ileal tumors in C57BL/6J‐Muc2‐deficient mice. In the duodenum of male Muc2 −/– mice, expression of Pla2g2a caused a 50% reduction in tumors in mice at 60 weeks of age. Duodenal tumor incidence was only half of that observed in the Muc2−/– class. In female Muc2−/– mice, expression of Pla2g2a completely abolished tumorigenesis in the duodenum (Table 1). A novel type of lesion found in Muc2 −/– mice on the C57BL/6 J background is a sessile adenoma that predominantly arises in the distal ileum, sometimes close to the ileocecal junction. These tumors are broad, very low lesions and they are difficult to identify and separate in whole‐mount tissue analysis (see Fig. 1d). The incidence and multiplicity of these tumors was modestly higher in Muc2−/– mice than in Muc2−/–Pla2g2a transgenic mice and the ileal tumors were more numerous in female mice than males. Histopathological analysis revealed that most of these tumors were tubular adenomas with high‐grade dysplasia. Our pathologist (I.M) did identify one likely ileal adenocarcinoma and several more potential adenocarcinomas based on observing glandular structures within the submucosa where the greatest difficulty was distinguishing glandular herniation from true invasion. These ileal tumors were not reported in Muc2−/– mice on the C57BL/6 J × 129/SvOla mixed background, thus the appearance of ileal tumors may be due to factors in the C57BL/6 J genetic background. Finally, we also found a few adenomas in the medial region of the small intestine of C57BL/6J‐Muc2 −/– mice.
Figure 1.
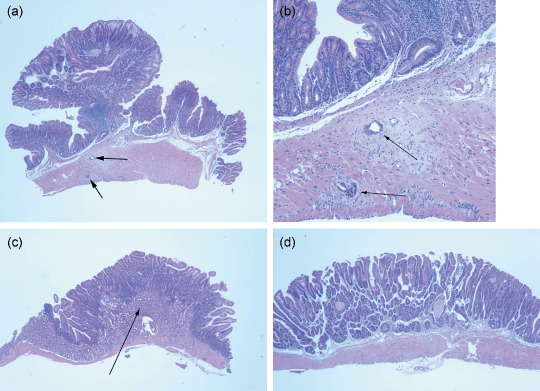
Histopathology of tumors from Muc2−/– mice. Histopathological analysis was performed on formaldehyde‐fixed tumors and adjacent normal tissues from the duodenum, ileum and colon obtained from Muc2−/– mice that were either positive or negative for the Pla2g2a transgene. Multiple sections were obtained from 18 duodenal tumors, 20 large intestinal tumors and 11 ileal tumors from both male and female Muc2−/– mice and six duodenal tumors, seven colon tumors and four ileal tumors from male Muc2 −/– Pla2g2a transgenic mice. In Muc2 −/–mice we observed adenocarcinomas in six of 20 tumors from the large intestine (virtually all from the distal colon); the remaining 14 tumors were described as tubular adenomas with high grade dysplasia. No adenocarcinomas were observed in 18 duodenal tumors. The duodenal tumors were generally described as tubular adenomas, sessile, with Brunner's gland hyperplasia. In the ileum, one of 11 tumors was described as an adenocarcinoma with the remaining tumors described as tubular adenomas, sessile with high grade dysplasia. Figure 1 shows representative images from the histopathological analysis of Muc2−/– tumors. (a) Adenocarcinoma from the distal colon (40×). (b) The same tumor with arrows indicating invasive areas (200×). (c) Duodenal adenoma with the arrow pointing to the extensive Brunner's gland hyperplasia (40×). (d) Adenoma from the ileum (40×). Pla2g2a transgenic mice did not develop adenocarcinomas and adenomas were generally identical to those seen in Muc2 −/– mice and generally were described as tubular adenomas.
Tumorigenesis in Muc2‐deficient mice is affected by genetic background. Previous work showed that Muc2 nullizygosity caused small intestinal tumors in some mice as early as 26 weeks of age (6 months).( 19 ) In that study, mice were on a mixed genetic background (C57BL/6J ×129/SvOla; F 2 to F 5 backcross to C57BL/6J). In the current study, all mice were on a fully isogenic C57BL/6J background. Notably, on the C57BL/6 J background, Muc2‐deficient mice developed at least 4‐fold more tumors overall. These increases were observed in both the small and large intestine but the difference was strongest in the large intestine. On the mixed genetic background, Muc2 knockout mice developed no tumors in the large intestine at 26 weeks and only 0.26 tumors at 52 weeks of age.( 19 ) On the C57BL/6 J background, Muc2 knockout mice (combined male and female classes) developed a mean average of 2.6 tumors in the large intestine at 30 weeks of age and 3.3 tumors at 60 weeks of age; thus, overall, Muc2 knockout mice on the C57BL/6 J background developed greater than 10‐fold more large intestinal tumors than on the C57BL/6 J × 129/SvOla mixed background. In addition, genetic background also affected the location of tumors. The rare tumors in the large intestine of older mice on the mixed background were most common in the distal rectum but on the C57BL/6 J background tumors were more often found in the medial large intestine/distal colon. However, we did still observe some tumors in the distal rectum of the C57BL/6J‐Muc2−/– mice. The corresponding tumor incidence was also much higher in Muc2 −/– mice on the C57BL/6 J strain. For example, in the combined sex groups, overall, 76% of mice on the C57BL/6 J background developed tumors in the large intestine versus only 11% of mice on the mixed background.( 19 )
Sex also significantly affected the phenotype of Muc2 knockout mice in our study. Male mice developed more tumors in both the large intestine and duodenum. For example, male knockout mice (30 and 60 weeks of age combined) developed a mean of 3.6 large intestinal tumors and 0.9 duodenal tumors compared with 1.5 and 0.3 tumors in female mice, respectively (Table 1).
Histopathology of Muc2‐deficient tumors. Similar to the previous report by Velcich et al.( 19 ) in the current study C57BL/6J‐Muc2−/– mice developed adenocarcinomas in the large intestine. Adenocarcinomas were scored only if there was clear invasion of submucosa or muscularis and an effort was made to distinguish these characteristics from gland herniation and tissue invasion (see Fig. 1a,b). Adenocarcinomas ranged from well to moderately differentiated. However, unlike the previous study we found adenocarcinomas only in the medial large intestine/distal colon and not in the distal rectum. We observed a frequency of adenocarcinomas in the large intestine of 30% (6/20) compared with 15% (3/19) in mice from the mixed genetic background.( 19 ) The remaining 70% of tumors in the large intestine were described as tubular adenomas with high grade dysplasia. Interestingly, we observed no adenocarcinomas in the duodenum of C57BL/6J‐Muc2−/ − (0/18) in mice at 60 weeks of age, unlike the report of duodenal adenocarcinomas in the mice on the mixed genetic background.( 19 ) Moreover, duodenal tumors were extremely rare in the C57BL/6 J class at 30 weeks of age, again, different than that reported in the mice on the mixed background. The duodenal adenomas that developed at 60 weeks in C57BL/6 J mice generally were described as sessile tubular adenomas, with high‐grade dysplasia and extensive Brunner's gland hyperplasia (see Fig. 1c).
Pla2g2a may prevent cancer progression. Muc2 −/– mice that expressed the Pla2g2a transgene failed to develop a single tumor that was classified as invasive (0/17, see Table 2). The rare lesions in the colon of Pla2g2a transgenic mice at 60 weeks of age were described as either tubular adenomas or as structures characterized by thickening of colonic mucosa suggestive of adenoma or diffuse, mild, crypt hyperplasia. None of the colonic lesions in the transgenic mice showed any evidence of invasion. Tumors from the duodenum of Pla2g2a transgenic mice were identical in histopathological description to tumors from non‐transgenics: tubular adenomas with Brunner's gland hyperplasia.
Table 2.
Tumor progression in Muc2−/– mice
| Class | Large intestine | Duodenum | Ileum |
|---|---|---|---|
| Muc2−/– | 30% | 0% | 9% |
| (6/20) | (0/18) | *(1/11) | |
| Muc2−/– Pla2g2a+ | 0 | 0 | 0 |
| (0/7) | (0/6) | (0/4) |
Tumors were obtained from 15 Muc2−/– mice (both males and females) and six Muc2−/– ‐Pla2g2a transgenic mice (males only for colon and duodenum tumors). All tumors from Muc2 −/– mice were from the 60‐week age class. Tumors from the Muc2−/–Pla2g2a transgenic mice include all of the colon and duodenum tumors that arose in these mice (at both 30 and 60 weeks). *Single asterisk indicates that several more ileal lesions were potential adenocarcinomas but a definitive classification could not be made.
Pla2g2a protein is expressed and secreted by cells of the goblet cell region of the large intestine in B6‐Muc2 −/– Pla2g2a transgenic mice. As Muc2 deficiency prevents the development of cells of goblet cell morphology, we wished to determine whether the expression pattern of Pla2g2a might be altered in the absence of Muc2. We found abundant expression of Pla2g2a in the epithelial cells and extracellular lumen of the large intestine, indicating that secretion of Pla2g2a is not dependent on Muc2 activity (see Fig. 2). The expression and secretion of Pla2g2a was generally not inhibited by the complete loss of goblet‐like cells in Muc2 −/– intestine, although immunohistochemical analysis did reveal a patchy pattern to Pla2g2a protein expression in comparison with the robust Pla2g2a protein expression found in Pla2g2a transgenic mice that are wildtype for Muc2 (see Fig. 1a,b).
Figure 2.
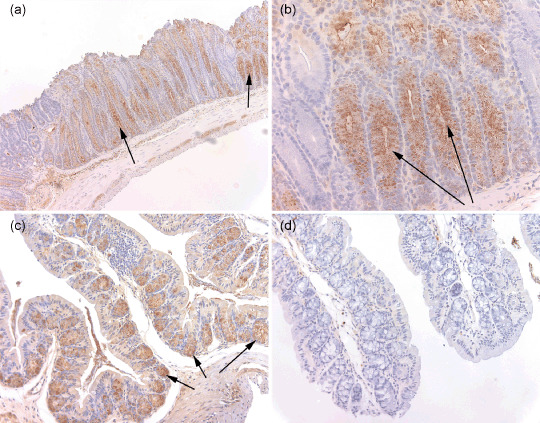
Immunohistochemistry for Pla2g2a in colon of Muc2−/– Pla2g2a transgenic mice. Expression patterns of Pla2g2a protein were determined by immunohistochemistry using a polyclonal rabbit antimouse antibody at 1:90 000 dilution and visualized with avidin–biotin complex reagents. Formaldehyde‐fixed tissues were obtained from the duodenum, jejunum and ileum and several regions of the colon. In the small intestine Pla2g2a was expressed normally, with strong staining seen at the bottom of crypts in the Paneth cell compartment (data not shown). Figure 2 depicts a representative tissue section from the large intestine. (a) Abundant secreted Pla2g2a protein found in the columnar epithelial cells and lumen of crypts is clearly lacking in cells of goblet cell morphology (200×). Only columnar epithelial cells can be seen lining the crypt. Arrows point to regions of Pla2g2a expression. (b) A higher resolution image of Pla2g2a staining in C57BL/6J‐Muc2−/– Plag2g2a transgenic mice (400×). Arrows point to Pla2g2a expression. (c) Pla2g2a immunostaining from tissue from a C57BL/6J Pla2g2a transgenic mouse that is wildtype for Muc2 (40×). Pla2g2a is clearly detected in the goblet cells but not the enterocytes at the crypt borders. (d) Immunostained tissue from a C57BL/6J mouse (200×). No Pla2g2a protein is detected.
Gene expression regulated by Pla2g2a in colon of Muc2‐deficient mice. Deficiency for Muc2 results in profound changes in gene expression in the colon of C57BL/6 mice. An extensive study of Muc2 knockout mice describing changes in gene expression in different regions of the gastrointestinal tract and at different ages is being published separately (A. Velcich, unpublished data). Here, using the Agilent 4 × 44K microarray platform, we took a snapshot of gene expression changes in the distal colon at 100 days of age, comparing C57BL/6J‐Muc2−/– mice with C57BL/6J‐Muc2 +/+‐Pla2g2a‐Tg and C57BL/6J‐Muc2−/–‐Pla2g2a‐Tg mice. First, comparing C57BL/6J‐Muc2−/– mice with C57BL/6J‐Muc2 +/+‐Pla2g2a‐Tg mice we observed that deficiency for both Muc2 and Pla2g2a caused the up‐regulation of groups of genes that are involved in the cell cycle (Cdc7, Cdca5, Ccna2, Cdca2, Cdca1, Cdkn3, Ccnf, Cdca3, Cdk2, Ccnb1), inflammation (S100a11, Il1rl1, Tnfrs1b, Il1b, S100a16) and oncogenicity (Has1, Nrg1, Fgfr4, Stc1, Mmp10, Baiap2, Afp, Bysl, Ptgs2, Mybl2, Egfr, Bmp7, Kras, Nras). Genes significantly down‐regulated in this comparison were involved in some of these same processes and included Igfbp6, Plp1, Grb14, Reck, Ssbp2, Glis2, Lefty1, Tgfb1l1, Efna3, Gtl2, Adam11, Sst, Ptger3 and Ppara. A select list of genes that are differentially expressed in the C57BL/6J‐Muc2 −/–/C57BL/6J‐Pla2g2a‐Tg comparison is provided in Suppl. Table S1.
We then compared the gene expression profiles of C57BL/6J‐Muc2 −/– mice with C57BL/6J‐Muc2−/–‐Pla2g2a‐Tg mice. Overall, 224 genes and transcripts were found to be significantly changed (a complete list of these genes, grouped by functional class, is included in Suppl. Table S2). First, we found that expression of Pla2g2a on the Muc2 knockout background significantly modulated a number of genes involved in intestinal lipid and energy metabolism. Genes in this category that were up‐regulated included Akr1c18, Aldh1l2, Mocos and Aqp5. Among the down‐regulated genes were two members of the chymotrypsin family, Ctrb1 and Ctrl, plus Enpp1, Cel, Cpa2 and Pnlip. Pla2g2a also modulated a number of genes involved in cell signaling and nuclear transactivation. Genes up‐regulated in this group included Mier2, Lhx1, Mlx1pL, Rgs3, Runx1 and Spp1. Genes down regulated in this category included Egfr, Reg2, Id4, Gli3 and Tgfb2. Eight Pla2g2a target genes have been reported to be Notch pathway targets including Adam 11, Lhx1, Spp1, Runx1 and Megf11 (up‐regulated), and Ela2a, Dtx1, Gli3, Myl9 (down regulated). Finally, genes involved in inflammatory and immune responses were differentially expressed in Pla2g2a positive Muc2−/– colon. Down regulated genes in this category included Clps and Cd248 and up‐regulated genes included Defb11, Ccr3, Ccl7, Ccl24 and Tm7sf4. Pla2g2a target genes were also involved in apoptosis and oxidative stress (e.g. Nudt15), protein degradation (e.g. Syvn1), intracellular trafficking (e.g. Praf2), cell structure and adhesion (e.g. Lmod1), transporters (e.g. Slc25a3) and proteases (e.g. Adam11). Finally, via subtraction of genes that were differentially expressed in the comparison C57BL/6J‐Pla2g2a‐Tg/C57BL/6 J (data not shown) from the genes differentially expressed in the comparison C57BL/6J‐Muc2 −/– ‐Pla2g2a‐Tg/C57BL/6J‐Muc2 −/–, we identified a set of 99 Pla2g2a target genes that were unique to the Muc2 −/– background. These Pla2g2a‐Muc2 interactor genes include Nfkbil1, Reg2, Limk1, Hoxb9, Ctrb1 and Ela2a. A complete list of these genes is provided in Suppl. Table S3. A subset of the most significant genes with a focus on genes involved in intestinal energy metabolism and inflammation were confirmed by quantitative reverse transcriptase–polymerase chain reaction (qRT‐PCR) (Table 3).
Table 3.
qRT‐PCR analysis of genes significantly changed in microarray studies
| Up‐regulated | Down‐regulated | ||||||
|---|---|---|---|---|---|---|---|
| Gene | Microarray (fold change) | P‐value (array) | qRT‐PCR (fold change) | Gene | Microarray (fold change) | P‐value (array) | qRT‐PCR (fold change) |
| Pla2g2a | +29.9 | 9.8 × 10−10 | +1264 | Reg2 | –5.0 | 1.6 × 10−8 | –10.1 |
| Mier2 | +2.6 | 4.1 × 10−10 | +5.8 | Ctrb1 | –1.8 | 4.9 × 10−6 | –24.2 |
| Defb11 | +1.5 | 4.8 × 10−6 | +4.0 | Cel | –10.0 | 6.4 × 10−6 | –2.9 |
| Syvn1 | +3.1 | 1.6 × 10−6 | +7.5 | Clps | –3.2 | 1 × 10−4 | –4.1 |
| Adam11 | +3.8 | 2.8 × 10−8 | +52.4 | Ela2a | –2.2 | 5.7 × 10−6 | –5.9 |
qRT‐PCR, quantitative reverse transcriptase–polymerase chain reaction.
Discussion
Muc2 plays a key role in protecting the intestinal mucosa and maintaining intestinal homeostasis. In the mouse, the targeted inactivation of Muc2 caused dysregulation of homeostasis as demonstrated by increased intestinal cell proliferation, decreased apoptosis, loss of goblet‐like cells in the large intestine and increased migration of intestinal epithelial cells, eventually resulting in tumors that progressed to invasive adenocarcinomas. Importantly, these tumors arose via an Apc/β‐catenin‐independent pathway.( 19 ) Like Muc2, in the large intestine the phospholipase Pla2g2a is exclusively expressed by the goblet cell population and its secreted proteins accumulate in the mucinous gel in the crypt lumen. Both these goblet cell specific proteins affect tumor formation in the large intestine as previous studies showed that expression of Pla2g2a significantly reduced tumor multiplicity in the large intestine in the ApcMin/ + mouse model. These data suggest that Pla2g2a and Muc2 may function in common biological pathways, raising the expectation that Pla2g2a would not be able to (strongly) suppress tumorigenesis in Muc2‐deficient mice. As the natural Pla2g2a gene is mutant in the two strains of mice (C57BL/6 J and 129/Sv) that have been previously tested for Muc2 deficiency, we examined whether expression of functional Pla2g2a as a transgene in C57BL/6 J mice provided resistance to Muc2‐deficient intestinal tumorigenesis. We here report that Pla2g2a significantly reduced tumor multiplicity, incidence and possibly tumor progression in the large intestine of Muc2 −/– mice. Moreover, these results are the first evidence that Pla2g2a can act to suppress tumors independent of the Apc/Wnt signaling pathway. Therefore our data suggest that Pla2g2a protects against tumorigenesis via modulation of biochemical pathways that are common in both the Apc and Muc2 mouse models. The ability of Pla2g2a to rescue the Muc2 mutant phenotype was somewhat surprising given that goblet cell morphology is absent in Muc2 −/– mice. Immunohistochemical analysis (Fig. 2) shows expression of Pla2g2a in the columnar epithelial cells and lumen of colonic crypts that contain no goblet‐like cells, reinforcing the concept that in Muc2−/ − mice the goblet cell lineage is not abrogated.
A second finding of our study is that the phenotype of Muc2 deficiency on the C57BL/6 J genetic background differs from the previous report using mice on a mixed (C57BL/6 J × 129SvOla) background. C57BL/6J‐Muc2 −/– mice developed more tumors in both the large intestine and duodenum than mice on the mixed background and the tumors in the large intestine appeared much earlier in C57BL/6J‐Muc2−/– mice. The region of the large intestine affected also varied by strain as C57BL/6J‐Muc2−/– mice developed tumors in a cluster in the middle of the large intestine whereas tumors in the C57BL/6 J × 129/SvOla‐Muc2−/– mice were generally restricted to the distal rectum. Another strain difference is that C57BL/6J‐Muc2 −/– mice did not develop adenocarcinomas in the duodenum (0/18), unlike the numerous invasive tumors found in Muc2 −/– mice on the mixed background. Finally, C57BL/6J‐Muc2−/– mice developed sessile adenomas and adenocarcinomas in the ileum that were not observed in the C57BL/6J ×129/SvOla‐Muc2−/– mice. It is likely then, that the C57BL/6J and 129/SvOla strains carry modifier genes that modulate the severity of the Muc2 deficiency.
Another result that may be linked to genetic background is that sex affected the Muc2 phenotype. Overall, male mice developed more tumors in both the duodenum and large intestine than female mice. This difference was also seen in mice carrying the Pla2g2a transgene where the rare tumors that were observed were only found in male mice as only one female Pla2g2a transgenic mouse developed a tumor in the colon and none developed tumors in the duodenum. Interestingly, we did observe more ileal tumors in female mice of both genotypes although the difference was not statistically significant. Sex differences in the phenotype of the ApcMin/+ mouse model have been shown in several studies. For example, it was recently reported that male Apc Min/+ mice developed more colon tumors than female mice but fewer tumors in the small intestine, especially in the ileum.( 31 ) Consistent with this finding there is growing evidence that estrogen is a protective factor in the mouse large intestine, as ovariectomized ApcMin/+ mice develop more tumors than littermate controls.( 32 ) Our new data indicates that female protection against tumorigenesis in the large intestine extends beyond the Apc/β‐catenin pathway. Finally, we also note that tumor multiplicity at 60 weeks of age was generally equal to or larger than at 30 weeks of age, conforming to expectations of an increased tumor load with age (Table 1). However, colon tumor multiplicity in female Muc2 knockout mice formed an exception, as tumor number appeared to be higher in mice at 30 weeks of age than at 60 weeks of age. Because tumor multiplicity is not expected to decrease over time, we assume this observation has occurred ‘by chance’ owing to random (stochastic) sampling of relatively small groups of mice, and may disappear when the number of mice within this group would be increased. Moreover, there is no published evidence of estrogen fluctuations in mice between 30 and 60 weeks (and estrogen levels begin to decline at ~ 16 months in female mice) thus differences in estrogen levels are not likely to underlie the difference in phenotypes in these mice.
The evidence that Pla2g2a prevents tumors arising from both Muc2 and Apc deficiency suggests that Pla2g2a affects very fundamental processes in tumorigenesis, at least to some extent independently from biochemical pathways affected by Muc2 or Apc. One possibility is that Pla2g2a acts outside the cell to protect the intestinal mucosa from environmental insult that can lead to inflammation and tumor promotion, for instance, in the absence of Muc2. Secreted Pla2g2a molecules form a part of the mucinous extracellular gel in the colonic crypt lumen where they may exert potent bactericidal activity.( 33 ) This could modulate biochemical signaling pathways. To find out what genes are affected by Pla2g2a in Muc2‐deficient mice, we performed microarray gene expression analyses. Our snapshot of gene expression in the distal colon at 100 days of age revealed that loss of Muc2 and Pla2g2a (C57BL/6J‐Muc2 −/–/C57BL/6J‐Muc2 +/+‐Pla2g2a‐Tg) resulted in the up‐regulation of a number of pro‐inflammatory genes (Il1rl1, Il1b, s100a11, Capg, s100a16), and up‐regulation of a number of genes associated with oncogenicity in human and rodent cancers (Ptgs2, Mmp10, Has1, Afp, Fgfr4, Birc5, Itga3, Nrg1, Reg2, Trim29, Baiap2). When we then compared C57BL/6J‐Muc2−/–‐Pla2g2a‐Tg mice with C57BL/6J‐Muc2 −/– littermate mice (C57BL/6J‐Muc2 −/– ‐Pla2g2a‐Tg/C57BL/6J‐Muc2−/– , a gain of Pla2g2a in the Muc2 −/– background) we found the reversal of some gene expression changes observed in the C57BL/6J‐Muc2 −/–/C57BL/6J‐Muc2 +/+‐Pla2g2a‐Tg comparison. Egfr, Ctrb1, Ela2a, Reg2, Ctrl, Dner, Cel, Cpa2,Yap1, Rnase1, Klf16, Limk1, Ssbp2, Aldh2, Akt1s1, Sst and Fbxl19 are genes that were either up‐ or down‐regulated by loss of Muc2 and Pla2g2a but which were then significantly changed in the opposite direction upon the gain of expression of Pla2g2a in the Muc2 knockout mice. Most of these reversal genes are Pla2g2a–Muc2 interactor genes that are specific to Muc2 deficiency, i.e., they are genes that are not differentially expressed in a C57BL/6J–Muc2 +/+–Pla2g2a–Tg/C57BL/6J–Muc2 +/+ comparison (unpublished data). Notably, the proto‐oncogene c‐Myc, a gene that is significantly up‐regulated by Muc2 deficiency,( 19 ) is not altered by expression of Pla2g2a.
When Pla2g2a target genes in Muc2 knockout mice were grouped into functional categories we found altered expression of genes involved in intestinal lipid and energy metabolism, cell signaling and transactivation and inflammation and immune responses (see Suppl. Table S2). Our microarray data indicate that cancers arise in Muc2 −/– mice following early dysregulation of many pro‐inflammatory genes and genes involved in intestinal energy metabolism. Pla2g2a and its target genes may prevent the development of these cancers by modulating inflammation and energy metabolism in the intestinal mucosa, via an effect in either the epithelial or stromal compartments (or both). To further investigate these specific pathways we employed qRT‐PCR to test Pla2g2a target genes involved in inflammatory and energy processes that were identified in the array studies (Table 3). One gene is Defb11 (Defensin B11, up‐regulated 4‐fold by qRT‐PCR in Pla2g2a + Muc2 knockout mice), a member of the defensin family, an important component of the innate immune system. Defensins have potent antimicrobial properties and have been described as the first line of defense in the human colon mucosa, and polymorphisms in defensin genes are linked to susceptibility to Crohn disease.( 34 , 35 , 36 , 37 , 38 , 39 ) Another Pla2g2a target gene is Reg2 (down‐regulated in the arrays and down‐regulated 10‐fold in qRT‐PCR). C57BL/6 mice (Pla2g2a negative) fed a high fat diet become obese, develop type‐2 diabetes and overexpress Reg2 in pancreatic cells. Reg2 is pro‐inflammatory and is significantly up‐regulated in rodent models of experimental colitis.( 40 , 41 ) A third gene analyzed by qRT‐PCR is Cel (carboxyl ester lipase, down‐regulated in the arrays and down‐regulated by 2.9 fold in qRT‐PCR), a secreted lipase found in the gastrointestinal tract that is involved in fat digestion and absorption, and insulin and cholesterol metabolism, and Cel knockout mice are resistant to obesity in mice caused by high fat diets.( 42 , 43 , 44 ) Other Pla2g2a target intestinal enzymes confirmed by qRT‐PCR were Clps (pancreatic colipase, down‐regulated 4‐fold), a type‐2 diabetes susceptibility gene;( 45 ) Ela2a (a GI tract elastase down‐regulated 5.9‐fold)( 46 ) and Ctrb1 (chymotrypsinogen B1, down‐regulated 24 fold).( 47 ) Although the role of each of these Pla2g2a target genes in susceptibility to intestinal neoplasia is unclear, considered together these highly significant target genes demonstrate that Pla2g2a exerts a strong influence on genes involved in intestinal energy metabolism and inflammation that may work in unison to maintain intestinal homeostasis. However, the exact role of Pla2g2a and its downstream effectors, such as PGE2, in inflammation and cancer in the gastrointestinal tract remains controversial. For example, it has been shown that pharmacological and genetic blockage of COX‐2 and/or prostaglandin receptors inhibits tumorigenesis in Apc mutant mice. Yet, there is also substantial evidence that PGE2 can prevent inflammatory bowel disease in mouse models,( 48 , 49 ) suggesting that PGE2, acting downstream of Pla2g2a activity, may also prevent gastrointestinal cancer by resolving chronic inflammation.
In summary, we now know that Pla2g2a can significantly prevent tumors independent of the Apc pathway and that its tumor prevention functions may involve interactions with Muc2, possibly in the extracellular environment, as well as Muc2‐independent mechanisms. More has also been learned about Muc2 because genetic background and sex modulate the severity of its deficiency phenotype. Finally, we have discovered gene expression profiles associated with Muc2 deficiency, and Pla2g2a target genes that likely underlie Pla2g2a's tumor resistance. Ongoing experiments are exploring the contributions of specific Pla2g2a target genes to tumor susceptibility.
Supporting information
Table S1. Select list of genes differentially expressed in C57BL/6J‐Muc2 −/– mice compared with C57BL/6J‐Pla2g2a‐Tg mice.
Table S2. Genes differentially expressed in C57BL/6J‐Muc2−/– Pla2g2a‐Tg mice compared with C57BL/6J‐Muc2−/– mice.
Table S3. Differentially expressed genes in C57BL/6J‐Muc2 −/–‐Pla2g2a‐Tg/C57BL/6J‐ Muc2 −/– colon that are unique from genes differentially expressed in C57BL/6J‐Pla2g2a‐Tg/C57BL/6J comparison.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
We wish to thank Dr Rita Mulherkar, Tata Institute, Mumbai, India for generously providing the Pla2g2a antiserum and Dr Wan Cai Yang, pathologist at the Albert Einstein College of Medicine, for additional histopathological analysis of tumors. We also acknowledge M. Tijssen and P. Eijk for their excellent technical assistance. Funding for this work was provided by the University of Minnesota Academic Health Center and the University of Minnesota Medical School (R.T.C.) and the1st AEGON International Scholarship in Oncology (R.J.A.F.).
References
- 1. Velcich A, Palumbo L, Selleri L, Evans G, Augenlicht L. Organization and regulatory aspects of the human intestinal mucin gene (MUC2) locus. J Biol Chem 1997; 272: 7968–76. [DOI] [PubMed] [Google Scholar]
- 2. Augenlicht LH, Mariadason JM, Wilson A et al . Short chain fatty acids and colon cancer. J Nutr 2002; 132 (12): 3804S–8S. [DOI] [PubMed] [Google Scholar]
- 3. Van Klinken BJ‐W, Einerhand AWC, Duits LA et al . Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol 1999; 276: G115–24. [DOI] [PubMed] [Google Scholar]
- 4. Gum JR, Hicks JW, Gillespie A‐M et al . Goblet cell‐specific expression mediated by the MUC2 mucin gene promoter in the intestine of transgenic mice. Am J Physiol 1999; 276: G666–76. [DOI] [PubMed] [Google Scholar]
- 5. Gratchev A, Siedow A, Bumke‐Vogt C et al . Regulation of the intestinal mucin MUC2 gene expression in vivo: evidence for the role of promoter methylation. Cancer Lett 2001; 168: 71–80. [DOI] [PubMed] [Google Scholar]
- 6. Kim DH, Kim JW, Cho JH et al . Expression of mucin core proteins, trefoil factors, APC and p21 in subsets of colorectal polyps and cancers suggests a distinct pathway of pathogenesis of mucinous carcinoma of the colorectum. Int J Oncol 2005; 27: 957–64. [PubMed] [Google Scholar]
- 7. Iwase T, Kushima R, Mukaisho K, Mitsufuji S, Okanoue T, Hattori T. Overexpression of CD10 and reduced MUC2 expression correlate with the development and progression of colorectal neoplasms. Pathol Res Pract 2005; 201: 83–91. [DOI] [PubMed] [Google Scholar]
- 8. Kim YS. Altered glycosylation of mucin glycoproteins in colonic neoplasia. J Cell Biochem 1992; S 16 G: 91–6. [DOI] [PubMed] [Google Scholar]
- 9. Li A, Goto M, Horinouchi M et al . Expression of MUC1 and MUC2 mucins and relationship with cell proliferative activity in human colorectal neoplasia. Pathol Int 2001; 51 (11): 853–60. [DOI] [PubMed] [Google Scholar]
- 10. Baldus SE, Hanisch FG, Putz C et al . Immunoreactivity of Lewis blood group and mucin peptide core antigens: correlations with grade of dysplasia and malignant transformation in the colorectal adenoma‐carcinoma sequence. Histol Histopathol 2002; 17: 191–8. [DOI] [PubMed] [Google Scholar]
- 11. Hara A, Saegusa M, Mitomi H et al . Colonic mucin‐carbohydrate components in colorectal tumors and their possible relationship to MUC2, p53 and DCC immunoreactivities. Pathol Res Pract 2000; 196: 159–66. [DOI] [PubMed] [Google Scholar]
- 12. Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metast Rev 2004; 23: 77–99. [DOI] [PubMed] [Google Scholar]
- 13. Biemer‐Huttmann A‐E, Walsh MD, McGuckin MA et al . Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res 2000; 6: 1909–16. [PubMed] [Google Scholar]
- 14. Li D, Semba S, Wu M, Yokozaki H. Molecular pathological subclassification of mucinous adenocarcinoma of the colorectum. Pathol Int 2005; 55: 766–74. [DOI] [PubMed] [Google Scholar]
- 15. Park SY, Lee HS, Choe G, Chung JH, Kim WH. Clinicopathological characteristics, microsatellite instability, and expression of mucin core proteins and p53 in colorectal mucinous adenocarcinomas in relation to location. Virchows Arch 2006. (Apri 28) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Pastrello C, Santarosa M, Fornasarig M et al . MUC gene abnormalities in sporadic and hereditary mucinous colon cancers with microsatellite instability. Dis Markers 2005, 121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sylvester PA, Myerscough N, Warren BF et al . Differential expression of the chromosome 11 mucin genes in colorectal cancer. J Pathol 2001; 195: 327–35. [DOI] [PubMed] [Google Scholar]
- 18. Inaba T, Sano H, Kawahito Y et al . Induction of cyclooxygenase‐2 in monocyte/macrophage by mucins secreted from colon cancer cells. Proc Natl Acad Sci USA 2003; 100: 2736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Velcich A, Yang W, Heyer J et al . Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002; 295: 1726–9. [DOI] [PubMed] [Google Scholar]
- 20. Yang W, Velcich A, Lozonschi I et al . Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2−/– mice. Am J Path 2005; 166: 1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dietrich WF, Lander ES, Smith JS et al . Genetic identification of Mom‐1, a major modifier locus affecting Min‐induced intestinal neoplasia in the mouse. Cell 1993; 75: 631–9. [DOI] [PubMed] [Google Scholar]
- 22. Cormier RT, Bilger A, Lillich AJ et al . The Mom1 AKR intestinal tumor resistance region consists of Pla2g2a and a locus distal to D4Mit64 . Oncogene 2000; 19: 3182–92. [DOI] [PubMed] [Google Scholar]
- 23. Gould KA, Luongo C, Moser AR et al . Genetic evaluation of candidate genes for the Mom1 modifier of intestinal neoplasia in mice. Genetics 1996; 144: 1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacPhee M, Chepenik KP, Liddell RA, Nelson KK, Siracusa LD, Buchberg AM. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin ‐induced intestinal neoplasia. Cell 1995; 81: 957–66. [DOI] [PubMed] [Google Scholar]
- 25. Cormier RT, Hong KH, Halberg RB et al . Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat Genet 1997; 17: 88–91. [DOI] [PubMed] [Google Scholar]
- 26. Koo JS, Kim YD, Jetten AM, Belloni P, Nettesheim P. Overexpression of mucin genes induced by interleukin‐1 beta, tumor necrosis factor‐alpha lipopolysaccharide, and neutrophil elastase is inhibited by a retinoic acid receptor alpha antagonist. Exp Lung Res 2002; 28: 315–32. [DOI] [PubMed] [Google Scholar]
- 27. Perrais M, Pigny P, Copin M‐C, Aubert J‐P, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal‐regulated kinase cascade and Sp1. J Biol Chem 2002; 277: 32258–67. [DOI] [PubMed] [Google Scholar]
- 28. Kim Y‐D, Kwon E‐J, Park D‐W, Song S‐Y, Yoon S‐K, Baek S‐H. Interleukin‐1B induces MUC2 and MUC5AC synthesis through cyclooxygenase‐2 in NCI‐H292 cells. Mol Pharmacol 2002; 62: 1112–18. [DOI] [PubMed] [Google Scholar]
- 29. Velcich A, Augenlicht LH. Regulated expression of an intestinal mucin gene in HT29 colonic carcinoma cells. J Biol Chem 1993; 19: 13956–61. [PubMed] [Google Scholar]
- 30. Fleming N, Mellow L. Arachidonic acid stimulates intracellular calcium mobilization and regulates protein synthesis, ATP levels, and mucin secretion in submandibular gland cells. J Dent Res 1995; 74: 1295–302. [DOI] [PubMed] [Google Scholar]
- 31. McAlpine CA, Barak Y, Matise I, Cormier . Intestinal‐specific PPARgamma deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer 2006; 119: 2339–46. [DOI] [PubMed] [Google Scholar]
- 32. Javid SH, Moran AE, Carothers AM, Redston M, Bertagnolli MM. Modulation of tumor formation and intestinal cell migration by estrogens in the ApcMin/+ mouse model of colorectal cancer. Carcinogenesis 2005; 26: 587–95. [DOI] [PubMed] [Google Scholar]
- 33. Sawaguchi A, Tojo H, Kawano JI, Okamoto M, Sugunuma T. Immunocytochemical demonstration of the secretory dynamics of zymogenic contents in rat gastric gland processed by high‐pressure freezing/freeze substitution, with special references to phospholipase A(2) and phospholipase Cgamma1. Histochem Cell Biol 2001; 116: 361–9. [DOI] [PubMed] [Google Scholar]
- 34. Lakatos PL, Altorjay I, Mandi Y et al . Interaction between seroreactivity to microbial antigens and genetics in Crohn's disease. is there a role for defensins? Tissue Antigens 2008. (Apri 7) [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Kocsis AK, Lakatos PL, Somogyvari F et al . Association of beta‐defensin 1 single nucleotide polymorphisms with Crohn's disease. Scand J Gastroenterol 2007. (Oct 18): 1–9 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36. Pazgier M, Prahl A, Hoover DM, Lubkowski J. Studies of the biological properties of human β‐defensin 1. J Biol Chem 2007; 282: 1819–29. [DOI] [PubMed] [Google Scholar]
- 37. Tollin M, Bergman P, Svenberg T, Jornvall H, Gudmundsson GH, Agerberth B. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 2003; 24: 523–30. [DOI] [PubMed] [Google Scholar]
- 38. Wehkamp J, Stange EF. A new look at Crohn's disease: breakdown of the mucosal antibacterial defense. Ann NY Acad Sci 2006; 1072: 321–31. [DOI] [PubMed] [Google Scholar]
- 39. Eckmann L. defence molecules in intestinal innate immunity against bacterial infections. Curr Opin Gastroenterol 2005; 21: 147–51. [DOI] [PubMed] [Google Scholar]
- 40. Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF‐kappaB inhibition in a mouse model of chronic colitis. J Immunol 2007; 179: 6988–7000. [DOI] [PubMed] [Google Scholar]
- 41. Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet‐induced diabetic mice. Mol Cell Proteomics 2005; 4: 1311–18. [DOI] [PubMed] [Google Scholar]
- 42. Gilham D, Labonte ED, Rojas JC, Jandacek RJ, Howles PN, Hui DY. Carboxyl ester lipase deficiency exacerbates dietary lipid absorption abnormalities and resistance to diet‐induced obesity in pancreatic triglyceride lipase knockout mice. J Biol Chem 2007; 282: 24642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reuss R, Aberle S, Klingel K et al . The expression of the carboxyl ester lipase gene in pancreas and pancreatic adenocarcinomas. Int J Oncol 2006; 29: 649–54. [PubMed] [Google Scholar]
- 44. Hui DY, Howles PN. Molecular mechanisms of cholesterol absorption and transport in the intestine. Sem Cell Dev Biol 2005; 16: 183–92. [DOI] [PubMed] [Google Scholar]
- 45. Lindner I, Helwig U, Rubin D et al . Putative association between a new polymorphism in exon 3 (Arg109Cys) of the pancreatic colipase gene and type 2 diabetes mellitus in two independent Caucasian study populations. Mol Nutr Food Res 2005; 49: 972–6. [DOI] [PubMed] [Google Scholar]
- 46. Szepessy E, Sahin‐Toth M. Inactivity of recombinant ELA2B provides a new example of evolutionary elastase silencing in humans. Pancreatology 2006; 6: 117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci 2007; 52: 1–17. [DOI] [PubMed] [Google Scholar]
- 48. Melgar S, Drmotova M, Rehnstrom E, Jansson L, Michaelsson E. Local production of chemokines and prostaglandin E2 in the acute, chronic and recovery phase of murine experimental colitis. Cytokine 2006; 35: 275–83. [DOI] [PubMed] [Google Scholar]
- 49. Kabashima K, Saji T, Murata T et al . The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest 2002; 109: 883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Select list of genes differentially expressed in C57BL/6J‐Muc2 −/– mice compared with C57BL/6J‐Pla2g2a‐Tg mice.
Table S2. Genes differentially expressed in C57BL/6J‐Muc2−/– Pla2g2a‐Tg mice compared with C57BL/6J‐Muc2−/– mice.
Table S3. Differentially expressed genes in C57BL/6J‐Muc2 −/–‐Pla2g2a‐Tg/C57BL/6J‐ Muc2 −/– colon that are unique from genes differentially expressed in C57BL/6J‐Pla2g2a‐Tg/C57BL/6J comparison.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


