Abstract
Tumor angiogenesis is a multistep interactive process in which vascular endothelial growth factor (VEGF) and its receptors have a major role. However, the clinical significance of these molecules in gastric cancer (GC) remains unclear. Our study group comprised 86 patients who underwent gastrectomy and subsequently received chemotherapy for recurrent or residual tumor. Using immunohistochemical techniques, we analyzed the expression of VEGF receptors (VEGF‐R) 1, 2, and 3. VEGF‐R1 expression (defined as >5% staining) was found in the tumor cells of 65 tumors (76%) and in the stromal vessels of 36 tumors (42%). VEGF‐R2 expression was found in tumor cells and stromal vessels of 0 and 46 tumors (0 and 53%), respectively, and VEGF‐R3 expression was found in tumor cells and stromal vessels of 0 and 75 tumors (0 and 87%), respectively. Univariate analysis revealed that VEGF‐R expression correlated with shorter survival (VEGF‐R1 in stromal vessels, P = 0.001; VEGF‐R2 in stromal vessels, P = 0.009; VEGF‐R3 in stromal vessels, P = 0.005) and lower response to S‐1 (VEGF‐R1 in stromal vessels, P = 0.039). Multivariate analysis of potential prognostic factors showed that VEGF‐R1 and VEGF‐R2 in stromal vessels were independent predictors of poor outcome. Our data suggest that VEGF‐R expression can be a predictor of unfavorable clinical outcome in GC. VEGF‐R are promising candidates as therapeutic targets. (Cancer Sci 2009; 100: 310–315)
Gastric cancer (GC) is the second leading cause of cancer‐related death worldwide, accounting for over 20 deaths per 100 000 population annually in East Asia (China, Japan), Eastern Europe, and parts of Central and South America.( 1 ) Recently, many chemotherapy regimens using new agents have been developed that show high response rates for advanced GC, and progress in basic research has revealed many factors and mechanisms implicated in sensitivity and resistance to chemotherapy.
Angiogenesis reportedly plays an important role in cancer invasion and metastasis. Vascular endothelial growth factor (VEGF) and VEGF receptor (VEGF‐R) represent important regulators of angiogenesis, and increased expression of this family of molecules has been documented in various cancer cell lines( 2 ) and tissues.( 3 , 4 ) Previous clinical studies have demonstrated that increased expression of VEGF or its family is associated with the grade of angiogenesis and the prognosis for various human cancers.( 5 , 6 , 7 , 8 , 9 )
In GC, several studies have found that expression of VEGF ligands and subtypes correlates with prognosis,( 10 , 11 , 12 ) and expression of soluble VEGF‐R1 is also a predictor of prognosis.( 13 ) However, the distribution, frequency, and prognostic value of VEGF‐R expression in GC have not been clarified. The present study investigated relationships between VEGF‐R expression and prognosis in patients with advanced GC.
Materials and Methods
Patients. Subjects were 86 patients who underwent surgery for primary GC and received chemotherapy for the treatment of recurrent or residual tumors at the National Cancer Center Hospital (NCCH). Inclusion criteria were as follows: histologically proven advanced GC; unresectable, locally advanced, or metastatic disease; no prior chemotherapy and no prior adjuvant or neoadjuvant chemotherapy; specimens of primary GC were obtained before the start of chemotherapy by surgical resection or biopsy at NCCH; radiographically measurable disease; first‐line chemotherapy was received from January 1995 to December 2004; tumor response and survival times were confirmed; adequate bone marrow, liver, and renal function; and written informed consent. The tissue samples were collected retrospectively from patients who met these criteria. Measurable disease was assessed by computed tomography. Response was evaluated according to the standard International Union against Cancer (UICC) guidelines as complete response (CR), partial response (PR), no change (NC), or progressive disease (PD). The response rate was calculated as the ratio of CR + PR to CR + PR + NC + PD.( 14 ) Written informed consent was obtained before treatment and evaluation of tumor samples.
Immunohistochemical staining. Serial 4‐µm sections were made from formalin‐fixed paraffin‐embedded tissue. Sections were dewaxed in xylene and rehydrated through a graded alcohol series. Antigen retrieval was carried out by incubating sections in target‐retrieval solution (Dako Japan, Tokyo, Japan) for 40 min in a 95°C water bath and cooling for at least 20 min.
After quenching endogenous peroxidase with peroxidase‐blocking reagent (Dako Japan) for 5 min and washing with Tris‐buffered saline containing Tween 20, sections were incubated with the primary antibody (Table 1).
Table 1.
Antibodies used for immunohistochemistry
| Antigen | Antibody | Manufacturer | Dilution | Incubation time (min) |
|---|---|---|---|---|
| CD34 | M 7165 | Dako Japan | 1:100 | 30 |
| D2‐40 | M 3619 | Dako Japan | 1:50 | 30 |
| CD31 | M 0823 | Dako Japan | 1:50 | Overnight |
| Factor XIII | N 1505 | Dako Japan | 1:2 | 30 |
| VEGF‐R1 | AF 321 | R&D | 1:150 | 15 |
| VEGF‐R2 | AF 357 | R&D | 1:50 | 15 |
| VEGF‐R3 | AF 349 | R&D | 1:50 | 15 |
Immunoreaction was detected using the following secondary antibody systems: CSA‐II (Dako Japan) for VEGF‐R1, VEGF‐R2, and VEGF‐R3; and the Envison + kit (Dako Japan) for CD34, D2‐40, CD31, and factor VIII, according to the instructions of the manufacturer. Sections were counterstained using Mayer's hematoxylin.
Evaluation of immunostaining. The entire specimen was examined at low magnification (×40), and positive cells were counted in areas with strong immunoreactivities at high magnification (×200). The number of immunoreactive cells was counted in three fields of view that exhibited the most positive staining, and the average ratio of immunoreactive cells to the total number of cancer cells per field was calculated. The number of immunoreactive vessels was counted in three fields of view that demonstrated the most positive staining, and the average ratio of immunoreactive vessels to the total number of CD34‐positive and D2‐40‐positive vessels per field was calculated. Staining results for VEGF‐R1, VEGF‐R2, and VEGF‐R3 were classified by estimating the percentage of epithelial cells and vessels showing specific immunoreactivity: negative (defined as <5% staining) or positive (defined as >5% staining).( 7 ) Two researchers evaluated the immunostaining results without being informed of the clinical data.
Statistical analysis. We examined objective tumor response to chemotherapy overall survival. Overall survival were calculated as the period from the start of first‐line chemotherapy until disease progression or death from any cause, respectively. If patients were lost to follow up, data were censored at the date of the last evaluation. Statistical analysis was carried out using Stat View version 5 software (SAS Institute, Cary, NC, USA). Pearson's correlations were used to assess VEGF and VEGF‐R expression, and a χ2‐test was used to assess relationships between VEGF and VEGF‐R expression and therapeutic effect. Each factor and overall survival were determined by Kaplan–Meier methods and analyzed using a log‐rank test. Multivariate analysis was carried out using a Cox proportional hazard model.
Results
Clinicopathological characteristics. The clinicopathological characteristics of the patients are shown in Table 2. Patients comprised 69 (80%) men and 17 (20%) women, with a median age of 61 years. Tumor stage (assessed according to TNM classification at the time of surgery) was I, II, or III in 35 patients, and distant metastasis was confirmed at the time of surgery (stage IV) in 51 patients. Histopathologically, 39 patients had intestinal‐type adenocarcinoma and 47 displayed diffuse‐type adenocarcinoma. All patients received chemotherapy; first‐line chemotherapy comprised S‐1 in 29 patients, 5‐fluorouracil (5‐FU) in 24 patients, cisplatin (CDDP) and irinotecan (CPT‐11) in 28 patients, and other agents in the remaining five patients. The median follow‐up time was 13.3 months (range 1.0–71.7 months).
Table 2.
Patient characteristics (n = 86)
| Characteristic | n |
|---|---|
| Sex | |
| Male | 69 |
| Female | 17 |
| Median age (years) | 61 (range 39–84) |
| Tissue type | |
| Intestinal | 39 |
| Diffuse | 47 |
| pStage † | |
| I | 2 |
| II | 11 |
| III | 22 |
| IV | 51 |
| ECOG performance status | |
| 0 | 42 |
| 1 | 41 |
| 2 | 3 |
| Metastases | |
| Liver | 25 |
| Abdominal lymph node | 43 |
| Peritoneum | 23 |
| Lung | 4 |
| Other | 4 |
| First‐line chemotherapy | |
| S‐1 | 29 |
| 5‐Fluorouracil | 24 |
| Cisplatin + irinotecan | 28 |
| Other | 5 |
Japanese classification. ECOG, Eastern Cooperative Oncology Group.
Expression of VEGF‐R1, VEGF‐R2, and VEGF‐R3. VEGF‐R1 was immunoreactive in tumor cells (not only in the membrane, but also in the cytoplasm) and tumor stromal vessels (Fig. 1a). VEGF‐R1 expression was found in tumor cells of 65 tumors (76%) and in stromal vessels of 36 tumors (42%) (Table 3).
Figure 1.
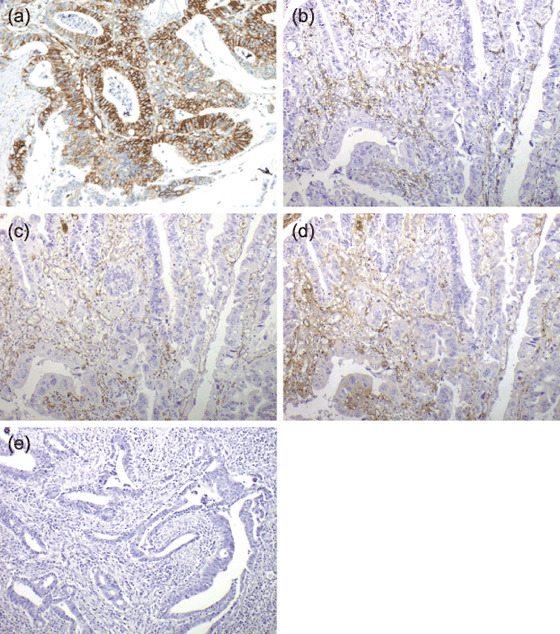
Typical examples of (a) CD34 staining, (b) D2‐40 staining, (c) CD31 staining, (d) factor VIII staining, and (e) negative controls. (a) Vascular endothelial growth factor receptor (VEGF‐R) 1 is mainly expressed in tumor cells, secondarily on stromal vessels. (b–d) VEGF‐R2 and VEGF‐R3 are mainly expressed on stromal vessels. Original magnification, ×200.
Table 3.
Distribution of vascular endothelial growth factor receptor (VEGF‐R) 1, VEGF‐R2, and VEGF‐R3 expression
| Status | VEGF‐R1 | VEGF‐R2 | VEGF‐R3 | |||||
|---|---|---|---|---|---|---|---|---|
| Cytoplasm | Vessel | Vessel | Vessel | |||||
| n | % | n | % | n | % | n | % | |
| Negative (<5%) | 21 | 24 | 50 | 58 | 40 | 47 | 11 | 13 |
| Positive (>5%) | 65 | 76 | 36 | 42 | 46 | 53 | 75 | 87 |
VEGF‐R2 and VEGF‐R3 were immunoreactive mainly in tumor stromal vessels (Fig. 1b–d). VEGF‐R2 expression was found in tumor cells and stromal vessels of 0 and 46 tumors (0 and 53%), respectively, and VEGF‐R3 expression was found in tumor cells and stromal vessels of 0 and 75 tumors (0 and 87%), respectively. The three types of VEGF‐R were not markedly correlated with each other in terms of expression.
Relationship of VEGF‐R expression with response to chemotherapy and survival. The response rate was 38% (11/29) in the S‐1 group, 4% (1/24) in the 5‐FU group, and 43% (12/28) in the CDDP and CPT‐11 group (Table 4). In the S‐1 group, the response rate was lower in the 15 patients in whom stromal vessels stained positive for VEGF‐R1 than in the 14 patients in whom stromal vessels did not (20 vs 57%, χ2‐test P = 0.039). In the other groups, the response rates were not markedly affected by expression of VEGF‐R.
Table 4.
Relationship between vascular endothelial growth factor receptor (VEGF‐R) expression and response to chemotherapy
| First‐line regimen | n | Total response (%) | VEGF‐R1 | VEGF‐R2 | VEGF‐R3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasm | Stromal vessels | Stromal vessels | Stromal vessels | |||||||
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | Positive (%) | Negative (%) | Positive (%) | Negative (%) | |||
| S‐1 | 29 | 38 | 32 | 57 | 20 | 57 | 31 | 44 | 37 | 50 |
| P = 0.234 | P = 0.039 | P = 0.474 | P = 0.715 | |||||||
| Cisplatin and irinotecan | 28 | 43 | 33 | 47 | 45 | 41 | 47 | 38 | 46 | 25 |
| P = 0.255 | P = 0.570 | P = 0.445 | P = 0.887 | |||||||
| 5‐Flurouracil | 24 | 4 | 0 | 4 | 0 | 4 | 4 | 0 | 4 | 0 |
| – | – | – | – | |||||||
To clarify the relevance of marker positivity in prediction of disease outcome, staining results for VEGF‐R1, VEGF‐R2, and VEGF‐R3 were correlated with patient survival according to the log‐rank test. A univariate analysis revealed that VEGF‐R expression correlated with shorter survival (VEGF‐R1 in stromal vessels, 11.2 vs 15.9 months, P = 0.001, Fig. 2a; VEGF‐R2 in stromal vessels, 11.0 vs 15.6 months, P = 0.009, Fig. 2b; VEGF‐R3 in stromal vessels, 12.8 vs 24.3 months, P = 0.005, Fig. 2c). Moreover, multivariate analysis of potential prognostic factors showed that VEGF‐R1 and VEGF‐R2 expression by stromal vessels were independent predictors of poor outcome in advanced GC (Table 5).
Figure 2.
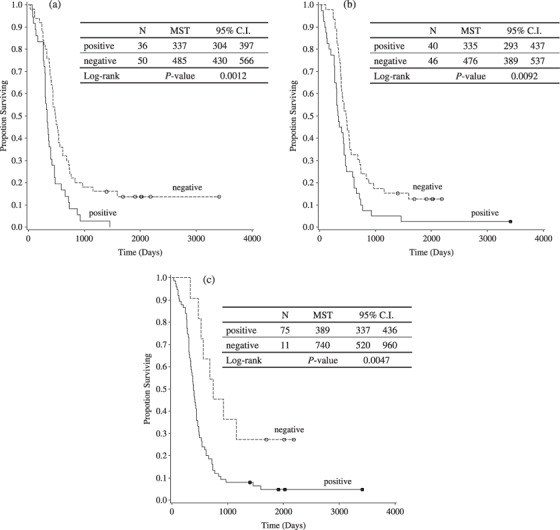
Impact of (a) vascular endothelial growth factor receptor (VEGF‐R) 1, (b) VEGF‐R2, and (c) VEGF‐R3 expression in stromal vessels on patient survival.
Table 5.
Impact of vascular endothelial growth factor receptor (VEGF‐R) expression on patient survival from first‐line chemotherapy (multivariate analysis)
| Parameter | Hazard ratio | 95% confidence interval. | P‐value | ||
|---|---|---|---|---|---|
| VEGF‐R1 (vessel) | 1.75 | 1.09 | 2.80 | 0.020 | |
| PS | 1, 2 versus 0 | 1.45 | 0.62 | 2.27 | 0.109 |
| Tissue type | Diffuse vs intestinal | 0.64 | 0.64 | 1.00 | 0.052 |
| Metastasis site | 2≥ versus 1 | 1.5 | 0.89 | 2.55 | 0.132 |
| VEGF‐R2 (vessel) | 1.76 | 1.12 | 2.75 | 0.014 | |
| PS | 1, 2 versus 0 | 1.56 | 1.00 | 2.46 | 0.052 |
| Tissue type | Diffuse versus intestinal | 0.64 | 0.41 | 1.01 | 0.055 |
| Metastasis site | 2≥ versus 1 | 1.69 | 1.01 | 2.81 | 0.045 |
PS, Performance Status.
Discussion
In the present study, we analyzed VEGF‐R expression levels in primary tumors from 86 patients with advanced GC. Our goal was to determine whether such expression levels are related to treatment outcomes such as survival and response. We found that expression of VEGF‐R1 and VEGF‐R2 in stromal vessels in GC specimens were significant predictors of poor survival in advanced GC. Recently, several studies have reported that the genetic profile of patients is related to the outcome of cancer therapy. In colorectal cancer, VEGF‐R2 expression for metastatic tumors was higher when compared to non‐metastatic tumors,( 5 ) and in head and neck cancer( 15 ) and breast cancer,( 16 ) some studies have documented that VEGF‐R3 expression correlates with lymph node metastasis and malignancy,( 7 , 9 , 14 , 17 ) whereas others have not observed this relationship.( 18 , 19 , 20 ) Further investigations are needed to clarify interactions among VEGF‐R subtypes and the effects of VEGF expression in stroma on angiogenesis and lymphangiogenesis. In GC, several studies have reported correlations between the expression of VEGF and poor prognosis, or lymphatic metastasis. However, most studies examined survival from the date of surgery to the time of event. In the present study, we examined the expression of VEGF‐R, objective tumor response to chemotherapy, and overall survival; the latter two being calculated as the period from the start of first‐line chemotherapy until disease progression or death from any cause, respectively.
After treatment with S‐1, patients with positive staining for VEGF‐R1 in stromal vessels showed a lower response rate (20 vs 57%, P = 0.039) and shorter survival (10.2 vs 20.2 months, hazard ratio = 3.62: data not shown) than those with negative staining, whereas there was no difference with CDDP and CPT‐11. The number of patients treated with S‐1 was small, but Boku et al. have reported the relationship between VEGF status and the effects of S‐1 and 5‐FU; patients expressing VEGF showed a slightly lower response rate and relatively shorter survival than those who did not.( 21 , 22 ) The mechanisms behind this relationship are unclear,( 23 ) but expression of VEGF‐R may become a prognostic marker relevant in deciding on a treatment strategy of 5‐FU‐based drugs.
Our analysis revealed that VEGF‐R expression was correlated with shorter survival (VEGF‐R1 in stromal vessels, P = 0.001; VEGF‐R2 in stromal vessels, P = 0.009; and VEGF‐R3 in stromal vessels, P = 0.005), and multivariate analysis of potential prognostic factors showed that VEGF‐R1 and VEGF‐R2 in stromal vessels were independent predictors of poor outcome. VEGF‐R2 is a potent regulator of vascular endothelial cells and has been directly linked to tumor angiogenesis and blood vessel‐dependent metastasis. VEGF‐R1 may contribute to pathological vascularization directly by stimulating endothelial cell function and indirectly by mediating recruitment of bone marrow progenitor cells.( 24 ) Furthermore, Carmeliet and coworkers demonstrated synergy between the VEGF‐R1‐ and VEGF‐R2‐specific ligands, indicative of cross‐talk between the receptors, allowing modulation of a variety of VEGF‐R‐dependent signals.( 25 ) In GC, the expression of VEGF or VEGF‐C, which are intimately involved in regulation of the lymphangiogenic process, has been reported to be correlated with a poor prognosis.( 10 , 11 , 26 ) Juttner et al. found that the presence of VEGF‐D and its receptor VEGF‐R3 was associated with lymphatic metastasis.( 12 ) Given these results, expression of the VEGF family appears to affect the prognosis of GC.
Our immunostaining evaluation revealed that VEGF‐R is expressed in tumor cells and tumor stromal vessels.VEGF‐R2, which is expressed primarily in vascular endothelial cells, is believed to be the major mediator of angiogenesis in human malignancy, as it regulates activation of downstream effector molecules such as the phosphoinositide 3‐kinase plus AKT and mitogen‐activated protein kinase pathways. It also potentiates endothelial differentiation, DNA synthesis, and proliferation.( 27 , 28 ) On the other hand, VEGF‐R3 is expressed primarily in lymphatic endothelial cells and regulates lymphangiogenesis.( 29 ) Recently, some studies have documented that the expression of VEGF‐R has been observed in tumor cells in several cancers,( 30 , 31 , 32 , 33 , 34 , 35 ) and in the autocrine VEGF–VEGFR loop in cancer cells. Fan et al. demonstrated that incubation with VEGF‐A or VEGF‐B significantly increased colorectal cancer cell migration; however, treatment with a VEGF‐R1 antibody blocked this effect.( 30 ) Giatromanolaki et al. demonstrated that phosphorylated VEGF‐R2 plus KDR receptors are largely expressed in colon cancer cells and intratumoral vasculature, and their expression is associated with tumor diameter and poor histological differentiation.( 31 ) In GC, Tian et al. demonstrated that VEGF‐R2‐positive tumor cells could be stimulated by exogenously added VEGF.( 32 ) In our study, patients with strong positive staining (defined as >50% staining) for VEGF‐R1 in the cytoplasm of tumor cells showed shorter survival (12.6 vs 14.2 m, P = 0.044; data not shown) than others. Thus, these results suggest that the autocrine VEGF–VEGF‐R loop function may contribute to cancer cell proliferation.
In conclusion, our study provides evidence that VEGF‐R expression in GC specimens is a risk factor for poor survival in patients with advanced GC. The results of our analysis can help to identify patient subgroups at higher risk for poor disease outcome in GC.
Acknowledgments
We would like to thank Mr K. Nagashima, Mr H. Sato, Mr T. Asakawa, and Ms A. Morita for their excellent technical assistance.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res 1986; 46: 5629–32. [PubMed] [Google Scholar]
- 3. Brown LF, Berse B, Jackman RW et al . Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993; 53: 4727–35. [PubMed] [Google Scholar]
- 4. Brown LF, Berse B, Jackman RW et al . Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995; 26: 86–91. [DOI] [PubMed] [Google Scholar]
- 5. White JD, Hewett PW, Kosuge D et al . Vascular endothelial growth factor‐D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res 2002; 62: 1669–75. [PubMed] [Google Scholar]
- 6. Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF‐C and VEGF‐D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci 2004; 95: 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yokoyama Y, Charnock‐Jones DS, Licence D et al . Expression of vascular endothelial growth factor (VEGF)‐D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res 2003; 9: 1361–9. [PubMed] [Google Scholar]
- 8. Yokoyama Y, Charnock‐Jones DS, Licence D et al . Vascular endothelial growth factor‐D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer 2003; 88: 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura Y, Yasuoka H, Tsujimoto M et al . Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long‐term follow‐up. Clin Cancer Res 2003; 9: 716–21. [PubMed] [Google Scholar]
- 10. Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF‐C in gastric carcinoma. J Surg Oncol 2001; 78: 132–7. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi A, Kono K, Itakura J et al . Correlation of vascular endothelial growth factor‐C expression with tumor‐infiltrating dendritic cells in gastric cancer. Oncology 2002; 62: 121–7. [DOI] [PubMed] [Google Scholar]
- 12. Juttner S, Wissmann C, Jons T et al . Vascular endothelial growth factor‐D and its receptor VEGFR‐3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006; 24: 228–40. [DOI] [PubMed] [Google Scholar]
- 13. Kosaka Y, Mimori K, Fukagawa T et al . Identification of the high‐risk group for metastasis of gastric cancer cases by vascular endothelial growth factor receptor‐1 overexpression in peripheral blood. Br J Cancer 2007; 96: 1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayward JL, Rubens RD, Carbone PP, Heuson JC, Kumaoka S, Segaloff A. Assessment of response to therapy in advanced breast cancer. A project of the programme on clinical oncology of the International Union against Cancer, Geneva, Switzerland. Eur J Cancer 1978; 14: 1291–2. [DOI] [PubMed] [Google Scholar]
- 15. Moriyama M, Kumagai S, Kawashiri S, Kojima K, Kakihara K, Yamamoto E. Immunohistochemical study of tumor angiogenesis in oral squamous cell carcinoma. Oral Oncol 1997; 33: 369–74. [DOI] [PubMed] [Google Scholar]
- 16. Valtola R, Salven P, Heikkila P et al . VEGF‐R3 and its ligand VEGF‐C are associated with angiogenesis in breast cancer. Am J Pathol 1999; 154: 1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer 2003; 7: 457–64. [DOI] [PubMed] [Google Scholar]
- 18. Gunningham SP, Currie MJ, Han C et al . The short form of the alternatively spliced flt‐4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin Cancer Res 2000; 6: 4278–86. [PubMed] [Google Scholar]
- 19. Jacquemier J, Mathoulin‐Portier MP, Valtola R et al . Prognosis of breast‐carcinoma lymphagenesis evaluated by immunohistochemical investigation of vascular‐endothelial‐growth‐factor receptor 3. Int J Cancer 2000; 89: 69–73. [DOI] [PubMed] [Google Scholar]
- 20. George ML, Tutton MG, Janssen F et al . VEGF‐A, VEGF‐C, and VEGF‐D in colorectal cancer progression. Neoplasia 2001; 3: 420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boku N, Ohtsu A, Nagashima F, Shirao K, Koizumi W. Relationship between expression of vascular endothelial growth factor in tumor tissue from gastric cancers and chemotherapy effects: comparison between S‐1 alone and the combination of S‐1 plus CDDP. Jpn J Clin Oncol 2007; 37: 509–14. [DOI] [PubMed] [Google Scholar]
- 22. Boku N, Ohtsu A, Yoshida S et al . Significance of biological markers for predicting prognosis and selecting chemotherapy regimens of advanced gastric cancer patients between continuous infusion of 5‐FU and a combination of 5‐FU and cisplatin. Jpn J Clin Oncol 2007; 37: 275–81. [DOI] [PubMed] [Google Scholar]
- 23. Boku N, Chin K, Hosokawa K et al . Biological markers as a predictor for response and prognosis of unresectable gastric cancer patients treated with 5‐fluorouracil and cis‐platinum. Clin Cancer Res 1998; 4: 1469–74. [PubMed] [Google Scholar]
- 24. Shibuya M, Claesson‐Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res 2006; 10: 549–60. [DOI] [PubMed] [Google Scholar]
- 25. Carmeliet P, Moons L, Luttun A et al . Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001; 7: 575–83. [DOI] [PubMed] [Google Scholar]
- 26. Yonemura Y, Endo Y, Fujita H et al . Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 1999; 5: 1823–9. [PubMed] [Google Scholar]
- 27. Gerber HP, McMurtrey A, Kowalski J et al . Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′‐kinase/Akt signal transduction pathway. Requirement for Flk‐1/KDR activation. J Biol Chem 1998; 273: 30 336–43. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C‐dependent, but Ras‐independent Raf‐MEK‐MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 1999; 18: 2221–30. [DOI] [PubMed] [Google Scholar]
- 29. Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet 2000; 67: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fan F, Wey JS, McCarty MF et al . Expression and function of vascular endothelial growth factor receptor‐1 on human colorectal cancer cells. Oncogene 2005; 24: 2647–53. [DOI] [PubMed] [Google Scholar]
- 31. Giatromanolaki A, Koukourakis MI, Sivridis E et al . Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest 2007; 37: 878–86. [DOI] [PubMed] [Google Scholar]
- 32. Tian X, Song S, Wu J, Meng L, Dong Z, Shou C. Vascular endothelial growth factor: acting as an autocrine growth factor for human gastric adenocarcinoma cell MGC803. Biochem Biophys Res Commun 2001; 286: 505–12. [DOI] [PubMed] [Google Scholar]
- 33. Higgins KJ, Liu S, Abdelrahim M et al . Vascular endothelial growth factor receptor‐2 expression is down‐regulated by 17β‐estradiol in MCF‐7 breast cancer cells by estrogen receptor α/Sp proteins. Mol Endocrinol 2008; 22: 388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdelrahim M, Baker CH, Abbruzzese JL et al . Regulation of vascular endothelial growth factor receptor‐1 expression by specificity proteins 1, 3, and 4 in pancreatic cancer cells. Cancer Res 2007; 67: 3286–94. [DOI] [PubMed] [Google Scholar]
- 35. Castro‐Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA 2004; 101: 11 432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


