Abstract
Little is known about the pathologic significance of epidermal growth factor receptor (EGFR) expression in malignant testicular germ cell tumors (TGCTs) in adults. From the primary tumor sites of a cohort of 110 TGCT cases, we obtained 209 histologically distinct components: 53 intratubular germ cell neoplasia unclassified (IGCNU) lesions, 83 seminomas (66 pure‐form seminomas and 17 seminoma components in the mixed‐form with nonseminomatous TGCTs), 27 embryonal carcinomas, eight choriocarcinomas, 18 yolk sac tumors, and 20 immature teratomas. Samples were analyzed for expression of EGFR protein and EGFR gene amplification by immunohistochemistry and fluorescence in situ hybridization (FISH), respectively. Overexpression of the EGFR protein was detected in 28% of seminomas (27% in the pure‐form and 29% in the mixed‐form), 11% of embryonal carcinomas, 88% of choriocarcinomas, 44% of yolk sac tumors, and none of the IGCNU lesions or immature teratomas. A higher copy number (≥4 copies per cell) and amplification of the EGFR gene were detected in 20% and 10% of seminomas, 13% and 0% of embryonal carcinomas, 71% and 60% of choriocarcinomas, 15% and 8% of yolk sac tumors, and none of the IGCNU lesions or immature teratomas, respectively. Both higher copy number and amplification of the EGFR gene were positively correlated with immunohistochemical overexpression of EGFR protein (each P < 0.0001). These results suggest that overexpression of EGFR protein and increased copy number or amplification of the EGFR gene occur relatively frequently in primary TGCTs, and may play roles in the formation of invasive cancer and in the progression, especially morphological evolution, of tumors. (Cancer Sci 2010)
Malignant testicular germ cell tumors are the most common form of malignancy in men between the ages of 15 and 45 years.( 1 ) In this age group, these tumors account for 60% of all malignancies and, despite their overall curability, are a major cause of cancer death.( 2 , 3 ) Poor prognosis of patients with testicular germ cell tumors generally results from their ability to metastasize, relapse of disease, or resistance to conventional platinum‐based chemotherapies.
From the clinicopathological viewpoint, testicular germ cell tumors can be subdivided into seminoma and nonseminomatous germ cell tumors (NSGCT).( 1 , 2 , 3 ) NSGCT are a heterogeneous group showing various stages of embryonic differentiation ranging from undifferentiated embryonic stem cells (embryonal carcinoma) to maturing somatic tissues (teratomas). Moreover, NSGCTs can also resemble extraembryonic tissues, that is yolk sac tumors and choriocarcinomas. Testicular germ cell tumors may present as a pure seminoma or as a mixture of seminoma and NSGCT; in this latter situation, seminoma and NSGCT components may be intimately admixed, adjacent to each other, or separately located.
Although the histogenesis of adult testicular germ cell tumors has not been fully evaluated, it is generally accepted that intratubular germ cell neoplasia unclassified (IGCNU) lesion is a substantial precursor lesion for both seminoma and embryonal carcinoma.( 4 ) Moreover, embryonal carcinoma has been considered as a precursor for other NSGCT components, that is choriocarcinoma and yolk sac tumor,( 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ) and the majority of embryonal carcinomas are thought to be derived from pre‐existing seminoma cells which may or may not be identified by histological examination.
Epidermal growth factor receptor (EGFR) is a member of the human epidermal growth factor receptor (HER) or Erb‐B family of type I receptor tyrosine kinases, and its signaling causes increased cell proliferation, decreased apoptosis, and enhanced tumor cell motility and neo‐angiogenesis.( 13 ) Aberrant EGFR protein expression has been implicated in the malignant transformation of non‐small‐cell lung cancers, astrocytomas and renal cell carcinoma; in the poor prognosis of patients with breast cancer, non‐small‐cell lung cancers and osteosarcoma; and in the acquisition of chemo‐resistance by breast cancer, pancreatic cancer, and osteosarcoma.( 14 , 15 , 16 , 17 , 18 , 19 ) However, little is known about the status of EGFR expression in primary testicular germ cell tumors in adults and its pathological significance.( 20 , 21 , 22 , 23 )
In the present study, we performed histological review of surgically resected specimens from 110 primary testicular germ cell tumors and selected a total of 209 histologically distinct components, including IGCNU, seminomas, embryonal carcinomas, choriocarcinomas, yolk sac tumors, and immature teratomas. We used immunohistochemistry and fluorescence in situ hybridization (FISH) to investigate EGFR protein expression and EGFR gene copy number alterations in these tumor components with the aim of answering the following questions: (i) whether overexpression of EGFR protein and increased copy number or amplification of the EGFR gene are common occurrences in primary testicular germ cell tumors; (ii) whether such alterations are already evident in the putative precursor lesion IGCNU; (iii) whether these EGFR alterations are common to pure seminomas and seminoma components in mixed tumors; and (iv) whether these EGFR alterations are common to the histologically distinct components in NSGCTs. Such information would allow us to determine whether changes in EGFR expression and gene copy number occur in association with tumorigenesis or as later events in tumor progression.
Materials and Methods
Cases. A total of 110 cases of primary testicular germ cell tumor were identified from files at the Department of Laboratory Medicine, National Defense Medical College Hospital, Tokorozawa, Saitama, Japan. All cases were surgically resected specimens obtained from 1986 to 2008, and the age of patients ranged from 18 to 79 (mean, 33.2; median, 33) years. Only one patient (1%) was over the age of 60 (79 years). The T factor was T1 in 76 tumors, T2 in 29, T3 in five, and none of the cases were staged T4. The N factor was N1 in 16 tumors, N2 in three tumors, and N3 in four tumors, according to TNM classification (2003). All pathology specimens were reviewed by two observers (K.M. and S.Y.) at our institution, and tumors were classified according to the World Health Organization criteria.( 1 ) The research protocol was approved by the Ethics Committee of the National Defense Medical College, Tokorozawa, Japan.
Tissue microarray. To construct tissue microarray (TMA) blocks, we selected formalin‐fixed paraffin‐embedded cancer tissue blocks containing the areas in which histological diagnosis for tumor components had been performed. Two core specimens for each histological component, 2.0 mm in diameter, were taken from these blocks and transferred to recipient blocks with a Tissue Microarrayer (Beecher Instrument, Silver Spring, MD, USA). These TMA blocks were then cut into 4.5‐μm‐thick sections and subjected to both immunohistochemistry and FISH analysis.
Immunohistochemistry. Expression of EGFR protein was analyzed by immunohistochemistry with a Dako EGFR pharmDx kit (Dako, Glostrup, Denmark), according to the manufacturer’s instructions. Briefly, deparaffinized sections were subjected to antigen retrieval with Proteinase K (Dako) digestion for 5 min at room temperature, and treated with 3% hydrogen peroxide for 5 min to inhibit endogenous peroxidase activity. Then, sections were incubated with a mouse monoclonal antibody against EGFR (clone 2‐18C9, ready‐to‐use) for 30 min at room temperature. The slides were then reacted with a dextran polymer reagent combined with secondary antibodies and peroxidase (Dako) for 30 min at room temperature. Specific antigen–antibody reactions were visualized with 0.2% diaminobenzidine tetrahydrochloride and hydrogen peroxide, and counterstaining was performed with Mayer’s hematoxylin. Non‐neoplastic matured placental tissue (i.e. chorionic villi) was used as a positive control. As negative controls, sections without the primary antibody were used.
Cytoplasmic and/or cell membranous immunoreactivity was taken into account for EGFR protein expression. According to the scoring system for EGFR immunostaining described by Adams et al.,( 24 ) intensities of EGFR immunoreactivity were classified into the following four categories: non‐staining (score 0), weak (score 1+), moderate (score 2+), and strong (score 3+). Immunoreactivity in the tumor was defined as overexpression of EGFR protein if 50% or more tumor cells evaluated in the TMA cores showed moderate (2+) to strong (3+) immunoreactivity.
Fluorescence in situ hybridization (FISH) analysis. Epidermal growth factor receptor (EGFR) SpectrumOrange/chromosome enumeration probe 7 (CEP7) SpectrumGreen DNA probes (Abbott Molecular, Chicago, IL, USA) were used for FISH analysis by the same methods as those for the PathVysion DNA probe kit (Abbott Molecular, Chicago, IL, USA), as described previously.( 25 ) Hybridization was performed between the denatured probes and denatured DNA in TMA sections at 37°C for 14–18 h. The sections were counterstained with 4,6‐diamidino‐2‐phenylindone (DAPI).
Analysis and counting of fluorescence signals on FISH analysis were performed for tumor cells corresponding to those with EGFR immunoreactivity, if the tumor cells showed partial immunostaining for EGFR. The number of fluorescence signals from both CEP7 and EGFR probes in 20 interphase tumor cell nuclei were counted independently by two observers (K.M. and S.Y.). The mean copy number of the EGFR gene per tumor cell nucleus for each tumor component was calculated by dividing the total counts of EGFR signals by the number of counted nuclear. The copy number of the EGFR gene was defined as higher and lower when the mean EGFR copy number was ≥4.0 and <4.0, respectively. The EGFR/CEP7 ratio was calculated by dividing the total counts of the EGFR signals by the total counts of the CEP7 signals. Epidermal growth factor receptor (EGFR) amplification was defined to be present if the EGFR/CEP7 ratio was equal or higher than 2.0. As a control, non‐neoplastic testicular tissue (seminiferous tubules containing non‐neoplastic spermatocytes) was used. When the judgments by two observers differed for a tumor, they counted further to a total of 40 nuclei and reached consensus by discussion.
Statistical analysis. Statistical analyses were performed by using Ekuseru‐Toukei 2008 software (SSRI, Tokyo, Japan). Comparisons between parameters were computed by the chi‐squared test. Differences at P < 0.05 were considered to be statistically significant.
Results
Histology of malignant germ cell tumor components identified. Of the 110 cases, 66, 33, and 11 cases were classified as pure seminomas, mixed tumors (cases with more than two components of seminoma or NSGCT), and pure NSGCTs, respectively. Moreover, a total of 209 histologically distinct components were identified in the 110 cases: 53 IGCNU lesions (30 in pure seminomas, 19 in mixed tumors, four as pure NSGCTs), 83 seminomas (66 as pure seminomas and 17 in mixed tumors), 27 embryonal carcinomas (22 in mixed tumors and five as pure NSGCTs), eight choriocarcinomas (seven in mixed tumors and one as pure NSGCT), 18 yolk sac tumors (14 in mixed tumors and four as pure NSGCTs), and 20 immature teratomas (19 in mixed tumors and one as pure NSGCT). Cases enrolled and histological components examined are summarized in Table 1.
Table 1.
Cases enrolled and distinct histological components analyzed in this study
| Tumor type | No. of cases | No. of histological components studied | |||||
|---|---|---|---|---|---|---|---|
| IGCNU | SE | EM | CH | YS | IT | ||
| Pure seminoma | 66 | 30 | 66 | – | – | – | – |
| Mixed tumor | 33 | 19 | 17 | 22 | 7 | 14 | 19 |
| Pure NSGCT | 11 | 4 | – | 5 | 1 | 4 | 1 |
| Total | 110 | 53 | 83 | 27 | 8 | 18 | 20 |
CH, choriocarcinoma; EM, embryonal carcinoma; IT, immature teratoma; IGCNU, intratubular germ cell neoplasia unclassified; NSGCT, nonseminomatous germ cell tumor; SE, seminoma; YS, yolk sac tumor.
Overexpression of EGFR protein. Of 110 tumor cases enrolled, 35 (32%) showed immunoreactivity for EGFR in at least one histological component: 18 (27%) of 66 pure seminomas, 14 (42%) of 33 mixed tumors, and three (27%) of 11 pure NSGCTs. Results of immunohistochemistry for all 209 histological components are summarized in Table 2.
Table 2.
Overexpression of EGFR protein in each distinct histological component of adult testicular germ cell tumors
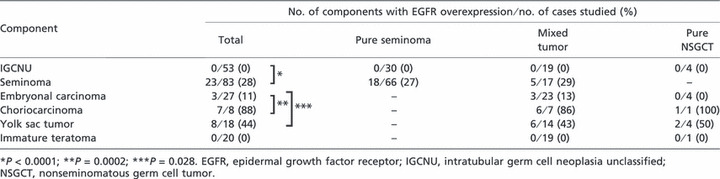
On a total component basis, overexpression of EGFR protein was identified in 23 (28%) of 83 seminoma components, seven (88%) of eight choriocarcinoma components, and eight (44%) of 18 yolk sac tumor components, but in only three (11%) of 27 embryonal carcinoma components and none of the immature teratoma or IGCNU components (Fig. 1). Although immature teratomas frequently showed immunoreactivity for EGFR, especially in the maturing epithelial foci, such as stratified squamous/squamoid epithelial foci or cuboidal to columnar epithelial foci, none of these cases were defined as overexpression because immunonegative mesenchymal teratomatous cells represented over 50% of the tumor cells assessed in these cases.
Figure 1.
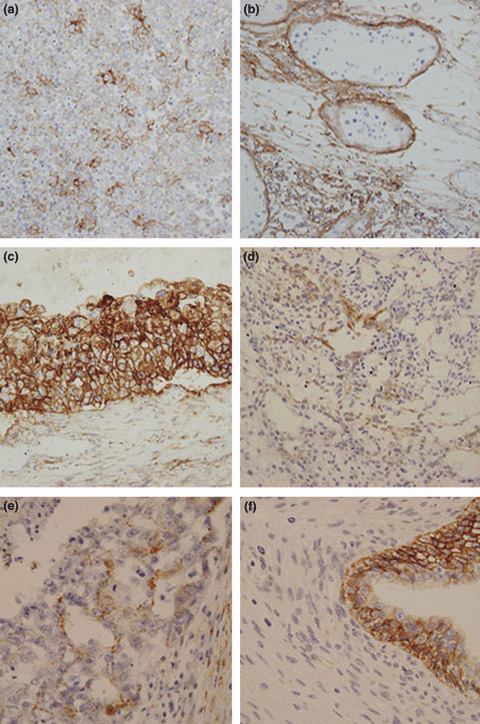
Representative immunohistochemistry for epidermal growth factor receptor (EGFR) protein expression in testicular germ cell tumors. (a) A case of pure seminoma showing overexpression of EGFR protein. (b) A component of intratubular germ cell neoplasia unclassified showing no immunoreactivity for EGFR. Note the surrounding invasive seminoma cells showing strong EGFR immunoreactivity. Cases of (c) choriocarcinoma, (d) yolk sac tumor, and (e) embryonal carcinoma, showing overexpression of EGFR protein. (f) A case of immature teratoma. Epithelial components were weakly to moderately immunoreactive for EGFR, but mesenchymal tumor cells were immuno‐negative. According to the criteria for EGFR protein expression, this case was not considered to show overexpression. Immunoperoxidase stain, original magnification × 200 for (a), (b), (c), and (d), and ×400 for (e) and (f).
Consequently, EGFR overexpression was more frequent in seminoma components than in IGCNU components (P < 0.0001). Likewise, choriocarcinoma and yolk sac tumor components showed more frequent EGFR overexpression than did embryonal carcinoma components (P = 0.0002 and P = 0.028, respectively). Although seminoma components showed relatively frequent overexpression of EGFR protein compared with embryonal carcinoma components, the difference was not statistically significant (P = 0.077).
In the 66 pure seminomas and 17 seminoma components of mixed tumors, overexpression of EGFR protein was identified in 18 (27%) and five (29%) of the seminoma components, respectively. There were no statistically significant differences between seminoma components in pure seminomas and those in mixed tumors (P = 0.86).
Fluorescence in situ hybridization (FISH) analysis. The results of FISH analysis are summarized in Table 3. Among 209 components subjected to immunohistochemical analysis, measurement of EGFR copy number was possible in 180 and measurement of EGFR/CEP7 ratio was possible in 176.
Table 3.
Higher copy number and amplification of the EGFR gene in each distinct histological component of adult testicular germ cell tumors
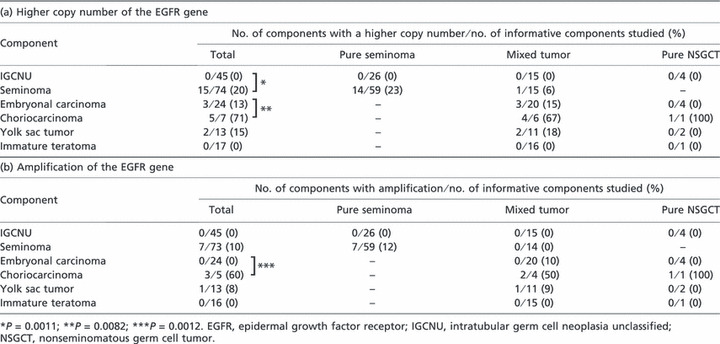
(a) Higher copy number of the EGFR gene in adult testicular germ cell tumors. Among 100 informative tumor cases, 23 (23%) showed higher copy numbers of the EGFR gene: 14 (23%) of 60 pure seminomas, eight (26%) of 31 mixed tumors, and one (13%) of nine pure NSGCTs. Among 180 informative tumor components, 15 (20%) of 74 seminomas, three (13%) of 24 embryonal carcinomas, five (71%) of seven choriocarcinomas, and two (15%) of 13 yolk sac tumors, but none of 17 IGCNU components, showed higher copy numbers of the EGFR gene (Fig. 1). In the 20 immature teratoma components, a higher copy number of the EGFR gene was not detected in mesenchymal or epithelial foci, even in the epithelial cells showing EGFR immunoreactivity. Consequently, the frequency of higher copy number of the EGFR gene differed significantly between IGCNU and seminoma components (0%vs 20%, respectively; P = 0.0011) and between embryonal carcinoma and choriocarcinoma components (13%vs 71%, respectively; P = 0.0082). There was no significant difference in the incidence of higher EGFR copy number between seminoma and embryonal carcinoma components (P = 0.58).
Of the 59 informative pure seminomas and 15 informative seminoma components of mixed tumors, 14 (23%) and one (6%) showed a higher copy number of the EGFR gene, respectively. There were no significant differences between pure seminomas and seminoma components in mixed tumors (P = 0.27).
(b) Amplification of the EGFR gene in adult testicular germ cell tumors. Among 98 informative tumor cases, 11 (11%) showed amplification of EGFR gene: seven (12%) of 60 pure seminomas, three (10%) of 29 mixed tumors, and one (13%) of nine pure NSGCTs. Among 176 informative tumor components, seven (10%) of 73 seminomas, three (60%) of five choriocarcinomas, and one (8%) of 13 yolk sac tumors showed amplification of the EGFR gene (Fig. 2). EGFR gene amplification was not detected in 45 IGCNU lesions, 24 embryonal carcinomas, and 16 immature teratoma components even in the epithelial cells showing EGFR immunoreactivity. Although the difference was not statistically significant, seminoma components tended to show amplification of the EGFR gene more frequently than did IGCNU and embryonal carcinoma components (P = 0.082 and P = 0.26, respectively). Consequently, there was a significant difference in the frequency of EGFR gene amplification between embryonal carcinoma and choriocarcinoma components (0%vs 60%, respectively; P = 0.0012).
Figure 2.
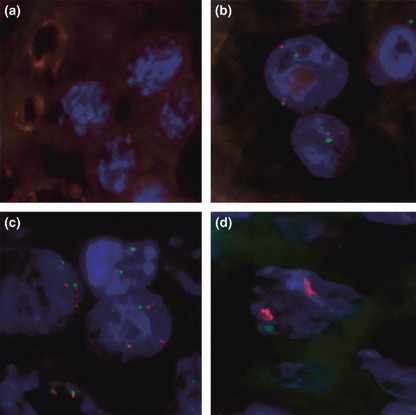
Status of copy number of the epidermal growth factor receptor (EGFR) gene determined by fluorescence in situ hybridization (FISH) in non‐neoplastic spermatocytes and testicular germ cell tumors. Red and green signals indicate the EGFR and CEP7 signals, respectively. (a) Non‐neoplastic spermatocytes, used as a control tissue for FISH analysis. One red signal and one green signal are noted. (b) A case of intratubular germ cell neoplasia unclassified showing two red signals and two green signals, suggesting disomy of chromosome 7. (c) A case of pure seminoma showing four to six signals for red and green, respectively, showing a higher copy number of the EGFR gene. (d) A case of choriocarcinoma showing 10 or more red signals and one or two green signals, showing amplification of the EGFR gene as well as higher copy number. 4,6‐Diamidino‐2‐phenylindone (DAPI)‐counterstained interphase nuclei are shown for each specimen (original magnification, × 1000).
Of the 59 informative pure seminomas and 14 seminoma components in mixed tumors, seven (12%) and none (0%) showed amplification of the EGFR gene, respectively. There were no significant differences between pure seminomas and seminoma components in mixed tumors (P = 0.38).
Correlation between EGFR gene alterations and EGFR protein expression. Among the 180 tumor components in which information on EGFR gene copy number was available by FISH analysis, EGFR gene copy number was compared with immunohistochemical expression of EGFR protein (Table 4). There was a highly positive correlation between EGFR gene copy number and immunohistochemical expression of the EGFR protein: 15 (60%) of 25 tumor components with a higher EGFR copy number, but only 23 (15%) of 155 tumor components with a lower EGFR copy number, showed overexpression of EGFR protein (P < 0.0001). Of the 10 tumor components showing a higher EGFR copy number but no overexpression of EGFR protein, eight (80%) were seminoma components and two (20%) were embryonal carcinoma components. Of the 23 components showing a lower EGFR copy number and overexpression of EGFR protein, 16 (70%) were seminoma components, two (9%) were embryonal carcinomas, one (4%) was a choriocarcinoma component, and four (17%) were yolk sac tumor components.
Table 4.
Correlation between the status of the EGFR gene and levels of EGFR protein expression in 209 distinct histological components of adult testicular germ cell tumors
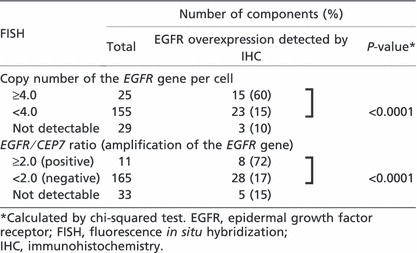
Among the 176 tumor components in which information on the EGFR gene amplification was available, the status of the EGFR gene was compared with immunohistochemical expression of EGFR protein (Table 4). Again, there was a highly positive correlation between EGFR gene amplification and immunohistochemical expression of EGFR protein: eight (73%) of 11 tumor components with EGFR gene amplification but only 28 (17%) of 165 tumor components without gene amplification showed overexpression of EGFR protein (P < 0.0001). All of the three tumor components showing amplification of the EGFR gene but no overexpression of EGFR protein were seminomas. Of the 28 tumor components showing no amplification of the EGFR gene but overexpression of EGFR protein, 19 (66%) were seminomas, three (10%) were embryonal carcinomas, one (3%) was a choriocarcinoma, and five (17%) were yolk sac tumor components.
Correlation between clinical tumor stages and EGFR aberrations. Overexpression of the EGFR protein was detected in 27 (36%) T1 tumors, five (17%) T2 tumors, and four (80%) T3 tumors. A higher copy number and amplification of the EGFR gene were detected in 17 (25%) and seven (11%) T1 tumors, seven (27%) and two (8%) T2 tumors, and one (20%) and one (20%) T3 tumors, respectively. No significant difference was observed between EGFR aberrations and T factor.
Overexpression of the EGFR protein was detected in six (38%) N1 tumors, two (67%) N2 tumors, and three (75%) N3 tumors. A higher copy number and amplification of the EGFR gene were detected in three (23%) and two (13%) N1 tumors, one (50%) and one (50%) N2 tumors, and one (33%) and none (0%) of the N3 tumors, respectively. There was no significant difference between EGFR aberrations and N factor.
Discussion
The EGFR family consists of four growth factor receptors with tyrosine kinase activity, including EGFR (HER1/erbB‐1), HER2 (erbB‐2/neu), HER3 (erbB‐3), and HER4 (erbB‐4). The EGFR gene, a proto‐oncogene located on chromosome 7p12–p22, can be activated in cancers by three principal mechanisms: increased gene copy number, amplification, or activating mutations.( 26 ) Activation of the EGFR induces a complex series of intracellular signals through the mitogen‐activated protein kinase (MAPK) and phosphatidylinositol 3‐kinase (PI3K) pathways, with effects on cell proliferation activity and angiogenesis, and decreased apoptosis.( 26 ) High expression of the EGFR protein has been reported to occur in a variety of human malignancies, and it is likely that EGFR overexpression plays significant roles in tumorigenesis and/or tumor progression.( 14 , 15 , 16 , 17 , 18 , 19 ) However, information on EGFR expression in adult testicular germ cell tumors is very limited.( 20 , 21 , 22 , 23 )
In the present study, which analyzed a total of 209 histologically distinct tumor components from a cohort of 110 testicular germ cell tumors, we demonstrated overexpression of the EGFR protein in 28% of seminoma components (27% of pure form and 29% of combined form), 11% of embryonal carcinoma components, 88% of choriocarcinoma components, and 44% of yolk sac tumor components. Moreover, higher copy number and amplification of the EGFR gene were detected in 20% and 10% of seminoma components, 13% and 0% of embryonal carcinoma components, 71% and 60% of choriocarcinoma components, and 15% and 8% of yolk sac tumor components, respectively. Both higher copy number and amplification of the EGFR gene were positively and highly significantly (P < 0.0001) correlated with immunohistochemical overexpression of the EGFR protein. These results demonstrate that overexpression of the EGFR protein is relatively common in primary malignant germ cell tumors of adults, and suggest that amplification or increased copy number of the EGFR gene are major mechanisms for its protein overexpression.
In some multistage developmental models of human malignancy, such as brain astrocytic tumors( 27 ) (from local astrocytoma to glioblastoma multiforme) and lung adenocarcinomas( 28 ) (from atypical adenomatous hyperplasia to invasive adenocarcinoma), EGFR overexpression or EGFR gene amplification has been reported to be a later event and was associated with malignant transformation or progression. Although the etiology of adult testicular germ cell tumors has not been fully evaluated, it is generally accepted that IGCNU is the precursor lesion for seminoma, and embryonal carcinoma can progress from IGCNU or seminoma by reprogramming of these tumor cells, and that embryonal carcinoma is the precursor lesion for other NSGCT components, that is choriocarcinoma and yolk sac tumor.( 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 )
All IGCNU components examined in this study were negative for EGFR overexpression or gene alterations, and the frequencies of EGFR overexpression and increased EGFR copy number were significantly higher in the invasive seminoma component than in the IGCNU component. Therefore, it is likely that EGFR alterations are later events during progression from IGCNU to invasive seminoma. However, there is a “missing link” which cannot be overcome by the present data. Recently, the entity of “intratubular seminoma” has been identified as the intermediate stage between IGCNU and invasive seminoma.( 11 ) To confirm the presented hypothesis, it is necessary to examine EGFR alterations in intratubular seminoma. However, morphological distinction was difficult between IGCNU and intratubular seminoma, or between the true “pre‐invasive” seminoma and the invasive seminoma involving adjacent seminiferous tubules. Therefore, the histological term “intratubular seminoma” was not set in this series.
In addition, in mixed tumors and pure NSGCTs, choriocarcinomas showed EGFR overexpression, gene copy number increase, and/or amplification especially frequently in comparison with embryonal carcinomas. These findings suggest that these EGFR alterations may be involved in the progression or acquisition of clonal diversity by mixed tumors or pure NSGCTs, especially from embryonal carcinoma to choriocarcinoma. Histological heterogeneity in these tumors appeared to be associated with EGFR alterations, not only at the protein expression level but also at the genetic level.
Three previous studies have suggested the possible oncogenic potential of EGFR in adult testicular germ cell tumors.( 20 , 21 , 23 ) Moroni et al. ( 20 ) immunohistochemically studied 18 NSGCTs, finding EGFR expression in 16 (89%) that also strongly correlated with β‐human chorionic gonadotropin (β‐HCG) expression in the NSGCT components. They also showed that 11 (69%) of the 16 EGFR‐positive NSGCTs were immunoreactive for tyrosine‐phosphorylated EGFR. Taken together with the present study, these observations suggest that, in adult testicular germ cell tumors, overexpression of EGFR might be involved in trophoblastic differentiation of the tumor cells and progression to choriocarcinoma component.
Wang et al. ( 21 ) investigated EGFR expression in 21 patients with metastatic and chemorefractory testicular embryonal carcinomas and detected immunoreactivity of EGFR protein, EGFR gene amplification, and copy number gain of the EGFR gene in 43%, 5%, and 71% of cases, respectively. In the present series, embryonal carcinoma at the primary sites showed relatively lower frequencies of EGFR protein overexpression (11%, 3 of 27 tumors) or higher copy number of the EGFR gene (13%, 3 of 24 informative tumors) than reported by Wang et al. ( 21 ) Because the probes and protocols used in FISH analysis were very similar in these studies, the discrepancy suggests that overexpression of EGFR protein and increased copy number of the EGFR gene in embryonal carcinoma components appeared to have been associated with the process of metastasis and the acquisition of chemo‐resistance.
When the altered expression of EGFR protein or of the EGFR gene were associated with tumor progression of adult testicular germ cell tumor, a correlation between these EGFR statuses and clinical tumor stages, that is T and N factors, is of interest. However, there was no relationship between EGFR aberrations and clinical tumor stages in the cases enrolled in the present study. Therefore, EGFR overexpression/amplification might be involved in histological tumor evolution rather than in locoregional tumor spreading.
The frequencies of overexpression of EGFR protein and increased copy number or amplification of the EGFR gene did not differ between pure seminomas and seminoma components in mixed tumors. From these data, it may be possible that invasive pure seminomas and invasive seminoma components in mixed tumors are similar in view of molecular aberrations.
In summary, the present data demonstrate that: (i) overexpression of EGFR protein is relatively common in primary malignant germ cell tumors in adults, and that increased copy number or amplification of the EGFR gene frequently underlie EGFR protein overexpression; (ii) these EGFR aberrations appear to be later events, not only during progression from IGCNU to invasive seminoma, but also during progression of NSGCTs, especially morphological evolution to choriocarcinoma; and (iii) the frequencies of these EGFR alterations are similar in pure seminomas and seminoma components in mixed tumors.
To our knowledge, this is the first report of EGFR gene amplification in adult primary testicular germ cell tumors. It is also the largest case series reporting immunohistochemical analysis of EGFR protein expression. In this report, although we have mainly analyzed and discussed the data on the basis of tumor components, the frequencies of overexpression of EGFR protein and higher copy number/amplification of the EGFR gene were not low on a case basis. The incidences of EGFR aberrations were not correlated with three tumor types, that is pure seminoma, mixed tumor, and pure NSGCT, but these incidences tended to be correlated with histologically distinct components regardless of these tumor types. From these results, EGFR aberrations as a late event appeared to occur in association with morphological evolution or differentiation in individual tumors, and did not appear to occur in an all‐or‐nothing manner throughout an individual tumor. Molecular approaches targeted to histologically distinct components in individual tumors may also provide an insight into novel targeted therapeutic strategies for patients with tumors that are refractory to conventional chemotherapy.( 29 , 30 )
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Promotion of Defense Medicine from the Ministry of Defense of Japan (S.Y., H.T., O.M.), and by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor, and Welfare of Japan (H.T.).
References
- 1. Mostofi FK, Sesterhenn IA. Germ cell tumour. Tumours of the testis and paratesticular tissue. In: Eble JN, Sauter G, Epstein MD, eds. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs (World Health Organization Classification of Tumours). Lyon: IARC press, 2004; 220–49. [Google Scholar]
- 2. Bosl GJ, Motzer RJ. Medical progress: testicular germ‐cell cancer. N Engl J Med 1997; 337: 242–53. [DOI] [PubMed] [Google Scholar]
- 3. Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol 1990; 8: 1777–81. [DOI] [PubMed] [Google Scholar]
- 4. Skakkebaek NE, Bethelsen JG, Giwercman A, Müller J. Carcinoma‐in‐situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 1987; 10: 19–28. [DOI] [PubMed] [Google Scholar]
- 5. Kiss F, Jubasz J. Testicular germ cell tumours, current problems of histogenesis and classification. Int Urol Nephrol 1985; 17: 85–95. [DOI] [PubMed] [Google Scholar]
- 6. Mostofi FK. Pathology and germ cell tumours of the testis. Cancer 1986; 45: 1735–54. [PubMed] [Google Scholar]
- 7. Friedman NB. The comparative morphogenesis of extragonadal and gonadal teratoid tumours. Cancer 1951; 4: 265–76. [DOI] [PubMed] [Google Scholar]
- 8. Raghavan D, Sullivan AL, Peckman MJ, Neville AM. Elevated serum alphafetoprotein and seminoma. Clinical evidence for a histologic continuum? Cancer 1982; 50: 982–9. [DOI] [PubMed] [Google Scholar]
- 9. Ulbright TM, Amin MB, Young RH. Tumors of the Testis, Adnexa, Spermatic cord, and Scrotum, 3rd Series Fascicle. Washington DC: Armed Forces Institute of Pathology, 1999. [Google Scholar]
- 10. Kernek KM, Ulbright TM, Zhang S et al. Identical Allelic losses in mature teratoma and other histologic components of malignant mixed germ cell tumors of the testis. Am J Pathol 2004; 163: 2477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oosterhuis JW, Looijenga LH. Current views on the pathogenesis of testicular germ cell tumours and perspectives for future research: highlights of the 5th Copenhagen Workshop on Carcinoma In Situ and Cancer of the Testis. APMIS 2003; 111: 280–9. [DOI] [PubMed] [Google Scholar]
- 12. Oosterhuis JW, Looijenga LH. Testicular germ‐cell tumours in a broader perspective. Nat Rev Cancer 2005; 5: 210–22. [DOI] [PubMed] [Google Scholar]
- 13. Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004; 59: 21–6. [DOI] [PubMed] [Google Scholar]
- 14. Bargava R, Gerald WL, Li AR et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER‐2 status and absence of EGFR‐activating mutations. Mod Pathol 2005; 18: 1027–33. [DOI] [PubMed] [Google Scholar]
- 15. Jimeno A, Tan AC, Coffa J et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res 2008; 68: 2841–9. [DOI] [PubMed] [Google Scholar]
- 16. Hirsch FR, Garcia MV, Bunn PA et al. Epidermal growth factor receptor in non‐small‐cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 2003; 20: 3798–807. [DOI] [PubMed] [Google Scholar]
- 17. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol 2007; 170: 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sargent ER, Gomella LG, Belldegrun A, Linehan WM, Kasid A. Epidermal growth factor receptor gene expression in normal human kidney and renal cell carcinoma. J Urol 1989; 142: 1364–8. [DOI] [PubMed] [Google Scholar]
- 19. Freeman SS, Allen SW, Ganti R et al. Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer 2008; 113: 1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moroni M, Veronese S, Schiavo R et al. Epidermal growth factor receptor expression and activation in nonseminomatous germ cell tumors. Clin Cancer Res 2001; 7: 2770–5. [PubMed] [Google Scholar]
- 21. Wang X, Zhang S, MacLennan GT et al. Epidermal growth factor receptor protein expression and gene amplification in the chemorefractory metastatic embryonal carcinoma. Mod Pathol 2009; 22: 7–12. [DOI] [PubMed] [Google Scholar]
- 22. Hechelhammer L, Storkel S, Odermatt B, Heitz PU, Jochum W. Epidermal growth factor receptor is a marker for syncytiotrophoblastic cells in testicular germ cell tumors. Virchows Arch 2003; 44: 28–31. [DOI] [PubMed] [Google Scholar]
- 23. Mandani A, Kemmer K, Sweeney C et al. Expression of KIT and epidermal growth factor receptor in chemotherapy refractory non‐seminomatous germ‐cell tumors. Ann Oncol 2003; 14: 873–80. [DOI] [PubMed] [Google Scholar]
- 24. Adams EJ, Green JA, Clark AH, Youngson JH. Comparison of different scoring systems for immunohistochemical staining. J Clin Pathol 1999; 52: 75–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsuda H, Akiyama F, Terasaki H et al. Detection of HER‐2/neu (c‐erb B‐2) DNA amplification in primary breast carcinoma. Interobserver reproducibility and correlation with immunohistochemical HER‐2 overexpression. Cancer 2001; 92: 2965–74. [DOI] [PubMed] [Google Scholar]
- 26. Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist 2002; 7: 31–9. [DOI] [PubMed] [Google Scholar]
- 27. Nicholas MK, Lukas RV, Jafri NF, Faoro L, Salgia R. Epidermal growth factor receptor‐ mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res 2006; 12: 7261–70. [DOI] [PubMed] [Google Scholar]
- 28. Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR‐mutated lung cancer. Cancer Res 2008; 68: 2106–11. [DOI] [PubMed] [Google Scholar]
- 29. Veronese ML, O’Dwyer PJ. Monoclonal antibodies in the treatment of colorectal cancer. Eur J Cancer 2004; 40: 1292–301. [DOI] [PubMed] [Google Scholar]
- 30. Xiong HQ, Rosenberg A, LoBuglio A et al. Cetiximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol 2004; 22: 2610–6. [DOI] [PubMed] [Google Scholar]


