Abstract
Bendamustine is a cytotoxic agent with a novel mechanism of action. This phase I, dose‐escalation study evaluated the safety, tolerability, efficacy, and pharmacokinetics of bendamustine in Japanese patients with relapsed/refractory indolent B‐cell non‐Hodgkin lymphoma (B‐NHL) or mantle cell lymphoma (MCL) without major organ dysfunction. Bendamustine 90 or 120 mg/m2 (dose escalation) was administered intravenously over 60 min on days 1 and 2 every 3 weeks for up to three cycles. Nine patients (eight indolent B‐NHL and one MCL) received per‐protocol treatment, three at 90 mg/m2 and six at 120 mg/m2. No dose‐limiting toxicities were observed; thus, the maximum‐tolerated dose was not reached. Grade 3/4 hematologic toxicities were neutropenia (33%) and leukopenia (33%). Non‐hematologic toxicities were grade 1/2 and included gastrointestinal events and fatigue. Peak plasma concentrations of bendamustine occurred near the end of infusion in both dose groups and were equivalent to therapeutic concentrations observed in vitro. Bendamustine was rapidly eliminated, with a mean elimination half‐life (t 1/2) of 29 min. Plasma concentrations of active metabolites M3 and M4 were approximately 4 and <1% of the plasma concentration of the parent molecule, with t 1/2 of 42 and 33 min, respectively. Two unconfirmed complete responses and six partial responses were observed for an overall response rate (ORR) of 89%. The recommended dose for this schedule in phase II trials is 120 mg/m2. The acceptable safety profile and high ORR warrant further investigation of bendamustine in relapsed or refractory indolent B‐NHL and MCL. (ClinicalTrials.gov ID: NCT00389051). (Cancer Sci 2010)
Bendamustine is a unique cytotoxic agent with a multifaceted mechanism of action. Chemically, bendamustine, 4‐{5‐[bis (2‐chloroethyl)amino]‐1‐methyl‐1H‐benzimidazole‐2‐yl} butanoic acid, is composed of a mechlorethamine (nitrogen mustard) group, a benzimidazole ring, and a butyric acid side chain (Fig. 1). The mechlorethamine group confers alkylator properties and produces double‐strand breaks in DNA, the benzimidazole ring is similar in structure to purine analogs and may confer antimetabolite properties, and the butyric acid side chain increases water solubility.
Figure 1.
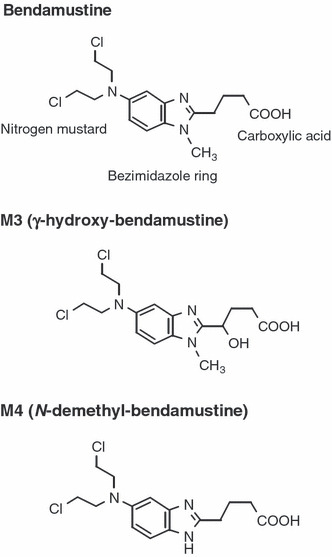
Structure of bendamustine and its active metabolites. The structure of bendamustine is shown, along with the structure of γ‐hydroxy‐bendamustine (M3) and N‐demethyl‐bendamustine (M4).
In vitro evidence indicates that bendamustine has a novel mechanism of action. In cancer cell lines, bendamustine shows incomplete cross‐resistance with other alkylators.( 1 ) Furthermore, the DNA damage produced by bendamustine is more extensive and more durable than that produced by other alkylators, and the cellular DNA repair mechanism that is activated in response to bendamustine is different from that activated by other alkylators.( 1 , 2 ) Gene‐expression profiling confirmed these findings, identifying a distinct pattern of activity in response to bendamustine that was unrelated to that of other alkylators.( 3 ) In addition to DNA damage, a cellular stress response, and apoptosis, bendamustine inhibits mitotic checkpoints and induces mitotic catastrophe.( 3 )
Bendamustine has demonstrated activity in patients with relapsed indolent B‐cell non‐Hodgkin lymphoma (B‐NHL) and mantle cell lymphoma (MCL) in North American and European clinical trials, both as a single agent( 4 , 5 , 6 , 7 ) and in combination with rituximab.( 8 , 9 ) Overall, bendamustine was well tolerated in these trials; reversible myelosuppression and infection were the most common severe (grade 3/4) toxicities associated with treatment. Non‐hematologic toxicities were typically mild and included gastrointestinal effects (e.g. nausea, vomiting, diarrhea) and fatigue.
Previous phase I studies of bendamustine administered in 3‐ or 4‐week cycles have recommended a wide range of doses, from 140 to 260 mg/m2 in patients with solid tumors( 10 , 11 , 12 ) and from 70 to 100 mg/m2 in patients with chronic lymphocytic leukemia.( 13 , 14 ) In phase II trials in patients with indolent B‐NHL, single‐agent bendamustine has been administered at a dose of 120 mg/m2 on days 1 and 2 of a 3‐week cycle;( 4 , 5 , 6 ) however, formal phase I testing in these patients is lacking.
The objectives of this study were to (i) identify the dose‐limiting toxicity (DLT) of bendamustine using two dose levels; (ii) describe the pharmacokinetic properties of bendamustine and its active metabolites; and (iii) define the antitumor effects of bendamustine 90 or 120 mg/m2 administered intravenously in Japanese patients with indolent B‐NHL and MCL, with the intent to provide a recommended dose for a phase II trial.
Materials and Methods
A multicenter phase I, dose‐escalation study was conducted. This study was performed in compliance with the Helsinki Declaration and the Japanese Pharmaceutical Affairs Law and related regulations.
Eligibility. Patients aged 20–75 years with measurable, histologically confirmed, relapsed or refractory indolent B‐NHL or MCL,( 15 ) were eligible if they had a life expectancy >12 weeks, an Eastern Cooperative Oncology Group performance status of 0–1,( 16 ) and adequate organ function. A 4‐week wash‐out period following prior treatment (12 weeks for antibody therapy) was required. Patients were excluded if they had apparent infection; serious hepatic, renal, cardiac, gastrointestinal, or nervous system disorder; human immunodeficiency virus–positive status; any active malignancy other than lymphoma; or autoimmune hemolytic anemia. Patients treated with cytokines or transfusions within 2 weeks prior to registration or with other investigational drugs within 3 months prior to registration were also excluded. All patients were required to provide written informed consent.
Treatment and dose escalation. Bendamustine was administered intravenously in a final volume of 250 mL of normal saline over 60 min on days 1 and 2 of a 3‐week cycle for up to three cycles. Two dose levels were planned: 90 and 120 mg/m2. The starting dosage of 90 mg/m2 was chosen based on the efficacy and tolerability observed at this dose level in combination with rituximab.( 8 , 17 ) As a single agent, bendamustine 120 mg/m2 has demonstrated safety and efficacy in a North American study( 5 ) and was anticipated to be the approved dose in the United States. Prophylaxis against anticipated adverse events was not permitted in cycle 1; however, in subsequent cycles, the use of prophylactics against those events observed in cycle 1 was allowed, including anti‐nausea medication. The use of granulocyte colony‐stimulating factor (G‐CSF) was allowed in patients experiencing grade 4 neutropenia or grade 3 neutropenia accompanied by fever (>38°C). Furthermore, after the first cycle, all patients received acyclovir and sulfamethoxazole with trimethoprim to prevent opportunistic infections, and general infection countermeasures (e.g. gargling with Isodine Gargle) were taken as necessary.
Dose escalation and determination of the maximum‐tolerated dose (MTD) were based on the number of patients who experienced DLT, which was defined as grade 4 neutropenia lasting >1 week and accompanied by fever (38°C or higher), platelet count <10 000/mm3 or bleeding tendency requiring platelet transfusion, any other grade 4 hematologic toxicity excluding lymphopenia and differential white blood cell count (%), or grade 3 or higher non‐hematological toxicity. Dose escalation was implemented as follows. Three patients were initially enrolled at the 90 mg/m2 dose level; if a DLT was observed in one or two patients, an additional three patients were enrolled at this level. If DLT was observed in 3/3, or ≥3/6 patients, the trial would be stopped and this dose designated as the MTD. If DLT was observed in 0/3 or ≤2/6 patients, dose escalation to 120 mg/m2 would proceed. Up to six patients were enrolled at 120 mg/m2, with a pause in enrollment after three patients to ensure that at least one patient did not experience DLT. If DLT was observed in 3/3 or ≥3/6 patients, this dose was designated as the MTD.
Assessment of toxicity and response. Physical examinations and laboratory tests were conducted on days of administration and weekly during the observation period. Computed tomography (CT) scans were performed at registration, 3 weeks after bendamustine administration and at discontinuation. Adverse events were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.( 18 )
Response was assessed according to International Working Group Criteria.( 19 ) Patients were classified according to best tumor response: complete response (CR), complete response unconfirmed (CRu), partial response (PR), stable disease, or disease progression. The overall response rate (ORR) was calculated as the proportion of patients who achieved a CR, CRu, or PR.
Pharmacokinetic analyses. Blood samples were collected 30 min after the start of bendamustine administration, upon completion of administration, and at 15, 30 min, 1, 2, 4, 6, and 23 h after completion of administration on day 1 of the first cycle of treatment. Urine was collected for 24 h after bendamustine administration. The concentrations of bendamustine and its two active metabolites (γ‐hydroxy‐bendamustine [M3] and N‐demethyl‐bendamustine [M4]) (Fig. 1),( 20 ) were measured in plasma and urine by high‐performance liquid chromatography/tandem mass spectrometry. The lower limit of detection was 0.5 ng/mL in plasma and 0.1 μg/mL in urine.
Peak concentration (C max), time to peak concentration (t max), area under the blood concentration–time curve (AUC), half‐life (t 1/2), volume of distribution (Vz), and clearance (Cl), were calculated using non‐compartmental analysis (Model 202) in statistical software WinNonlin (version 5.0.1; Pharsight Corporation, Mountain View, CA, USA). The urinary excretion rate was calculated as the total quantity collected in urine, expressed as a percentage of the administered dosage.
Results
Patients and exposure. Ten patients were registered at four medical centers between October 2006 and January 2007; one patient was not treated due to low platelet count and was excluded from all analyses. Among treated patients, the median age was 56 (range, 36–68) years (Table 1). Disease histologies included follicular lymphoma in eight patients and MCL in one patient; 44% had stage III or IV disease. No patients had evidence of histologic transformation at enrollment. Patients received a median of two prior regimens (range, 1–7).
Table 1.
Patient demographics and baseline characteristics
| 90 mg/m2 (n = 3) | 120 mg/m2 (n = 6) | All patients (n = 9) | |
|---|---|---|---|
| Sex | |||
| Male | 2 | 3 | 5 |
| Female | 1 | 3 | 4 |
| Age (years) | |||
| 20–29 | 0 | 0 | 0 |
| 30–39 | 0 | 1 | 1 |
| 40–49 | 1 | 2 | 3 |
| 50–59 | 0 | 1 | 1 |
| 60–69 | 2 | 2 | 4 |
| Histology (WHO classification) | |||
| Follicular | 2 | 6 | 8 |
| MCL | 1 | 0 | 1 |
| Clinical stage (Ann Arbor classification) | |||
| I | 1 | 2 | 3 |
| II | 1 | 1 | 2 |
| III | 0 | 2 | 2 |
| IV | 1 | 1 | 2 |
| Number of previous regimens | |||
| 1 | 1 | 2 | 3 |
| 2 | 0 | 3 | 3 |
| 3+ | 2 | 1 | 3 |
MCL, mantle cell lymphoma; WHO, World Health Organization.
Three patients were treated at the 90 mg/m2 dose level, and six patients were treated at the 120 mg/m2 dose level. A total of 24 cycles of bendamustine were administered: all treated patients received at least two treatment cycles, and most patients received the maximum three cycles (two patients in the 90 mg/m2 and four patients in the 120 mg/m2 group).
Safety. Overall, bendamustine was well tolerated. No DLT was observed at either dose level (Table 2); therefore, the MTD of bendamustine could not be determined. Grade 3/4 events (excluding lymphopenia, which was observed in all patients) were neutropenia in three patients (one at 90 mg/m2 and two at 120 mg/m2) and leukopenia in four patients (one at 90 mg/m2 and three at 120 mg/m2) (Table 2). Other hematologic toxicities included grade 1/2 thrombocytopenia (8/9 patients) and grade 1/2 anemia (8/9 patients). Supportive treatment with G‐CSF was not required in any patients.
Table 2.
Dose‐limiting and other grade 3/4 toxicity observed during bendamustine treatment
| Dose level | DLT criteria | No. of cases | Other grade 3/4 toxicities (non–dose limiting) |
|---|---|---|---|
| 90 mg/m2 | Grade 4 neutropenia† | 0 | Lymphocytopenia: 1 grade 4, 2 grade 3 Neutropenia: 1 grade 3 Leukopenia: 1 grade 3 |
| Platelets <10 000/mm3‡ | 0 | ||
| Other grade 4 hematologic toxicity§ | 0 | ||
| Grade ≥3 non‐hematologic toxicity | 0 | ||
| 120 mg/m2 | Grade 4 neutropenia† | 0 | Lymphocytopenia: 3 each grades 3 and 4 Neutropenia: 1 each grades 3 and 4 Leukopenia: 3 grade 3 |
| Platelets <10 000/mm3‡ | 0 | ||
| Other grade 4 hematologic toxicity§ | 0 | ||
| Grade ≥3 non‐hematologic toxicity | 0 |
†Grade 4 neutropenia lasting >1 week and accompanied by fever (38°C or higher). ‡Grade 4 thrombocytopenia or hemorrhage requiring platelet transfusion. §Any other grade 4 hematological toxicity excluding lymphopenia and differential white blood cell count (%).
Non‐hematologic toxicities were exclusively grades 1 and 2. The most common of these were anorexia (9/9 patients), nausea (9/9 patients), constipation (5/9 patients; one at 90 mg/m2 and four at 120 mg/m2), diarrhea (4/9 patients; two at 90 mg/m2 and two at 120 mg/m2), fatigue (4/9 patients; two at 90 mg/m2 and two at 120 mg/m2), and vomiting (3/9 patients; one at 90 mg/m2 and two at 120 mg/m2).
One serious adverse event was observed: one patient in the 120 mg/m2 group was diagnosed with grade 2 interstitial pneumonia (IP) during the third cycle of treatment, based on CT findings and symptoms including exertional breathlessness, palpitation, and chest pain. Although interstitial change and shadow were observed on the chest CT performed at study entry, a causal relationship with bendamustine could not be ruled out. The patient died of lymphoma progression and Pneumocystis jiroveci after subsequent treatment with intensive polychemotherapy consisting of cyclophosphamide, doxorubicin, vincristine, prednisolone, high‐dose methotrexate, and rituximab.
Efficacy. Among the nine bendamustine‐treated patients, eight (89%) responded to treatment: 3/3 patients in the 90 mg/m2 group and 5/6 in the 120 mg/m2 group (Table 3). Two patients (22%; both in the 90 mg/m2 group) achieved a CRu. Seven of eight patients with follicular lymphoma responded (one CRu and six PR); a CRu was achieved in the patient with MCL.
Table 3.
Response to bendamustine
| n | Best response, n | CR/CRu, % | ORR, % | |||
|---|---|---|---|---|---|---|
| CR | CRu | PR | ||||
| 90 mg/m2 | 3 | 0 | 2 | 1 | 67 | 100 |
| 120 mg/m2 | 6 | 0 | 0 | 5 | 0 | 83 |
| All patients | 9 | 0 | 2 | 6 | 22 | 89 |
CR, complete response; CRu, complete response unconfirmed; ORR, overall response rate; PR, partial response.
Pharmacokinetics. The mean (±SD) C max of bendamustine was 7.2 ± 3.3 μg/mL and 8.6 ± 4.5 mg/mL following administration of 90 and 120 mg/m2 doses, respectively (Table 4). In both dose groups, the C max was observed near the end of bendamustine administration, at approximately 1 h (Fig. 2).
Table 4.
Pharmacokinetic parameters of bendamustine and its metabolites†
| n | Measured species | C max (μg/mL) | t max (h) | t 1/2 (h) | AUC (μg·h/mL) | |
|---|---|---|---|---|---|---|
| 90 mg/m2 | 3 | Bendamustine | 7.2 ± 3.3 | 0.83 ± 0.29 | 0.53 ± 0.06 | 8.3 ± 3.6 |
| M3 | 0.25 ± 0.07 | 1.3 ± 0.00 | 0.63 ± 0.06 | 0.36 ± 0.11 | ||
| M4 | 0.05 ± 0.02 | 1.3 ± 0.00 | 0.57 ± 0.06 | 0.07 ± 0.03 | ||
| 120 mg/m2 | 6 | Bendamustine | 8.6 ± 4.5 | 0.92 ± 0.20 | 0.47 ± 0.05 | 10.2 ± 5.8 |
| M3 | 0.38 ± 0.05 | 1.2 ± 0.15 | 0.73 ± 0.23 | 0.64 ± 0.09 | ||
| M4 | 0.07 ± 0.02 | 1.2 ± 0.20 | 0.53 ± 0.05 | 0.12 ± 0.04 |
†Values reported as mean ± SD. AUC, area under the blood concentration‐time curve; C max, peak concentration; t max, time to peak concentration; t 1/2, half‐life.
Figure 2.
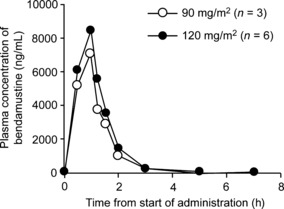
Mean plasma concentration of bendamustine over time. Plasma concentration of bendamustine (ng per mL of blood) was measured at 0.5, 1, 1.25, 1.5, 2, 3, 5, and 7 h after the start of administration. In both the 90 mg/m2 group (open circles) and the 120 mg/m2 group (filled circles), peak bendamustine concentration occurred at approximately 1 h, coinciding with the completion of administration.
The mean C max of M3 and M4, the two known active metabolites of bendamustine, was 3.4–4.4% and 0.6–0.9% of the C max of the parent molecule, respectively. The t max for both metabolites was 1.2–1.3 h, approximately 20 min after the t max of the unchanged drug. The AUC for unchanged bendamustine was 8.3 ± 3.6 and 10.2 ± 5.8 μg·h/mL in patients receiving 90 and 120 mg/m2, respectively.
Bendamustine was rapidly eliminated from the plasma, with a mean half‐life of 28–32 min in the two dose groups. The mean half‐lives of M3 (38–44 min) and M4 (32–34 min) were similar to that of unchanged bendamustine. Mean (±SD) Vz was 16.7 ± 8.7 L. Plasma Cl was 24.1 ± 13.3 L/h.
At the 90 mg/m2 dose level, 3.7, 0.3, and 0.1% of the administered dose was excreted in the urine unchanged and as M3 and M4 metabolites, respectively. At the 120 mg/m2 dose level, corresponding values were 1.6, 0.2, and 0.1%.
Discussion
In this phase I study, bendamustine was well tolerated in patients with relapsed or refractory indolent B‐NHL and MCL. DLT was not observed at either dose level studied. The most clinically significant adverse event was reversible grade 3/4 neutropenia, observed in 33% of patients. This finding is supported by the recent North American phase II trials in indolent B‐NHL patients, which reported grade 3/4 neutropenia in 54–61% of patients.( 5 , 6 ) Grade 3/4 thrombocytopenia and anemia, which were reported at 25 and 10–12%, respectively, in the two North American phase II trials, were not observed in this study, possibly due in part to the smaller number of patients in this study. The non‐hematologic toxicity profile of bendamustine was excellent, with no grade 3/4 events observed. Common grade 1/2 events were mainly gastrointestinal, which is consistent with previous results in this population.( 4 , 5 , 6 , 7 )
Grade 2 IP was reported as a serious adverse event in one patient, and a causal relationship with bendamustine treatment could not be ruled out. Other cases of bendamustine‐induced IP have not been specifically documented in the literature; however, in the North American pivotal trial of bendamustine 120 mg/m2, four patients (4%) experienced pneumonia as a serious adverse event resulting in death,( 6 ) while in the North American phase II trial, four patients (5%) experienced non‐fatal grade 3 pneumonia.( 5 ) These findings suggest that bendamustine may be associated with a risk of serious infectious complications, including pneumonia.
Bendamustine showed promising efficacy, with 89% of patients demonstrating an objective response, including a CRu in two patients (22%). These response rates were similar to 73–82% ORR and 11–39% CR/CRu observed in indolent B‐NHL patients in North American and European phase II trials.( 4 , 5 , 6 , 7 ) High response rates were observed at both dose levels. It is worth noting that a CRu was achieved in the patient with relapsed MCL. Although conclusions cannot be drawn based on one patient, this observation is consistent with other studies that have demonstrated the efficacy of bendamustine‐based regimens in MCL patients.( 8 , 17 , 21 )
Mean peak plasma bendamustine concentrations of 7.2 and 8.6 μg/mL were reached with 90 and 120 mg/m2 dose levels, respectively; these findings are comparable to peak plasma levels of 8.9–9.0 μg/mL observed after administration of 120 mg/m2 bendamustine in two patients with solid tumors, despite different infusion times.( 10 ) In vitro studies have determined the IC50 of bendamustine in lymphoma cell lines to be 20 μm,( 3 ) which corresponds to 7.2 μg/mL; therefore, plasma concentrations reached with the 120 mg/m2 dose should be sufficient to achieve an antitumor effect. Both the C max and AUC of bendamustine and its metabolites increased in a dose‐dependent manner.
Bendamustine was rapidly eliminated. The peak plasma concentration of bendamustine approximately coincided with the end of the 60‐min intravenous infusion; correspondingly, shorter t max (32–35 min) has been reported with infusion durations of 30 min.( 10 , 11 ) The t 1/2 of bendamustine observed in this study (28–32 min) was similar to that reported previously (32–41 min).( 10 , 11 , 22 , 23 ) Neither the t max nor the t 1/2 displayed dose dependency. However, patients with renal or hepatic impairment were not included in this study and no formal studies have been published in this population.
In conclusion, bendamustine was safe and well tolerated in Japanese patients with relapsed or refractory indolent B‐NHL and MCL when administered at 90 or 120 mg/m2 on two consecutive days every 3 weeks. Taking into consideration the toxicity profile, pharmacokinetic data, and ORR observed in this study, a dose of 120 mg/m2 is recommended for phase II trials.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AUC
area under the blood concentration‐time curve
- CR
complete response
- CRu
complete response unconfirmed
- MCL
mantle cell lymphoma
- ORR
overall response rate
- PR
partial response
- WHO
World Health Organization
Acknowledgments
The authors thank the patients, doctors, nurses, and staff members who participated in this multicenter trial for their excellent cooperation. Participating institutions of the Japanese Bendamustine Lymphoma Study Group: National Cancer Center Hospital, Tokai University, Nagoya Daini Red Cross Hospital, and Kyoto Prefectural University of Medicine. Protocol Committee members: Drs Kenichi Ishizawa (Tohoku University), Kensei Tobinai, Takashi Watanabe, Kiyoshi Ando, Michinori Ogura, and Masafumi Taniwaki. Medical writing assistance was provided by Jill Luer, PharmD, and Janis Leonoudakis, PhD, of ApotheCom, with support from Symbio Pharmaceuticals and Eisai Co. The authors, however, were fully responsible for the content and editorial decisions for this manuscript.
References
- 1. Strumberg D, Harstrick A, Doll K, Hoffmann B, Seeber S. Bendamustine hydrochloride activity against doxorubicin‐resistant human breast carcinoma cell lines. Anticancer Drugs 1996; 7: 415–21. [DOI] [PubMed] [Google Scholar]
- 2. Niemeyer CC, Bailey B, Riefert J. SDX‐105 (bendamustine is a clinically active chemotherapeutic agent with a distinct mechanism of action. Proc Am Assoc Cancer Res 2004; 45: 1129. [Google Scholar]
- 3. Leoni LM, Bailey B, Reifert J et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 2008; 14: 309–17. [DOI] [PubMed] [Google Scholar]
- 4. Heider A, Niederle N. Efficacy and toxicity of bendamustine in patients with relapsed low‐grade non‐Hodgkin’s lymphomas. Anticancer Drugs 2001; 12: 725–9. [DOI] [PubMed] [Google Scholar]
- 5. Friedberg JW, Cohen P, Chen L et al. Bendamustine in patients with rituximab‐refractory indolent and transformed non‐Hodgkin’s lymphoma: results from a phase II multicenter, single‐agent study. J Clin Oncol 2008; 26: 204–10. [DOI] [PubMed] [Google Scholar]
- 6. Kahl B, Carbone PP, Bartlett N, Leonard J, Chen L. Bendamustine is effective therapy in patients with rituximab‐refractory indolent B‐cell non‐Hodgkin’s lymphoma: results from a multicenter study. Cancer 2010; 116: 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bremer K. High rates of long‐lasting remissions after 5‐day bendamustine chemotherapy cycles in pre‐treated low‐grade non‐Hodgkin’s‐lymphomas. J Cancer Res Clin Oncol 2002; 128: 603–9. [DOI] [PubMed] [Google Scholar]
- 8. Robinson KS, Williams ME, Van Der Jagt RH et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B‐cell and mantle cell non‐Hodgkin’s lymphoma. J Clin Oncol 2008; 26: 4473–9. [DOI] [PubMed] [Google Scholar]
- 9. Rummel MJ, Niederle N, Maschmeyer G, Banat A, Von Gruenhagen U. Bendamustine plus rituximab is superior in respect of progression free survival and CR rate when compared to CHOP plus rituximab as first‐line treatment of patients with advanced follicular, indolent, and mantle cell lymphomas: final results of a randomized phase III study of the Stil (Study Group Indolent Lymphomas, Germany). Blood (ASH Annual Meeting Abstracts) 2009; 114: 405. [Google Scholar]
- 10. Rasschaert M, Schrijvers D, Van den Brande J et al. A phase I study of bendamustine hydrochloride administered day 1 + 2 every 3 weeks in patients with solid tumours. Br J Cancer 2007; 96: 1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rasschaert M, Schrijvers D, Van den Brande J et al. A phase I study of bendamustine hydrochloride administered once every 3 weeks in patients with solid tumors. Anticancer Drugs 2007; 18: 587–95. [DOI] [PubMed] [Google Scholar]
- 12. Schoffski P, Hagedorn T, Grunwald V et al. Repeated administration of short infusions of bendamustine: a phase I study in patients with advanced progressive solid tumours. J Cancer Res Clin Oncol 2000; 126: 41–7. [DOI] [PubMed] [Google Scholar]
- 13. Bergmann MA, Goebeler ME, Herold M et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica 2005; 90: 1357–64. [PubMed] [Google Scholar]
- 14. Lissitchkov T, Arnaudov G, Peytchev D, Merkle K. Phase I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre‐treated patients with B‐chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy. J Cancer Res Clin Oncol 2006; 132: 99–104. [DOI] [PubMed] [Google Scholar]
- 15. Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of Hematopoietic and Lymphoid Tissues, 3rd edn. Geneva, Switzerland: International Agency for Research on Cancer (IARC) Press; 2001: 119–187. [Google Scholar]
- 16. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55. [PubMed] [Google Scholar]
- 17. Rummel MJ, Al‐Batran SE, Kim SZ et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low‐grade non‐Hodgkin’s lymphoma. J Clin Oncol 2005; 23: 3383–9. [DOI] [PubMed] [Google Scholar]
- 18. Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda, MD: National Cancer Institute, US National Institutes of Health; 2006. Available from URL: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf [Google Scholar]
- 19. Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non‐Hodgkin’s lymphomas. NCI sponsored international working group. J Clin Oncol 1999; 17: 1244–53. [DOI] [PubMed] [Google Scholar]
- 20. Teichert J, Baumann F, Chao Q et al. Characterization of two phase I metabolites of bendamustine in human liver microsomes and in cancer patients treated with bendamustine hydrochloride. Cancer Chemother Pharmacol 2007; 59: 759–70. [DOI] [PubMed] [Google Scholar]
- 21. Herold M, Schulze A, Niederwieser D et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non‐Hodgkin’s lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO# 19). J Cancer Res Clin Oncol 2006; 132: 105–12. [DOI] [PubMed] [Google Scholar]
- 22. Owen JS, Melhem M, D’Andrea D, Darwish M. Population pharmacokinetics of bendamustine and metabolites in patients with indolent non‐Hodgkin lymphoma. Pharmacol Ther 2008; 83(Suppl 1): S54. [Google Scholar]
- 23. Matthias M, Preiss R, Sohr R, Possinger K. Pharmacokinetics of bendamustine in patients with malignant tumors. Proc Am Soc Clin Oncol 1995; 14: 458. [Google Scholar]


