Abstract
Nephronectin (POEM) was originally identified as a factor involved in tissue morphogenesis. POEM has several characteristics of a matrix protein including an arg‐gly‐asp binding domain site that is recognized by integrins. Recently, deregulation of POEM was found in breast cancer. We therefore speculate that deregulation of POEM expression plays a role in the development or progression of malignant melanoma. Thus, we evaluated melanoma cell lines and tissue samples of malignant melanoma for POEM transcription. We found that POEM expression was reduced or lost in most cell lines and in all tumor samples analyzed. Reduced POEM expression occurred as early as in primary tumors detected by both immunohistochemical and reverse transcription‐polymerase chain reaction analyses. Functional assays with stable POEM transfected cell lines revealed that POEM expression increased cell adhesion and decreased cell migration and invasion supporting a role of POEM in tumor progression. Interestingly, integrin α‐8 expression, which was described as a receptor for POEM, is enhanced in malignant melanoma. Our studies thus indicate that loss of POEM expression may contribute to melanoma progression. (Cancer Sci 2008; 99: 229–233)
Progressive invasion into the dermis and subsequent metastatic dissemination are the hallmarks of melanoma progression and characterize malignant melanoma as the most aggressive form of skin cancer.( 1 ) Ultraviolet‐ (UV) light exposure and genetic susceptibility are considered important predisposing factors that trigger a continuous proliferation of the melanocytes, leading to the development of melanoma.( 2 , 3 , 4 ) Several integrins and matrix proteins are known to play a role in melanoma development and progression. Fibronectin is one of the matrix molecules that was found to be strongly upregulated in malignant melanoma in comparison to melanocytes.( 5 , 6 ) Just recently osteopontin was shown to be involved in malignant melanoma and to be useful as a prognostic marker.( 5 , 7 , 8 , 9 )
PreOsteoblast EGF‐like repeat protein with MAM domain (POEM, alternative: nephronectin) was originally identified in developing mouse organs particularly prominent at epithelial–mesenchymal interfaces in tissues undergoing morphogenesis.( 10 , 11 ) The protein was determined to be associated with cells or with the extracellular matrix but not found in culture medium leading to the hypothesis of POEM as a matrix protein binding to the cell surface. This is in accordance with an arg‐gly‐asp binding domain integrin binding site found in the amino acid sequence and a study showing that integrin α‐8 β‐1 binds to POEM.( 11 ) Binding to other integrin molecules was not intensively characterized until today. In a recent study by Eckhardt et al. POEM was first described to be implicated in cancer.( 12 ) They applied a genomic analysis to cell lines derived from a spontaneous breast cancer model in mice. POEM expression was shown to be clearly related to metastasis. In breast cancer, strong POEM expression was found in the tumor epithelium of high‐metastasis tumors. A decrease of POEM expression in a highly aggressive cell line inhibited spontaneous metastasis. We therefore became interested in analyzing the potential role of POEM in malignant melanoma.
Materials and Methods
Cells and cell culture. The melanoma cell lines Mel Im, Mel Ei, Mel Wei, Mel Ho, Mel Juso, SK Mel28, SK Mel3, HMB2 and HTZ19d were described previously.( 13 ) The cell lines Mel Ei, Mel Wei, Mel Ho, HMB2 and Mel Juso were derived from a primary cutaneous melanoma. Mel Im, SK Mel3, SK Mel28 and HTZ19d were derived from metastases of malignant melanomas.( 14 ) Cells were maintained in Dulbecco's modified eagle medium supplemented with penicillin (400 U/mL), streptomycine (50 µg/mL), L‐glutamine (300 µg/mL) and 10% fetal calf serum (FCS; Sigma, Deisenhofen, Germany) and split at a 1:5 ratio every 3 days. Normal human epidermal melanocytes (NHEM) derived from normal skin (Promocell, Heidelberg, Germany) were cultivated in melanocyte medium MGM‐3 (Gibco, Eggenstein, Germany) under a humidified atmosphere of 5% CO2 at 37°C. Purity of melanocytes was proven by Affimetrix cDNA array confirming expression of melanocytic markers like tyrosinase, TRP‐1 and E‐Cadherin and lack of keratinocytic markers like cytokeratin 5, –10 or –14 and keratinocyte growth factor receptor. Cells were used between passages three to six and not later than 3 days after trypsinization. 5‐azacytidine treatments (10 µm, 24 h) for the analysis of promoter methylation were carried out as previously described.( 15 )
Construction of expression vectors. The POEM expression vector and that with the mutated RGD sequence (POEM RGE) were a generous gift from the group of Ken‐ichi Tezuka (Research Institute for Biological Sciences, Science University of Tokio).( 10 ) The murine POEM cDNA was subcloned into pcDNA3 including a His tag.( 12 )
Isolation of tumorous and non‐tumorous human tissues. Tissue samples from primary human melanoma, and melanoma metastasis obtained from patients undergoing surgical treatment were immediately snap frozen and stored at –80°C. Melanoma cells were selectively retrieved from tumor samples with PALM microlaser technology (PALM, Bernried, Germany) under microscopic control. Informed consent was obtained from all patients and the study was approved by the local Ethics Committee.
Expression analysis. Isolation of total cellular RNA from cultured cells and tissues and reverse transcription (RT) were carried out as described previously.( 16 ) Quantitative real time‐polymerase chain reaction (PCR) was carried out with specific primers for POEM (hPOEMfor: AGC CAA CAA CAA GAC CTA CAC; hPOEM rev: GCC GTG GAA TGA ACA CAA TCT C; based on human POEM: NM_001033047; hITGA8for: CAA ATC TCC TGT GCA GTG GGA CGA CT, hITGA8 rev: CTG TTC CCT GTC GGT CAT GTC CTC C) and β‐actin applying Lightcycler technology (Roche, Mannheim, Germany) as described.( 16 ) Specificity of amplification was confirmed by sequencing of the PCR product.
Protein analysis in vitro and in vivo. Protein extraction, analysis and Western blotting were carried out as described previously( 17 ) applying the following primary antibodies: polyclonal anti‐POEM (1:500, Cosmo Bio, Carlsbad, USA), or antiβ‐actin (1:5000; Sigma, Deisenhofen, Germany).
Immunohistochemistry of paraffin‐embedded tissues applying anti‐POEM antibody: (1:75) anti integrin α‐8 (1:100, Santa Cruz, USA) or anti‐tyrosinase was carried out as described.( 18 )
Proliferation, migration and invasion assay. Cell proliferation was determined by means of XTT assays (Roche, Mannheim, Germany). Migration and invasion assays were carried out as previously described.( 16 ) Colony forming and ‘scratch’ assay were carried out as previously described.( 19 )
Transfection of melanoma cell lines. Expression plasmids of POEM and POEM‐RGE were used for transient and stable transfection applying the Lipofectamin plus method (Invitrogen, Carlsbad, USA).( 19 ) To achieve stably transfected cells, cells were cultured under selective conditions using G418 (Sigma) in a concentration of 50 µg/mL. Controls received pcDNA3 alone. After 25 days of selection, individual G418‐resistant colonies were subcloned. POEM expression levels of these clones were determined by RT‐PCR and Western blot analysis.
Statistical analysis. Values are presented as the means SEM and statistical differences were determined using Student's t‐test.
Results
POEM expression is down‐regulated in melanoma. Nephronectin is expressed in fetal cochlea, eye, heart, lung, and in embryonic kidney cells. It is also expressed in adult lung, kidney, brain, pregnant uterus, placenta, thyroid gland, and blood vessels and was originally identified as a tumor oncogene that may have a role in the promotion of metastasis of highly metastatic breast cancer cells.
Here, we analyzed the expression and function of POEM in malignant melanoma. Initially, we evaluated POEM mRNA expression in nine human melanoma cell lines compared to normal human epidermal melanocytes (NHEM) using quantitative RT‐PCR analysis (Fig. 1a). Strong reduction or loss of POEM expression was found in all tumor cell lines compared to NHEM except of Mel Juso. In line with the reduced POEM mRNA levels, loss or strong reduction of POEM expression was observed by Western Blot (data not shown). This finding was in accordance with the loss of expression determined in melanoma tissues (Fig. 1b). Here, POEM mRNA expression was quantified in melanoma cells isolated by microdissection from primary melanomas of seven patients (PT1–PT7), and three distant metastases of malignant melanomas (M1, M2, M3), respectively. As compared to NHEM and RNA extracted from the basal cell layer of the epidermis, POEM mRNA expression was almost undetectable in the primary melanomas and in distant metastasis.
Figure 1.
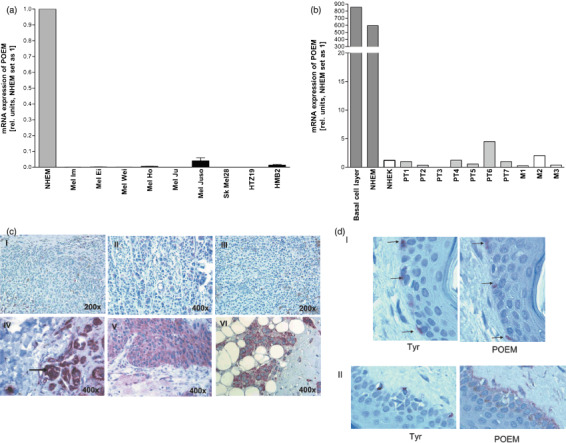
Reduced nephronectin (POEM) expression in malignant melanoma. POEM expression was measured during the development of malignant melanoma by (a) quantitative real time polymerase chain reaction (PCR) analysis in nine different human melanoma cell lines as compared with normal human epidermal melanocytes (NHEM) and (b) quantitative real time PCR analysis for seven primary melanomas and three metastases in comparison with NHEM. (c) Immunohistochemistry was carried out to detect the POEM expression. Primary malignant melanoma (I, II), melanoma metastasis (III) and nevi (IV, V) are presented. As a positive control intensive staining in mamma carcinoma is shown (V). (d) Staining of tyrosinase (Tyr) and POEM on serial section of normal skin was used to confirm POEM expression in melanocytes in situ (marked by arrows) (I). In areas of melanocyte accumulation also staining of the basal membrane (arrow) was detectable (II).
In agreement with the reduced POEM mRNA levels in primary melanomas and metastases, POEM protein expression was strongly reduced in malignant melanoma (I, II) and melanoma metastasis (III) as determined by immunohistochemistry, whereas nevi POEM expression was detectable (IV, V) (Fig. 1c). To confirm POEM expression in melanocytes in vivo, co‐immunofluorescence staining of POEM and tyrosinase was carried out on normal skin (Fig. 1d). Here, expression of POEM was found in melanocytes of the basal cell layer whereas keratinocytes are POEM negative (Fig. 1d.I). In some areas with a high amount of melanocytes also staining of the basal membrane as expected by a secreted matrix molecule was observed (Fig. 1d.II).
Regulation of POEM expression. Since loss of expression of the POEM gene by promoter methylation can be speculated, we analyzed silenced gene expression in the tumor cell lines. Cells were exposed to 5‐azacytidine for demethylation and POEM expression was quantified. In none of the cell lines induction of POEM expression was observed after demethylation indicating that expression of POEM is most likely reduced by transcriptional control of the promoter (data not shown).
Analysis of the POEM promoter region revealed binding sites of the transcriptional repressor Snail and AP‐2. Snail is known to be upregulated and AP‐2 to be lost in malignant melanomas and both proteins were found to be important in melanoma progression.( 20 , 21 , 22 , 23 ) We therefore determined the effect of Snail and AP‐2 on POEM expression in melanoma cells. Mel Im melanoma cells stably transfected with antisense Snail expression plasmids( 23 ) or transiently transfected with an AP‐2 expression construct revealed no regulation of POEM mRNA expression. Obviously, other regulators of transcription must be responsible for reduction of POEM expression in malignant melanoma.
POEM regulates migratory potential of melanoma cells. Next, we aimed to assay whether altered POEM levels have an effect on proliferation and invasion of melanoma cells. We therefore carried out functional experiments using mock and POEM stably transfected cells. As an additional control POEM carrying a mutation was used changing the RGD side known to interact with integrins into an RGE motive. Cell clones were analyzed by quantitative RT‐PCR and Western Blotting for re‐expression of POEM or the POEM mutant (Fig. 2). These assays confirmed expression of POEM or POEM RGE in the respective cell clones.
Figure 2.
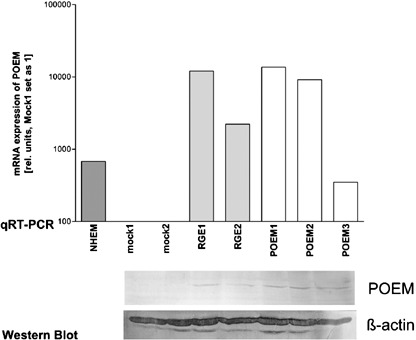
Generation of stable nephronectin (POEM) expressing cell clones. Three stable transfected POEM Mel Im cell clones and two arg‐gly‐asp binding domain sequence‐mutated clones (RGE1 and RGE2) were generated and expression was controlled by quantitative real time polymerase chain reaction and Western Blot.
Proliferation assays did not reveal any differences comparing POEM re‐expressing to mock or RGE‐POEM transfected cells (data not shown). In accordance, colony formation was not changed significantly (Fig. 3a). Migration assays using Boyden Chambers were carried out to examine the effect of POEM on the migratory potential. Comparing mock treated cells, POEM‐RGE expressing cells, and POEM over‐expressing cells, in two clones a significant effect on migration was observed, and a trend to reduced migration of the third POEM re‐expressing cell clones (POEM1) was visible (Fig. 3b). In so called ‘scratch assays’ a clear reduction of migration in a two dimensional system was observed for the POEM re‐expressing cells, whereas no difference was found comparing mock and POEM‐RGE cell clones (Fig. 3c). The invasive potential of the cells was measured in a Boyden Chamber invasion model using matrigel as a basement membrane equivalent (Fig. 3d). Clones with re‐expression of POEM revealed a strongly reduced invasive potential.
Figure 3.
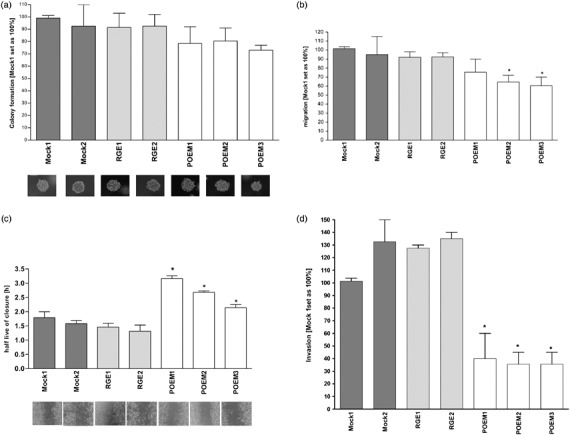
Functional assays with the Mel Im cell clones RGE1, RGE2, POEM1, POEM2 and POEM3 in comparison to the controls mock1 and mock2. (a) Analysis of cell–cell and cell–matrix independent growth in soft agar colony formation assay. The graph shows the colony sizes in per cent and mock1 cell clone was set as 100%. (b) Analysis of migration in Boyden Chamber assays. The mock1 cell clone was set as 100%. (*P < 0.05). (c) Analysis of migration in a two dimensional ‘scratch assay’ system where the closure of a scratched ‘wound’ was measured in half live of closure (hours). The wild type POEM‐expressing cell clones showed a slower wound closure in comparison to the controls. (*P < 0.05). (d) Analysis of invasive potential in Boyden Chamber assays. The POEM‐expressing cell clones and the RGD sequence mutated cell clones were analyzed in comparison to the mock cell clones. The control mock1 cell clone was set as 100%. (*P < 0.05).
The only known receptor of POEM today is integrin α‐8 β‐1, however, other integrins can also potentially bind POEM due to the RGD site. We analyzed integrin α‐8 expression in malignant melanoma. We revealed strong expression in RNAs extracted from primary tumors and metastasis in comparison to primary melanocytes (NHEM) except of PT3 (Fig. 4a). Analysis of gene expression data in GEOprofiles revealed that expression of integrin α‐8 is supported by GDS1989.( 24 ) Here, upregulation of integrin α‐8 during progression of malignant melanoma was confirmed (Fig. 4b). To confirm this finding we stained integrin α‐8 in skin, primary melanoma and melanoma metastasis (Fig. 4c). Here, lack of integrin α‐8 expression in melanocytes was revealed, whereas melanoma samples showed integrin α‐8 expression.
Figure 4.
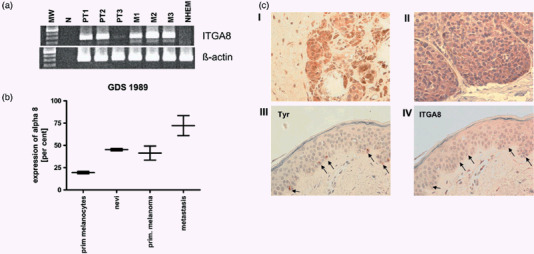
Increased integrin α‐8 expression in malignant melanoma. Integrin α‐8 expression was measured in samples of malignant melanoma by (a) quantitative real time polymerase chain reaction (PCR) analysis in three primary melanomas and three melanoma metastasis in comparison to normal human epidermal melanocytes (NHEM) and (b) by GEOprofiles array GDS1989. Integrin α‐8 expression is upregulated during progression of malignant melanoma. (c) Immunohistochemistry was carried out to detect the integrin α‐8 expression in melanoma in situ. Primary malignant melanoma (I) and melanoma metastasis (II) are presented. Staining of tyrosinase (Tyr) and ITGA8 on serial section of normal skin was used to confirm absence of integrin α‐8 expression in melanocytes in situ (marked by arrows).
Discussion
Generally, as a result of its structure (EGF‐like repeat structure, RGD sequence) POEM belongs to the family of extracellular matrix molecules (ECM) and has been implicated in cell–matrix adhesion, cell spreading, survival‐promoting activities and morphogenesis. Its expression was found in the skin by in situ hybridization using embryonic mice day 16.5. The localization of POEM is on the cell surface, where it can bind with the help of the MAM‐domain and an RGD site.( 10 , 11 ) Eckhardt et al. (2005) revealed expression of POEM during cancer development in highly metastatic breast cancer cells that metastasize into kidney, lung, and bone in a (4T1.2) mouse model. They describe the role of POEM in promoting spontaneous breast tumor metastasis.
In contrast to breast cancer we found a reduction of POEM expression in malignant melanoma in comparison with primary melanocytes. We detected no correlation between loss of POEM expression and the state of tumor progression, however, nevi showed expression of POEM. This suggested that reduction of POEM expression may be not associated to proliferation but to transformation of melanocytes. In the case of malignant melanoma POEM expression was generally lost in primary tumors and metastasis leading to the hypothesis that loss of POEM expression is an early event in melanoma development.
Interestingly, we revealed a potential role of POEM in regulating cell migration and invasion, but not in proliferation, which is in agreement with the function in breast cancer. Scratch assays and Boyden Chamber assays to determine migration and invasion displayed the importance of the RGD sequence for the tumor suppressor potential of POEM in malignant melanoma. The cell clones with wildtype POEM showed less invasive and migratory potential in comparison with the controls, whereas the potential is unchanged in clones that are stably transfected with RGD‐mutated‐POEM. In conclusion, the tumor suppressor function of POEM is dependent on the RGD site and therefore potentially on the regulation of cell adhesion via integrin expression.
Because of this, we analyzed integrin α‐8 expression, which is known to bind POEM. In malignant melanoma we determined strong expression of integrin α‐8 in cell lines and melanoma tissues. In this case the regulation of POEM and integrin α‐8 is antagonistic. This is in disagreement with data in breast cancer, where expression of POEM correlates with integrin α‐8 expression. In highly metastatic breast cancer cells the coexpression of α‐8 and POEM leads to transmission of the adhesive and survival potential of the cancer cells. Probably, the α‐8 integrin has other binding partners in malignant melanoma than POEM. Several integrins and matrix proteins are known to play a role in melanoma development and progression. Fibronectin is one of the matrix molecules, which was found to be strongly expressed in malignant melanoma in comparison to melanocytes.( 5 , 6 ) Just recently, osteopontin was shown to be involved in malignant melanoma and to be useful as a prognostic marker.( 5 , 7 , 8 , 9 ) These are two additional ECMs that are capable of connecting to α‐8. Interestingly, other integrins also know to bind to POEM as integrin α‐v‐β‐3 is known to be strongly induced in tumor progression of malignant melanoma.
In summary, we have characterized POEM as a tumor suppressor that is lost early in malignant melanoma development.
Acknowledgments
We are indebted to Dr J. Johnson (Institute for Immunology, Ludwig Maximilians University of Munich, Germany) for providing melanoma cell lines and to Robin Anderson (Cancer Biology Laboratory, Peter MacCallum Cancer Center, Melbourne, Australia) for providing the POEM constructs. AB was supported in part by grants from the Deutsche Forschungsgemeinschaft and the Deutsche Krebshilfe.
References
- 1. Marks R. Epidemiology of melanoma. Clin Exp Dermatol 2000; 25: 459–63. [DOI] [PubMed] [Google Scholar]
- 2. Curtin JA, Fridlyand J, Kageshita T et al . Distinct sets of genetic alterations in melanoma. N Engl J Med 2005; 353: 2135–47. [DOI] [PubMed] [Google Scholar]
- 3. De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res 2004; 64: 6372–6. [DOI] [PubMed] [Google Scholar]
- 4. Noonan FP, Recio JA, Takayama H et al . Neonatal sunburn and melanoma in mice. Nature 2001; 20: 271–2. [DOI] [PubMed] [Google Scholar]
- 5. Jaeger J, Koczan D, Thiesen HJ et al . Gene expression signatures for tumor progression, tumor subtype, and tumor thickness in laser‐microdissected melanoma tissues. Clin Cancer Res 2007; 13: 806–15. [DOI] [PubMed] [Google Scholar]
- 6. Gaggioli C, Robert G, Bertolotto C et al . Tumor‐derived fibronectin is involved in melanoma cell invasion and regulated by V600E B‐Raf signaling pathway. J Invest Dermatol 2007; 127: 400–10. [DOI] [PubMed] [Google Scholar]
- 7. Packer L, Pavey S, Parker A et al . Osteopontin is a downstream effector of the PI3‐kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinogenesis 2006; 27: 1778–86. [DOI] [PubMed] [Google Scholar]
- 8. Zhou Y, Dai DL, Martinka M et al . Osteopontin expression correlates with melanoma invasion. J Invest Dermatol 2005; 124: 1044–52. [DOI] [PubMed] [Google Scholar]
- 9. Geissinger E, Weisser C, Fischer P, Schartl M, Wellbrock C. Autocrine stimulation by osteopontin contributes to antiapoptotic signalling of melanocytes in dermal collagen. Cancer Res 2002; 62: 4820–8. [PubMed] [Google Scholar]
- 10. Morimura N, Tezuka Y, Watanabe N et al . Molecular cloning of POEM: A novel adhesion molecule that interacts with α8β integrin. J Biol Chem 2001; 276: 42 172–81. [DOI] [PubMed] [Google Scholar]
- 11. Brandenberger R, Schmidt A, Linton J et al . Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β in the embryonic kidney. J Cell Biol 2001; 154: 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckhardt BL, Parker BS, Van Laar RK et al . Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res 2005; 3: 1–13. [PubMed] [Google Scholar]
- 13. Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res 2005; 65: 448–56. [PubMed] [Google Scholar]
- 14. Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene, 2007; 26: 4158–70. [DOI] [PubMed] [Google Scholar]
- 15. Denk AE, Bettstetter M, Wild PJ et al . Loss of maspin expression contributes to a more invasive potential in malignant melanoma. Pigment Cell Res, 2007; 20: 112–19. [DOI] [PubMed] [Google Scholar]
- 16. Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene 2006; 25: 5027–36. [DOI] [PubMed] [Google Scholar]
- 17. Kuphal S, Wallner S, Schimanski CC et al . Expression of Hugl‐1 is strongly reduced in malignant melanoma. Oncogene 2006; 25: 103–10. [DOI] [PubMed] [Google Scholar]
- 18. Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK. Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene 2006; 26: 4158–70. [DOI] [PubMed] [Google Scholar]
- 19. Arndt S, Bosserhoff AK. TANGO is a tumor suppressor of malignant melanoma. Int J Cancer 2006; 119: 2812–20. [DOI] [PubMed] [Google Scholar]
- 20. Tellez CS, Davis DW, Prieto VG et al . Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein‐2α and protease‐activated receptor‐1 expression during melanoma progression. J Invest Dermatol 2007; 127: 387–93. [DOI] [PubMed] [Google Scholar]
- 21. Berger AJ, Davis DW, Tellez C et al . Automated quantitative analysis of activator protein‐2α subcellular expression in melanoma tissue microarrays correlates with survival prediction. Cancer Res 2005; 65: 11 185–92. [DOI] [PubMed] [Google Scholar]
- 22. Tellez C, McCarty M, Ruiz M, Bar‐Eli M. Loss of activator protein‐2alpha results in overexpression of protease‐activated receptor‐1 and correlates with the malignant phenotype of human melanoma. J Biol Chem 2003; 278: 46 632–42. [DOI] [PubMed] [Google Scholar]
- 23. Poser I, Dominguez D, De Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E‐cadherin expression in melanoma cells involves up‐regulation of the transcriptional repressor Snail. J Biol Chem 2001; 276: 24 661–6. [DOI] [PubMed] [Google Scholar]
- 24. Smith AP, Hoek K, Becker D. Whole‐genome expression profiling of the melanoma progression pathway reveals marked molecular differences between nevi/melanoma in situ and advanced‐stage melanomas. Cancer Biol Ther 2005; 4: 1018–29. [DOI] [PubMed] [Google Scholar]


