Abstract
Like gastric and intestinal mucins, the tight junction proteins called claudins can be used to determine the differentiation of gastric mucosa. We investigated the expression of claudins in gastric cancer and proposed a new claudin‐based gastric cancer classification system. The expression of gastric (claudin‐18) and intestinal (claudin‐3 and claudin‐4) claudins in non‐neoplastic gastric mucosa (with intestinal metatplasia [IM], 78 cases; without IM, 88 cases) and 94 gastric cancers was analyzed immunohistochemically, as was the expression of gastric (MUC5A and MUC6) and intestinal (CD10 and MUC2) mucins. Heterogeneous expression of claudin‐3, claudin‐4 and claudin‐18 was detected in advanced gastric cancer; however, there was no significant association between the claudins and the clinicopathological parameters. These gastric cancer tissues were also subclassified into claudin‐based phenotypes: gastric claudin (G‐CLDN), 28 cases (30%); intestinal claudin (I‐CLDN), 41 cases (44%); and unclassified claudin (U‐CLDN), 25 cases (26%). Interestingly, the U‐CLDN gastric cancers had worse malignancy grades, not only in size and invasiveness but also in potential metastatic ability and patient outcome. Although the mucin‐based gastric cancer classification was also assessed, no significant correlation was found between mucin production and clinicopathological parameters. These observations suggest that loss of claudin expression may enhance the grade of malignancy of gastric cancer in vivo. Classification of gastric cancers using gastric and intestinal claudins is a good biomarker for assessing the risk of poor prognosis. (Cancer Sci 2007; 98: 1014–1019)
Abbreviations:
- GC
gastric cancer
- G‐CLDN
gastric claudin
- G‐MUC
gastric mucin
- IM
intestinal metaplasia
- I‐CLDN
intestinal claudin
- I‐MUC
intestinal mucin
- TJ
tight junction
- U‐CLDN
unclassified claudin
- U‐MUC
unclassified mucin.
Based on a tendency of gland formation, GC is classified histologically as differentiated type versus undifferentiated type or as intestinal type versus diffuse type.( 1 , 2 ) Immunohistochemical examinations have demonstrated that gastric and intestinal mucin phenotypic cell markers are widely expressed in GC, irrespective of the histological characteristics: human gastric mucins (MUC5AC and MUC6), which are specifically expressed in gastric surface mucous cells and pyloric gland cells, and human intestinal mucins (CD10 and MUC2), which are closely correlated with mature intestinal epithelial cells and goblet cells, have been detected in various types of GC.( 3 , 4 ) Although tumors with gastric mucin expression are associated with poor patient outcome and greater malignancy potential in the incipient phase of invasion and metastasis compared with other tumor phenotypes,( 3 ) there is little understanding of whether or not mucin phenotypic classification could be used for evaluating tumor aggressiveness and the grade of GC malignancy.
The Cdx2 homeobox gene is important in the early differentiation, maintenance and proliferation of intestinal epithelial cells in mice.( 5 , 6 ) In fact, aberrant expression of Cdx2 has been observed consistently in IM of gastric mucosa and in a subset of GC.( 7 ) In addition, Cdx2‐mediated transactivation of the MUC2 promoter controls gastric cell differentiation.( 8 ) Thus, Cdx2 plays an important role in the aberrant intestinal differentiation program of IM and GC. Interestingly, Cdx2 can upregulate the levels of claudin‐2, a claudin TJ molecule, by activating the CLDN2 promoter.( 9 ) These findings suggest a possible relationship between mucin phenotype and claudins in controlling the differentiation of stomach epithelium, most likely by Cdx2 transcription activity.
TJs are located on the most apical side of the intercellular adherent structure of epithelial cells in the gastrointestinal tract,( 10 ) and claudins are crucial components of TJ in the formation of tightly connected cell sheets, the creation of physiological barriers separating the apical and basolateral spaces, and the control of electrolytes permeability across a paracellular barrier via the formation of hetero‐ or homodimers.( 11 , 12 ) Claudins also bind to ZO‐1, a TJ protein that promotes interaction between TJ and the actin cytoskeleton.( 13 ) Therefore, claudins are believed to determine cell polarity through cytoskeleton rearrangement.( 14 , 15 )
In general, the dissociation of cancer cells from the primary nests is a crucial step in metastasis. Suppression of cell‐to‐cell adhesiveness may trigger the release of cancer cells from primary cancer nests and confer invasive properties on tumors.( 16 ) Therefore, dysfunction of TJ by altered expression of claudins is thought to promote cancer cell invasion and metastasis.( 17 , 18 , 19 , 20 ) In the present study, on the hypothesis that disruption of TJs may enhance the grade of malignancy of cancer cells, we examined the expression of both ‘gastric’ (claudin‐18) and ‘intestinal’ (claudin‐3 and claudin‐4) claudins in primary GC at the invasive front.( 21 , 22 )
Materials and Methods
Tissue samples. A total of 94 cases of sporadic human GC surgically removed at Kobe University Hospital from 1995 to 2003 were used in this study. Formalin‐fixed and paraffin‐embedded specimens were used for immunohistochemical analyses. Informed consent was obtained from all patients. Histological examination was carried out according to the Japanese Classification of Gastric Carcinoma 2nd edn ( 23 ) along with the International Union Against Cancer classification.( 24 ) Non‐neoplastic gastric mucosa adjacent to GC (with IM, 78 cases; without IM, 88 cases) were also used to examine the expression of claudins and Cdx2.
Immunohistochemical analysis. Immunohistochemistry was carried out using the streptavidin–biotin–peroxidase method with an LSAB kit (DAKO, Carpinteria, CA, USA).( 25 ) Briefly, deparaffinized and rehydrated 4‐µm sections were autoclaved to retrieve antigenicity. After blocking endogenous peroxidase with H2O2 and non‐specific binding sites with bovine serum albumin, antibodies against claudin‐3, claudin‐4 and claudin‐18 (Zymed, San Francisco, CA, USA) were applied to sections as the primary antibody and incubated, as well as antibody against Cdx2 (BioGenex, San Ramon, CA, USA). Subsequently, sections were incubated with biotinylated goat antimouse–rabbit IgG and streptavidin conjugated to horseradish peroxidase (HRP). Chromogenic fixation was carried out by immersing the sections in a solution of 3,3‐diaminobenzidine tetrahydrochloride. The sections were then counterstained with Mayer's hematoxylin. Antibodies against gastric (MUC5AC [Novocastra, Newcastle‐upon‐Tyne, UK] and MUC6 [Novocastra]) and intestinal (MUC2 [Santa Cruz Biotechnology, Santa Cruz, CA, USA] and CD10 [Novocastra]) mucins were also used to determine the mucin‐based GC phenotypes.( 26 )
Phenotypic classification of GC according to the expression of claudins and mucins. The immunoreactivity of claudins in each primary GC at the invasive front was graded according to the number of stained cells and the staining intensity in individual cells: negative, almost no positive cells or <30% of tumor cells showed weak immunoreactivity; or positive, >30% of tumor cells showed intense immunoreactivity. The results of immunohistochemical analyses were evaluated by three independent observers (Y. M., S. S. and H. Y.) and all of the sections were scored twice to confirm the reproducibility of the results. According to the combination of claudin expression patterns, three ‘phenotypes’ were determined: the G‐CLDN phenotype, in which carcinomas expressed claudin‐18 but not claudin‐3 or claudin‐4; the I‐CLDN phenotype, in which carcinomas expressed claudin‐3 and/or claudin‐4 but not claudin‐18; and the U‐CLDN phenotype, in which carcinomas did not express any of these claudins. When a tumor expressed both a gastric claudin (claudin‐18) and intestinal claudins (claudin‐3 and claudin‐4), the predominant pattern of claudin expression was evaluated. Similarly, the mucin phenotype was estimated and cases were classified into three mucin phenotypes: the G‐MUC phenotype, in which carcinomas expressed MUC5AC and/or MUC6; the I‐MUC phenotype, in which carcinomas expressed CD10 and/or MUC2; and the U‐MUC phenotype, in which carcinomas did not express any of these mucins.( 3 , 26 , 27 )
Statistical analysis. We used χ2‐test to evaluate the relationship between claudin immunoreactivity and clincopathological characteristics in 94 cases of GC. Survival curves were drawn according to the Kaplan–Meier method, and differences between the curves were analyzed by applying the log‐rank test. P‐values less than 0.05 were considered statistically significant.
Results
Expression of claudin‐3, claudin‐4 and claudin‐18 in non‐neoplastic gastric mucosa and GC. The characteristics and differentiation of gastric mucosa in the presence or absence of IM were analyzed with the expression of claudins. In normal gastric mucosa, positive immunoreactivity was detected for claudin‐18 but not for claudin‐3 or claudin‐4. In IM, however, cells expressed both claudin‐3 and claudin‐4 but not claudin‐18, in accordance with Cdx2 expression (Fig. 1). The expression patterns of these claudins were correlated with the expression patterns of the gastric and intestinal mucins (Supplementary Fig. S1). Induction of claudin‐3 and claudin‐4 by Cdx2 was confirmed by transfection of a human Cdx2‐expressing vector in vitro (Supplementary Fig. S2).
Figure 1.
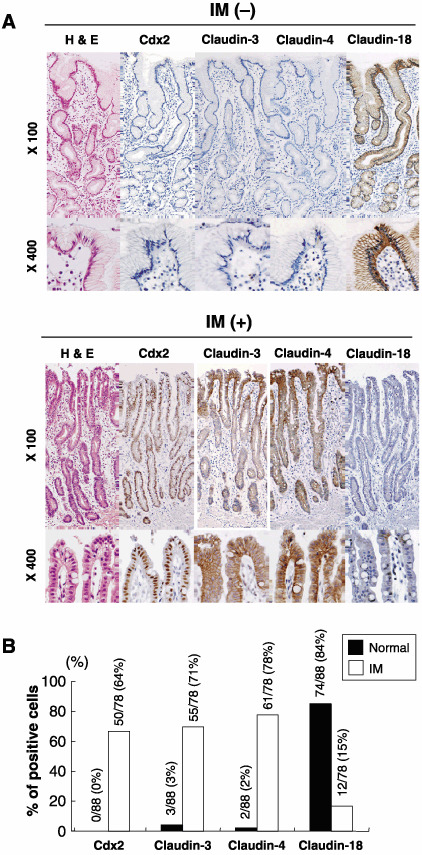
Expression of claudin‐3, claudin‐4 and claudin‐18 in non‐neoplastic gastric mucosa. (A) Representative illustrations of the expression of claudins and Cdx2 in normal pyloric gastric mucosa. (B) Summary of the expression of Cdx2, claudin‐3, claudin‐4 and claudin‐18 in non‐neoplastic gastric mucosa.
In GC samples, these claudins were mainly located in the cell surface, whereas claudins were distributed not only in the cell membrane but also in the cytoplasm (Fig. 2). In advanced GC, the immunoreactivity of claudins was heterogenous; strong immunoreactivity of claudins on the surface of GC tissues was decreased in the cancer cells at the invasive front (Fig. 2). The loss of claudin‐4 expression was correlated with advanced clinicopathological stage (P = 0.047); however, no statistical significance was observed for claudin‐3 and claudin‐18 expression (Supplementary Table S1).
Figure 2.
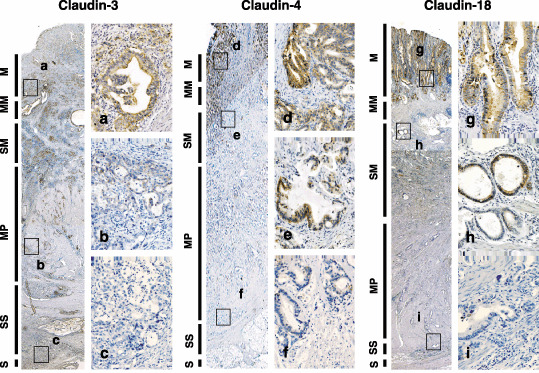
Heterogeneous expressions of claudin‐3, claudin‐4 and claudin‐18 in advanced gastric cancer. Representative results of the expression of claudin‐3 (×20; a–c, ×200), claudin‐4 (×20; d–f, ×200) and claudin‐18 (×20; g–i, ×200) are shown. M, mucosa; MM, muscularis mucosae; MP, muscularis propria; S, serosa; SM, submucosa; SS, subserosa.
Claudin‐based GC classification at the invasive front of GC and diagnostic implications. These GC were subclassified into the G‐CLDN (28 cases [30%]), I‐CLDN (41 cases [44%]) and U‐CLDN (25 cases [26%]) phenotypes. Statistically, the U‐CLDN phenotype of GC demonstrated a higher grade of malignancy involving tumor size (P = 0.010), depth of invasion (P = 0.003), venous vessel infiltration (P = 0.046) and lymph node metastasis (P = 0.028; Table 1). A comparative study of the relationship between the claudin‐based and mucin‐based phenotypic classifications is illustrated in Fig. 3. No significant change was detected between the MUC phenotypes and clinicopathological findings (Table 1).
Table 1.
Relationship between claudin and mucin phenotypes and clinicopathological parameters in gastric cancer
| CLDN phenotypes † | P‐value* | MUC phenotypes † | P‐values* | |||||
|---|---|---|---|---|---|---|---|---|
| G‐CLDN n (%) | I‐CLDN n (%) | U‐CLDN n (%) | G‐MUC n (%) | I‐MUC n (%) | U‐MUC n (%) | |||
| Total | 28 (30) | 41 (44) | 25 (26) | 22 (23) | 47 (50) | 25 (27) | ||
| Sex | ||||||||
| Male | 19 (20) | 30 (32) | 19 (20) | 0.793 | 16 (17) | 34 (35) | 18 (19) | 0.998 |
| Female | 9 (10) | 11 (12) | 6 (6) | 6 (7) | 13 (14) | 7 (8) | ||
| Age (years) | ||||||||
| ≥68 | 15 (16) | 13 (14) | 10 (10) | 0.191 | 10 (11) | 15 (16) | 12 (13) | 0.330 |
| <68 | 13 (14) | 28 (30) | 15 (16) | 12 (13) | 32 (33) | 13 (14) | ||
| Size (mm) | ||||||||
| ≥65 | 19 (20) | 31 (34) | 10 (11) | 0.010* | 14 (15) | 32 (33) | 15 (16) | 0.783 |
| <65 | 8 (8) | 10 (11) | 15 (16) | 8 (9) | 15 (16) | 10 (11) | ||
| Location ‡ | ||||||||
| Upper | 7 (8) | 12 (12) | 3 (3) | 0.541 | 5 (5) | 13 (14) | 4 (4) | 0.784 |
| Middle | 11 (12) | 18 (19) | 13 (14) | 10 (11) | 21 (21) | 11 (12) | ||
| Lower | 10 (10) | 11 (12) | 9 (10) | 7 (8) | 13 (14) | 10 (11) | ||
| Histological type ‡ | ||||||||
| W | 10 (10) | 19 (20) | 15 (16) | 0.332 | 8 (9) | 22 (23) | 14 (15) | 0.337 |
| P | 18 (19) | 22 (25) | 10 (10) | 15 (16) | 25 (25) | 11 (12) | ||
| Depth of invasion ‡ | ||||||||
| m + sm | 13 (14) | 8 (10) | 2 (2) | 0.003* | 8 (9) | 10 (11) | 4 (4) | 0.229 |
| mp + ss | 15 (16) | 33 (35) | 23 (23) | 14 (15) | 37 (38) | 21 (22) | ||
| Vessel infiltration | ||||||||
| Lymphatic vessels | ||||||||
| Negative | 7 (8) | 7 (8) | 1 (1) | 0.110 | 2 (2) | 9 (10) | 3 (3) | 0.645 |
| Positive | 21 (22) | 34 (36) | 24 (25) | 20 (21) | 47 (48) | 25 (26) | ||
| Venous vessels | ||||||||
| Negative | 13 (14) | 11 (11) | 4 (4) | 0.046* | 8 (9) | 13 (14) | 6 (7) | 0.629 |
| Positive | 15 (17) | 30 (32) | 21 (22) | 14 (15) | 34 (35) | 19 (20) | ||
| Lymph node metastases | ||||||||
| Negative | 11 (12) | 5 (4) | 2 (2) | 0.028* | 6 (7) | 7 (8) | 4 (4) | 0.438 |
| Positive | 17 (18) | 36 (40) | 23 (23) | 16 (17) | 40 (42) | 21 (22) | ||
| Clinicopathological stage ‡ | ||||||||
| I + II | 14 (15) | 13 (14) | 5 (5) | 0.065 | 10 (11) | 16 (17) | 6 (7) | 0.359 |
| III + IV | 14 (15) | 28 (30) | 20 (21) | 13 (14) | 30 (32) | 19 (20) | ||
Statistical analyses were carried out using the χ2‐test. P‐values less than 0.05 were considered to be statistically significant.
† The classification of gastric cancers (GC) with claudin and mucin phenotypes was carried out as described in the text.
Location, histological type, depth of invasion and clinicopathological stage were determined according to the Japanese Classification of Gastric Carcinoma 2nd edn.( 23 ) G‐CLDN, gastric claudin; G‐MUC, gastric mucin; I‐CLDN, intestinal claudin; I‐MUC, intestinal mucin; m, mucosa; mp, muscularis propria; P, poorly differentiated adenocarcinomas including signet‐ring cell carcinomas and mucinous adenocarcinomas; sm, submucosa; ss, subserosa; U‐CLDN, unclassified claudin; U‐MUC, unclassified mucin; W, well‐differentiated adenocarcinomas including papillary adenocarcinomas and tubular adenocarcinomas.
Figure 3.
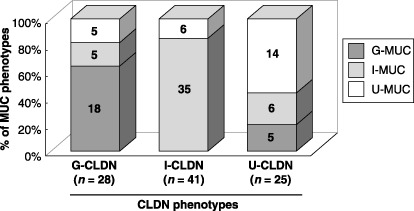
The association between mucin phenotypic classification and claudin phenotypic classification. Comparative study for the relationship between the claudin‐based and mucin‐based phenotypic classification is illustrated.
We examined whether or not the claudin‐based GC classification is associated with patient survival. Patients with stage I GC were excluded from this study. Among the 50 patients who underwent curative surgery and who received follow‐up care at Kobe University Hospital (Kobe Japan), the mean follow‐up time for the 35 surviving patients was 496 days (range: 55–1071 days). The remaining 15 patients died between 38 and 888 days after surgery (mean: 289 days). A significant difference in the survival rates of patients with GC was detected among the claudin phenotypes; the U‐CLDN phenotype exhibited poorer prognosis (P < 0.001), as has been shown in the G‐MUC phenotype (P = 0.015; Fig. 4).
Figure 4.
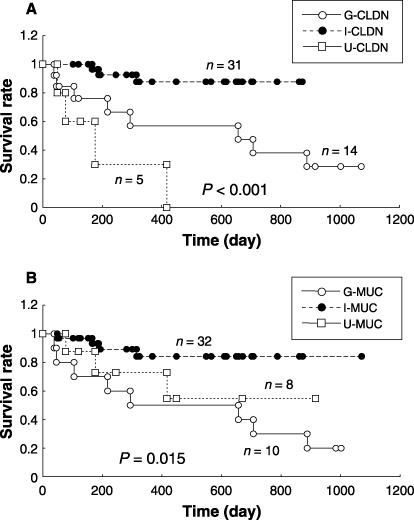
Kaplan–Meier survival curves of patients with gastric cancer (GC) analyzed in this study. (A) The claudin‐based GC classification. (B) The mucin‐based GC classifications.
Discussion
Evidence of altered claudin expression in various human malignancies has been accumulating: overexpression of claudins has been detected frequently in ovarian cancers,( 17 , 28 ) whereas reduced or loss of expression of claudin family members has been found to promote cell invasion and metastasis in malignant tumors, including those of the breast,( 29 ) pancreas( 18 ) and gastrointestinal tract.( 30 ) Thus, dysregulation of claudin expression is likely to be associated with cancer cell invasion and metastasis. However, little is known about the mechanism through which the altered expression of claudins may contribute to cancer cell behavior. In the present study, we demonstrated frequent reduction of gastric and intestinal claudin expression at the invasive front of GC, with a close correlation with carcinoma progression and subsequent metastatic events. This was similar to the loss of claudin‐7 expression in breast( 20 ) and esophageal cancer( 31 ) and to the loss of claudin‐4 in pancreatic( 18 ) and colorectal cancers.( 30 ) Because gastric mucosa can differentiate into the G‐CLDN and I‐CLDN phenotypes, the combined examination of the expression levels of gastric and intestinal claudins is required for speculation on GC aggressiveness and potential metastatic ability. Therefore, we propose that differentiation of GC monitored by the expression of gastric and intestinal claudins may be used as a novel method for the prediction of GC malignancy grade. Tumors with gastric mucin expression are associated with poor patient outcome and greater malignancy potential in the incipient phase of invasion and metastasis,( 3 ) and the mucin‐based GC classification has revealed a relationship with genetic alterations (p53 mutations or microsatellite instability).( 4 , 27 ) However, these experiments were conducted only with intramucosal well‐differentiated‐type neoplastic lesions. It is still unknown whether mucin phenotypic classification could be useful for evaluating tumor aggressiveness and the grade of GC malignancy.
The biological functions of claudins, particularly in the development and progression of human malignancies, are poorly understood. The functions of the TJ are to maintain a luminar barrier, paracellular transport and signal transduction; therefore, disruption of TJ can cause the loss of cell polarity, resulting in an abnormal influx of growth factors, which could provide autocrine and paracrine stimulation to tumorigenic epithelial cells. In differentiated human airway epithelia, it has been shown that disruption of TJ by injury increases epithelial permeability, resulting in altered distribution of erbB2‐4 and the activation of these receptors for cell survival.( 32 ) In addition, Fedwick et al. have demonstrated that the Helicobacter pylori strain SS1 can increase paracellular permeability by disrupting the TJ proteins occuldin, claudin‐4 and claudin‐5 in gastric epithelial cells.( 33 ) The disruption of TJ therefore is considered to be an important mechanism in stomach carcinogensis. In the present study, the U‐CLDN tumors had higher grades of malignancy than the G‐CLDN and I‐CLDN tumors. Because claudin family members are crucial components of TJ, alteration of claudin expression may affect permeability at TJ, possibly increasing the diffusion of nutrients and other extracellular growth factors to promote cancer cell growth, survival and motility.
Information about the functions and regulatory mechanisms of claudin‐3, claudin‐4 and claudin‐18 in cancers has been accumulating: (1) phosphorylation of claudin‐3 by cAMP‐dependent protein kinase( 34 ) and claudin‐4 by EphA2 receptor( 35 ) can modulate cell‐to‐cell contact; (2) knockdown of claudin‐3 and claudin‐4 expression alters matrix metalloproteinase‐2‐mediated cell invasiveness;( 36 ) and (3) expression of claudin‐4 and claudin‐18 is tightly regulated by hypermethylation of the promoter region of CLDN4 genes and the T/EBP/NKX2.1 transcription factor, respectively.( 21 , 37 ) As shown in the present study, overexpression of Cdx2 upregulated both claudin‐3 and claudin‐4 expression in GC cells; however, it remains unclear whether the regulation of Cdx2‐modulated intestinal claudins is direct or indirect.
Supporting information
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (14‐7) and by the Terry Fox Run Foundation for Cancer Research.
References
- 1. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 2. Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann 1968; 59: 251–8. [PubMed] [Google Scholar]
- 3. Tatematsu M, Ichinose M, Miki K, Hasegawa R, Kato T, Ito N. Gastric and intestinal phenotypic expression of human stomach cancers as revealed by pepsinogen immunohistochemistry and mucin histochemistry. Acta Pathol Jpn 1990; 40: 494–504. [DOI] [PubMed] [Google Scholar]
- 4. Endoh Y, Sakata K, Tamura G et al . Cellular phenotypes of differentiated‐type adenocarcinomas and precancerous lesions of the stomach are dependent on the genetic pathways. J Pathol 2000; 191: 257–63. [DOI] [PubMed] [Google Scholar]
- 5. James R, Erler T, Kazenwadel J. Structure of the murine homeobox gene cdx‐2. Expression in embryonic and adult intestinal epithelium. J Biol Chem 1994; 269: 15 229–37. [PubMed] [Google Scholar]
- 6. Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 1997; 386: 84–7. [DOI] [PubMed] [Google Scholar]
- 7. Almeida R, Silva E, Santos‐Silva F et al . Expression of intestine‐specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol 2003; 199: 36–40. [DOI] [PubMed] [Google Scholar]
- 8. Mesquita P, Jonckheere N, Almeida R et al . Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J Biol Chem 2003; 278: 51 549–56. [DOI] [PubMed] [Google Scholar]
- 9. Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin‐2 5′‐flanking region revealed a TATA‐less promoter with conserved binding sites in mouse and human for caudal‐related homeodomain proteins and hepatocyte nuclear factor‐1α. J Biol Chem 2002; 277: 21 361–70. [DOI] [PubMed] [Google Scholar]
- 10. Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 2000; 149: 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four‐transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999; 96: 511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down‐regulation of the mitogen‐activated protein kinase pathway in ras‐transformed Madin–Darby canine kidney cells. Mol Biol Cell 2000; 11: 849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO‐1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 1998; 273: 29 745–53. [DOI] [PubMed] [Google Scholar]
- 14. Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction‐associated MAGUKs, ZO‐1, ZO‐2, and ZO‐3, with the COOH termini of claudins. J Cell Biol 1999; 147: 1351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2: 285–93. [DOI] [PubMed] [Google Scholar]
- 16. Hirohashi S. Inactivation of the E‐cadherin‐mediated cell adhesion system in human cancers. Am J Pathol 1998; 153: 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rangel LB, Agarwal R, D'Souza T et al . Tight junction proteins claudin‐3 and claudin‐4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res 2003; 9: 2567–75. [PubMed] [Google Scholar]
- 18. Michl P, Barth C, Buchholz M et al . Claudin‐4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res 2003; 63: 6265–71. [PubMed] [Google Scholar]
- 19. Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin‐3 and claudin‐4 in prostate cancer epithelium. Cancer Res 2001; 61: 7878–81. [PubMed] [Google Scholar]
- 20. Kominsky SL, Argani P, Korz D et al . Loss of the tight junction protein claudin‐7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 2003; 22: 2021–33. [DOI] [PubMed] [Google Scholar]
- 21. Niimi T, Nagashima K, Ward JM et al . Claudin‐18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung‐ and stomach‐specific isoforms through alternative splicing. Mol Cell Biol 2001; 21: 7380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. Down‐regulation of the claudin‐18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol 2006; 208: 633–42. [DOI] [PubMed] [Google Scholar]
- 23. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma – 2nd English edition – Response assessment of chemotherapy and radiotherapy for gastric carcinoma: clinical criteria. Gastric Cancer 2001; 4: 1–8. [DOI] [PubMed] [Google Scholar]
- 24. Sobin LH, Wittekind CH. UICC TNM Classification of Malignant Tumor, 5th edn. New York: John Wiley and Sons, 1997. [Google Scholar]
- 25. Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002; 94: 3127–34. [DOI] [PubMed] [Google Scholar]
- 26. Hasuo T, Semba S, Li D et al . Assessment of micosatellite instability status for the prediction of metachronous recurrence after initial endoscopic submucosal dissection for early gastric cancer. Br J Cancer 2007; 96: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer 2001; 94: 166–70. [DOI] [PubMed] [Google Scholar]
- 28. Zhu Y, Brannstrom M, Janson PO, Sundfeldt K. Differences in expression patterns of the tight junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface epithelium as compared to epithelia in inclusion cysts and epithelial ovarian tumours. Int J Cancer 2006; 118: 1884–91. [DOI] [PubMed] [Google Scholar]
- 29. Hoevel T, Macek R, Swisshelm K, Kubbies M. Reexpression of the TJ protein CLDN1 induces apoptosis in breast tumor spheroids. Int J Cancer 2004; 108: 374–83. [DOI] [PubMed] [Google Scholar]
- 30. Ueda J, Semba S, Chiba H et al . Heterogeneous expression of claudin‐4 in human colorectal cancer: decreased claudin‐4 expression at the invasive front correlates with cancer invasion and metastasis. Pathobiology 2007. (in press). [DOI] [PubMed]
- 31. Usami Y, Chiba H, Nakayama F et al . Reduced expression of claudin‐7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol 2006; 37: 569–77. [DOI] [PubMed] [Google Scholar]
- 32. Vermeer PD, Einwalter LA, Moninger TO et al . Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003; 422: 322–6. [DOI] [PubMed] [Google Scholar]
- 33. Fedwick JP, Lapointe TK, Meddings JB, Sherman PM, Buret AG. Helicobacter pylori activates myosin light‐chain kinase to disrupt claudin‐4 and claudin‐5 and increase epithelial permeability. Infect Immun 2005; 73: 7844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D'Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin‐3 at threonine 192 by cAMP‐dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem 2005; 280: 26 233–40. [DOI] [PubMed] [Google Scholar]
- 35. Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin‐4 and mediates paracellular permeability. J Biol Chem 2005; 280: 42 375–82. [DOI] [PubMed] [Google Scholar]
- 36. Agarwal R, D'Souza T, Morin PJ. Claudin‐3 and claudin‐4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase‐2 activity. Cancer Res 2005; 65: 7378–85. [DOI] [PubMed] [Google Scholar]
- 37. Honda H, Pazin MJ, Ji H, Wernyj RP, Morin PJ. Crucial roles of Sp1 and epigenetic modifications in the regulation of the CLDN4 promoter in ovarian cancer cells. J Biol Chem 2006; 281: 21 433–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item


