Abstract
Hepatocellular carcinoma (HCC) associated with chronic liver disease evolves from precancerous lesions and early HCC to more malignant forms. Despite the demonstrated importance of cell‐cycle regulators in tumor biology, there have been few studies of their role in multistep hepatocarcinogenesis. Expression of p27Kip1 and a degradation pathway associated protein, S‐phase kinase‐interacting protein 2 (Skp2), was therefore evaluated in surgically resected specimens of eight adenomatous hyperplasias, 16 early HCC and 126 classical HCC. Immunohistochemistry revealed no p27Kip1 expression in the majority of hepatocytes from normal and cirrhotic liver, whereas positive staining for p27Kip1 protein was found in 75.0% and 93.8% of adenomatous hyperplasias and early HCC, respectively. The average p27Kip1 labeling indices (LI) for adenomatous hyperplasias, early HCC, well differentiated HCC, moderately differentiated HCC and poorly differentiated HCC were 36.99, 43.59, 47.73, 49.24, and 30.21, respectively. Real‐time quantitative reverse transcription–polymerase chain reaction (RT‐PCR) analyses confirmed the increases. Skp2 LI were also significantly elevated in accordance with stepwise progression of hepatocarcinogenesis. Increased expression of Skp2 mRNA was observed most frequently in less differentiated tumors and Kaplan–Meier survival analysis showed a significantly association with a poor prognosis (P = 0.0496). In conclusion, a high level of p27Kip1 expression is evident from early stages of hepatocarcinogenesis, indicating that this parameter could be a useful diagnostic marker for precancerous lesions and early HCC. In addition, Skp2 expression correlates with tumor dedifferentiation and may contribute to biological aggression in HCC. (Cancer Sci 2008; 99: 2152–2159)
The hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide and a leading cause of cancer death in Japan. As with other cancers, HCC are characterized by an obvious multistage process of tumor progression.( 1 , 2 , 3 ) It has been shown that small, nodular hypercellular lesions known as adenomatous hyperplasias (AH) appear in damaged liver infected with hepatitis virus B or C. These lesions develop into early HCC, which correspond to carcinoma in situ or microinvasive carcinoma, in which the portal tracts within nodules are preserved, and then into progressed HCC (classical HCC) through the transition stage of nodule‐in‐nodule‐type HCC (progressed HCC within early HCC).( 4 , 5 )
The proliferation of cancer cells has been found to be closely related to abnormalities in expression of cell‐cycle regulators. p27Kip1 a member of the CIP/KIP family, causes G1 arrest by inhibiting the activities of G1 cyclin–cyclin‐dependent kinases. As a negative regulator of the cell cycle, p27Kip1 is a new class of tumor suppressor controlling cell‐cycle progression.( 6 , 7 , 8 ) Recent findings indicate that decreased expression of p27Kip1 protein is associated with high aggressiveness and a poor prognosis in a large variety of cancers, including classical HCC.( 9 , 10 , 11 ) There are a number of key molecules involved in post‐translational regulation of p27Kip1. One is S‐phase kinase‐interacting protein 2 (Skp2), which has been shown to be required for ubiquitin‐mediated degradation of p27Kip1.( 12 , 13 ) Overexpression of Skp2 has been observed to be reciprocally correlated with p27Kip1 expression in some malignancies.( 9 , 14 , 15 , 16 )
Despite the demonstrated importance of cell‐cycle regulators in tumor biology, to our knowledge there have been no studies of cell‐cycle regulators in liver precancerous lesions and early HCC. In the present study, we therefore investigated the possible role of cell‐cycle regulators, especially p27Kip1 and the degradation related Skp2, in surgically resected specimens of AH, early HCC and classical HCC.
Materials and Methods
Patients. The present study involved 122 patients with precancerous lesions and/or HCC (150 lesions) undergoing hepatic resection between 1990 and 2006 at Keio University Hospital, Tokyo, and Kagawa University Hospital, Kagawa, Japan. The median age of the patients was 65.1 years (range 36–82 years), and the male : female ratio was 6.6 : 1. Of these patients, 28 were hepatitis B surface antigen (HBs‐Ag) positive, 63 were hepatitis C antibody (HCV‐Ab) positive, 2 were positive for both HBs‐Ag and HCV‐Ab, 15 were negative for both HBs‐Ag and HCV‐Ab in the presence of chronic liver disease, and the remaining 3 without chronic liver disease were negative for both HBs‐Ag and HCV‐Ab (unknown, 11 patients). With regard to background liver disease, 67 and 42 patients had chronic hepatitis and liver cirrhosis, respectively, and 13 patients had a normal liver. Informed consent for the use of resected tissues was obtained from every patient before the study was started and the study protocol confirms to the ethical guidelines of the Declaration of Helsinki in 1995.
Tissue samples histological evaluation. The tumors were classified in accordance with the World Health Organization (WHO) classification,( 17 ) and that proposed by the Liver Cancer Study Group of Japan.( 18 ) Each tumor was re‐evaluated for macroscopic type, microscopic vascular invasion, intrahepatic metastasis and degree of histological differentiation. In total, eight AH samples, 16 early HCC samples and 126 classical HCC samples were examined. When progressed HCC areas within early HCC are growing expansively, the nodules often have a ‘nodule‐in nodule’ appearance.( 5 , 17 , 18 ) Among the classical HCC, six were of the nodule‐in‐nodule type and 13 had early HCC‐like areas in the nodule periphery.
AH, also known as dysplastic nodules, are characterized by a moderate increase in cell density with a slightly irregular trabecular pattern.( 17 ) There are many portal tracts within the nodules, but no invasion into the portal tracts is evident. Early HCC are vaguely nodular with macroscopically indistinct margins. They are characterized by increased cell density with an increased nuclear/cytoplasmic ratio, increased staining intensity (eosinophilic or basophilic), an irregular, thin trabecular pattern with a frequent acinar or pseudoglandular pattern and fatty changes.( 17 , 18 ) Many portal tracts are present within the tumor nodules and tumor cell invasion into some portal tracts can be seen. At the tumor boundary, neoplastic cells proliferate as though they are replacing normal hepatocytes (‘replacing growth’) and there is no capsule formation. On the other hand, well differentiated HCC are distinctly nodular macroscopically. Although they are also composed of cells with minimal atypia and increased nuclear/cytoplasmic ratio in a thin trabecular pattern, they don't contain Glisson's components, including the bile duct and portal vein, within the tumor nodule miroscopically.( 17 , 18 ) The clinicopathologic characteristics of our eight AH, 16 early HCC and 126 classical HCC are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of eight adenomatous hyperplasias (AH), 16 early hepatocellular carcinoma (HCC) and 126 classical HCC
| Characteristic | AH (8 patients, 8 lesions) | Early HCC (13 patients, 16 lesions) | Classical HCC (112 patients, 126 lesions) |
|---|---|---|---|
| Mean age, years (SE) | 60.86 (2.81) | 62.46 (2.67) | 65.35 (0.91) |
| Range | 51–74 | 49–78 | 36–82 |
| Sex | |||
| Males | 5 | 10 | 99 |
| Females | 3 | 3 | 13 |
| Backgroud liver disease | |||
| Normal liver | 0 | 0 | 13 |
| Chronic hepatitis | 3 | 4 | 61 |
| Cirrhosis | 5 | 9 | 38 |
| Mean size, mm (SE) | 8.5 (1.5) | 15.8 (1.4) | 43.5 (3.0) |
| Range, mm | 7–10 | 10–27 | 5–155 |
| Macroscopic type | |||
| Simple nodular type | 38 | ||
| Simple nodular type with extranodular growth | 41 | ||
| Confluent multinodular type | 31 | ||
| Infiltrative type | 0 | ||
| Massive type | 1 | ||
| Diffuse type | 0 | ||
| Nodule in nodule | 6 | ||
| Unknown | 9 | ||
| Histological grading | |||
| Well | 16 | ||
| Moderately | 74 | ||
| Poorly | 36 | ||
| Portal vein invasion | |||
| Negative | 8 | 16 | 77 |
| Positive | 0 | 0 | 49 |
| Intrahepatic metastasis | |||
| Negative | 8 | 16 | 91 |
| Positive | 0 | 0 | 23 |
| Unknown | 12 | ||
Immunohistochemistry. Tissue specimens were fixed in 10% buffered formaldehyde, routinely processed for embedding in paraffin and sectioned at 4 µm. The sections were immunostained for Ki‐67, p27Kip1 and Skp2 by the avidin–biotin complex (ABC) method, all staining processes from deparaffinization to counterstaining with hematoxylin being performed automatically using the Ventana Discovery staining system (Ventana Medical Systems, Tucson, AZ, USA). An antihuman Ki‐67 mouse monoclonal antibody (Clone MIB‐1, diluted 1 : 50, Dako Cytomation, Glostrup, Denmark), an antihuman p27Kip1 mouse monoclonal antibody (diluted 1 : 1000, Transduction Laboratories, Lexington, KY, USA) and an antihuman Skp2 rabbit polyclonal antibody (diluted 1 : 100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used.
At least five randomly selected high‐power fields containing a minimum of 2000 cells were evaluated for each case, and the labeling index (LI) was calculated as the percentage of positive cell nuclei as reported previously.( 10 ) Sections of human tonsil were used as positive controls for Ki‐67 and Skp2 immunohistochemistry.( 19 ) The bile duct epithelium, infiltrating lymphocytes and sinusoidal endothelial cells always showed strong staining and thus served as an internal control for positive p27Kip1 staining. Reactive small lymphocytes in each case were regarded as internal positive controls for Ki‐67 and Skp2.( 20 ) Equal or more intense nuclear staining in comparison with the internal positive controls was considered to indicate positivity for each protein.
Real‐time quantitative reverse transcription–polymerase chain reaction (RT‐PCR) analysis. Forty‐seven paired specimens of HCC and corresponding non‐cancerous liver were obtained fresh from patients at the time of surgical resection at Kagawa University Hospital. The specimens were immediately cut into small pieces, snap‐frozen in liquid nitrogen and stored until use for real‐time quantitative RT‐PCR analysis. To synthesize complementary DNA (cDNA), total RNA were isolated from 47 specimens (two early HCC, four well differentiated, 29 moderately differentiated and 12 poorly differentiated HCC) and paired distant non‐cancerous liver tissue using RNeasy Plus Mini Kits (Qiagen, Hilden, Germany) following the manufacturer's protocol, then subjected to DNase treatment and stored at –80°C. For p27Kip1 the primer set 5′‐CAAATGCCGGTTCTGTGGAG‐3′ (forward) and 5′‐TCCATTCCATGAAGTCAGCGATA‐3′ (reverse) was designed. For Skp2 the primer set 5′‐GCTGAAGAGCAAAGGGAGTGAC‐3′ (forward) and 5′‐ATCAGACGCTAGGCGATACCAC‐3′ (reverse) was designed. For standardization of the amount of RNA, the expression of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) in each sample was quantified using the primer set 5′‐GCACCGTCAAGGCTGAGAAC‐3′ (forward) and 5′‐ATGGTGGTGAAGACGCCAGT‐3′ (reverse). The reverse transcription reaction (25°C for 10 min, 48°C for 30 min and 95°C for 5 min) was performed using a random 6‐mer and TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA, USA). The templates obtained were then used for real‐time PCR (95°C for 10 min for 1 cycle; 95°C for 15 s, and 60°C for 1 minute for 40 cycles) employing SYBR Green I with the Power SYBR Green PCR Master Mix (Applied Biosystems). An equivalent amount of cDNA sample, derived from 7.5 ng total RNA, was used for each PCR reaction. The ratio of the p27Kip1 or Skp2 messenger RNA (mRNA) expression level in the tumor relative to that in the corresponding non‐cancerous tissue (T : N ratio; p27Kip1 or Skp2/GAPDH in T divided by p27Kip1 or Skp2/GAPDH in N) was calculated in each case.
Statistical analysis. Statistical analyses were performed using the Mann–Whitney U‐test. The Spearman's rank correlation was used to determine the relationship between p27Kip1 and Skp2 expression. Survival analysis was performed by the Kaplan–Meier method, and survival curves were compared with the log‐rank test. Data are expressed as mean ± standard error (SE). Differences at P < 0.05 were considered significant. All the statistical analyses were performed using Stat View (Version 5.0) software (Abacus Concepts, Berkeley, CA, USA).
Results
Ki‐67 antigen is known to be preferentially expressed during all active phases of the cell cycle (G1, S, G2 and M phases), but is absent in resting cells.( 21 ) In the present series of samples, the Ki‐67 LI of hepatocytes in non‐cancerous liver was elevated in line with the degree of severity of the underlying liver disorder (normal liver, 0.83 ± 0.18; chronic hepatitis, 2.18 ± 0.06; liver cirrhosis, 2.25 ± 0.42). The Ki‐67 LI in the tumor increased in accordance with the degree of de‐differentiation of tumors (Fig. 1). The average LI in early HCC‐like areas in the periphery of nodules and early HCC components of nodule‐in‐nodule‐type HCC were 5.03 ± 1.31, and 3.00 ± 1.65, respectively, similar to the value for early HCC (3.78 ± 0.83). The Ki‐67 LI was significantly higher in 49 classical HCC with microscopic portal vein invasion than in 77 classical HCC without such invasion (29.97 ± 3.04 versus11.58 ± 1.48, P < 0.0001).
Figure 1.
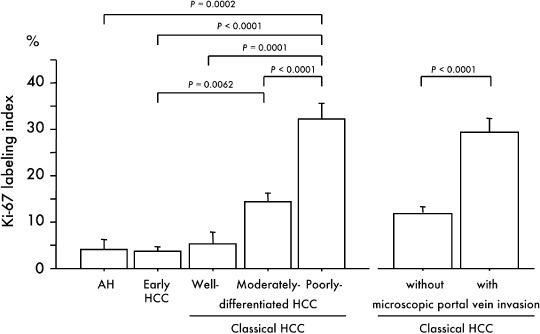
Relationship between immunohistochemical expression of Ki‐67 and multistep hepatocarcinogenesis and microscopic portal vein invasion. Bars = SE.
Immunohistochemistry revealed no p27Kip1 expression in the majority of hepatocytes from normal and cirrhotic liver, whereas positive staining for p27Kip1 protein was found in 75.0% (6/8) and 93.8% (15/16) of AH and early HCC, respectively. The average p27Kip1 LI in AH, early HCC, well differentiated HCC, moderately differentiated HCC and poorly differentiated HCC were 36.99, 43.59, 47.73, 49.24, and 30.21, respectively (2, 3, 4). The average p27Kip1 LI in early HCC‐like areas in the periphery of classical HCC nodules and early HCC components of nodule‐in‐nodule‐type HCC was 47.87 ± 10.02, and 53.17 ± 16.84, respectively, similar to that in early HCC (43.59 ± 7.16). Large regenerative nodules showed no p27Kip1 immunoreactivity (data not shown). The p27Kip1 LI did not differ significantly between AH and early HCC (P = 0.6174). In contrast, the p27Kip1 LI in 36 poorly differentiated HCC was significantly lower than that in 74 moderately differentiated HCC (P = 0.0069) (Fig. 4a). The immunoreactivity was localized primarily in the nuclei of tumor cells, but was evident in both the cytoplasm and nuclei in four AH, 10 early HCC and 58 classical HCC. In early HCC, as illustrated in Figure 2d, p27Kip1 staining in areas without fatty change, frequently observed in both the nucleus and cytoplasm, was stronger than that in areas with fatty change.
Figure 2.
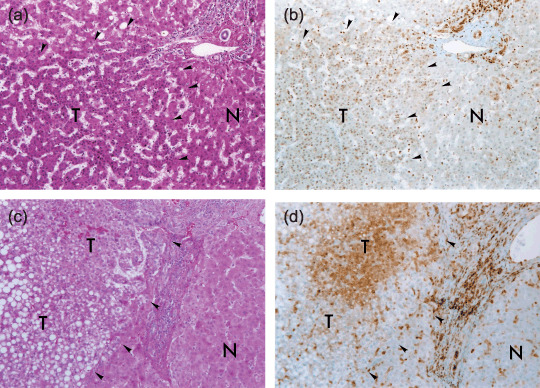
Typical immunohistochemical staining profiles of p27Kip1 in early hepatocellular carcinoma (HCC). Representative histology of early HCC (a and c) (hematoxylin‐eosin stain, original magnification, ×100), and p27Kip1 immunostaining of corresponding serial sections (b and d) (original magnification, ×100). Hepatocytes in non‐cancerous liver in b and d show only focal and faint p27Kip1 staining. Bile duct epithelia, infiltrating lymphocytes and sinusoidal endothelial cells serve as positive controls. The borders between early HCC (T) and non‐cancerous liver (N) are indicated by arrowheads. The early HCC in b shows p27Kip1 immunoreactivity. In the early HCC in d, p27Kip1 staining in areas without fatty change, frequently in both the nucleus and cytoplasm, is stronger than that in areas with fatty change.
Figure 3.
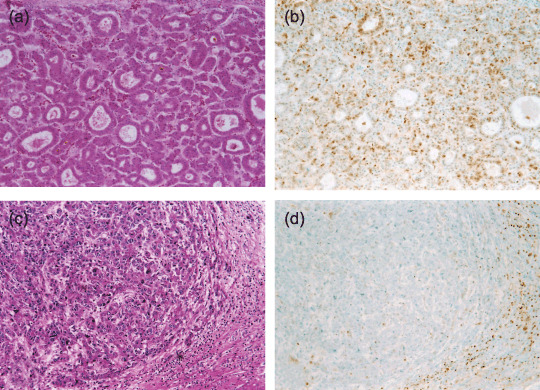
Typical immunohistochemical staining profiles of p27Kip1 in classical hepatocellular carcinoma (HCC). Representative histology of a moderately differentiated HCC (a) and a poorly differentiated HCC (c) (hematoxylin‐eosin stain, original magnification, ×100), and p27Kip1 immunostaining of corresponding serial sections (b and d) (original magnification, ×100). The moderately differentiated HCC (b) shows p27Kip1 immunoreactivity, but this is only weak in the poorly differentiated HCC in d.
Figure 4.
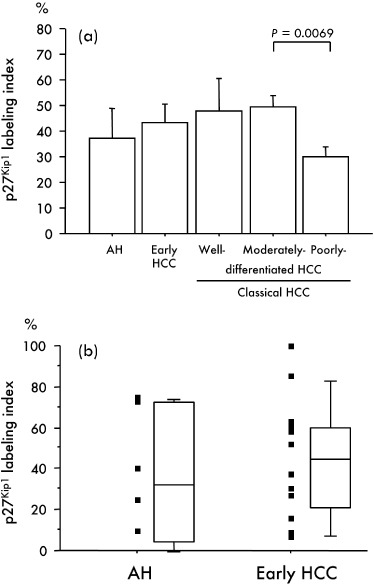
(a) Relationship between immunohistochemical expression of p27Kip1 and multistep hepatocarcinogenesis. Bars = SE. (b) A scattergram and a box plot show p27Kip1 labeling indices in adenomatous hyperplasias (AH) and early hepatocellular carcinoma (HCC). The top and bottom of the boxes are the first and third quartiles, respectively. The lines through the middle of the boxes represent the median values. The maximum and minimum values within 1.5 × interquartile range (IQR) are shown as whisker caps.
To confirm our findings, we next analyzed the level of p27Kip1 mRNA in 47 fresh specimens of HCC (two early, four well differentiated, 29 moderately differentiated and 12 poorly differentiated HCC) using real‐time quantitative RT‐PCR. The p27Kip1 mRNA in all early HCC and well differentiated HCC was increased in comparison with the matched non‐tumor liver specimen. On the other hand, the expression level of p27Kip1 mRNA in the tumor tissue was down‐regulated in comparison with that in the matched non‐cancerous liver tissue in 21 of the 29 moderately differentiated HCC and in 10 of the 12 poorly differentiated HCC. The average ratio of the p27Kip1 mRNA expression level in the tumor relative to that in the matched sample of non‐cancerous tissue (T : N ratio) was 1.66 ± 0.37, 1.73 ± 0.43, 0.79 ± 0.09 and 0.58 ± 0.11 in early, well, moderately and poorly differentiated HCC, respectively. The p27Kip1 mRNA expression level was significantly lower in moderately differentiated HCC (P = 0.0028) and poorly differentiated HCC (P = 0.0017) than in well differentiated HCC (Fig. 5). Kaplan–Meier survival analysis showed that overexpression of p27Kip1 gene (T : N ratio > 1) was associated with a good prognosis of patients but without statistical significance (P = 0.0604) (Fig. 6a).
Figure 5.
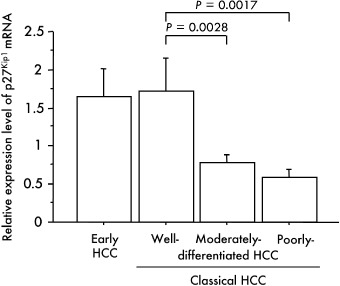
Relationship between the p27Kip1 mRNA expression level and multistep hepatocarcinogenesis. The ratios of the p27Kip1 mRNA expression level in the tumor relative to that in the corresponding non‐cancerous tissue (T : N ratio; p27Kip1/glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) in T divided by p27Kip1/GAPDH in N) were calculated for each case. Bars = SE.
Figure 6.
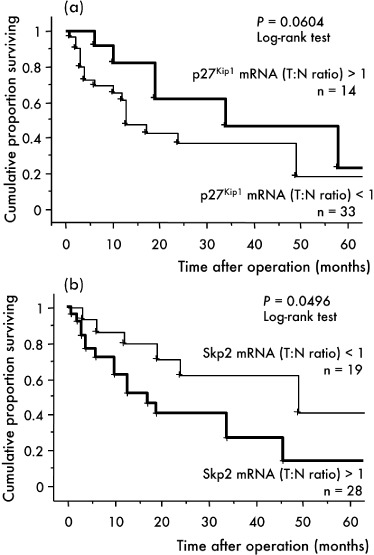
Correlation between p27Kip1 (a) and Skp2 gene (b) expression and cumulative survival rate in patients with hepatocellular carcinoma (HCC) (Kaplan–Meier method). The ratios of the p27Kip1 or Skp2 mRNA expression level in the tumor relative to that in the corresponding non‐cancerous tissue (T : N ratio; p27Kip1 or Skp2/glyceraldehyde‐3‐phosphate dehydrogenase [GAPDH] in T divided by p27Kip1 or Skp2/GAPDH in N) were calculated for each case.
Immunohistochemistry revealed no Skp2 expression in the majority of hepatocytes in non‐cancerous normal liver tissue, chronic hepatitis or cirrhosis. The LI of Skp2 increased in accordance with the degree of the de‐differentiation of tumors and was significantly higher in poorly differentiated HCC than in AH (P = 0.0158), early HCC (P = 0.0008), well (P = 0.0228) and moderately differentiated HCC (P < 0.0001) (Fig. 7). The Skp2 LI in 49 classical HCC with microscopic portal vein invasion was also significantly higher than that in 77 classical HCC without such invasion (10.39 ± 1.68 versus 3.52 ± 0.60, P < 0.0001) (Fig. 7b). Immunoreactivity was localized primarily in the nuclei of tumor cells, but a cytoplasmic reaction without a nuclear reaction was also observed in one well differentiated HCC (6.3%), 17 moderately differentiated HCC (23.0%) and eight poorly differentiated HCC (24.0%). Skp2 overexpression was observed especially at the tumor boundary and/or tumor thrombi in portal veins (Fig. 7a) and tended to be detected more frequently in classical HCC with a low p27Kip1 LI.
Figure 7.
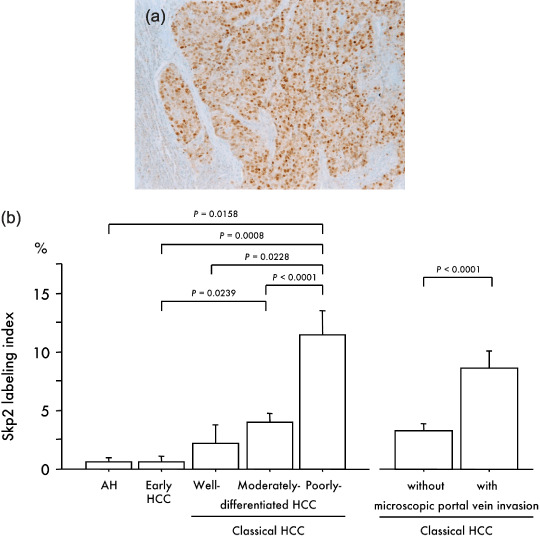
(a) Immunohistochemical localization of Skp2 in a poorly differentiated hepatocellular carcinoma (HCC) (original magnification, ×100). (b) Relationship between immunohistochemical expression of Skp2 and multistep hepatocarcinogenesis and microscopic portal vein invasion. Bars = SE.
The Skp2 mRNA expression level assessed by real‐time quantitative RT‐PCR was significantly higher in poorly differentiated than in moderately differentiated HCC (P = 0.0107) (Fig. 8). The Spearman coefficient revealed an inverse correlation between p27Kip1 and Skp2 mRNA expression levels in classical HCC (P = 0.0443). Kaplan–Meier survival analysis further showed that overexpression of Skp2 gene was significantly associated with a poor prognosis (P = 0.0496) (Fig. 6b).
Figure 8.
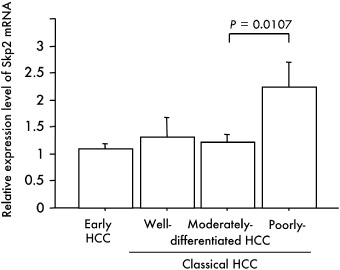
Relationship between the Skp2 mRNA expression level and multistep hepatocarcinogenesis. The ratio of the Skp2 mRNA expression level in the tumor relative to that in the corresponding non‐cancerous tissue (T : N ratio; Skp2/glyceraldehyde‐3‐phosphate dehydrogenase [GAPDH] in T divided by Skp2/GAPDH in N) was calculated for each case. Bars = SE.
Discussion
In the present study, hepatocytes in non‐cancerous normal liver tissue, chronic hepatitis or cirrhosis showed no p27Kip1 immunostaining or only focal and faint staining in the nuclei, as has been reported previously.( 22 , 23 ) However, high p27Kip1 expression was found in AH and early HCC, whereas the Ki‐67 LI were relatively low. In early HCC‐like areas in the periphery of classical HCC nodules and in early HCC components of nodule‐in‐nodule‐type HCC, p27Kip1 expression was similarly elevated. To our knowledge, this is the first demonstration that p27Kip1 is overexpressed in very early stages of hepatocarcinogenesis.
Early HCC generally show a very well differentiated histology with minimal atypia and lack definite invasive or destructive growth. Therefore, it is often difficult, even for an experienced hepatopathologist, to distinguish precancerous lesions and early HCC from regenerative nodules. For this reason, the discovery of an objective molecular marker that could help to standardize the histologic diagnosis of precancerous lesions and early HCC and lead to appropriate treatment has been eagerly anticipated.( 24 ) In the present study, we showed that p27Kip1 could be a sensitive marker for the differential diagnosis of AH and early HCC from non‐cancerous liver tissue.
In the present study, the p27Kip1 LI did not differ significantly between AH and early HCC, suggesting the difficulty of differential diagnosis between precancerous lesions and early HCC by immunohistochemical examination of p27kip1. Chuma et al. reported that immunohistochemical examination of heat‐shock protein 70 (HSP70) reveals its significant overexpression in early HCC compared with precancerous lesions.( 24 ) Shibata et al. also demonstrated that cyclase‐associated protein 2 (CAP2) is up‐regulated in early HCC when compared with precancerous lesions.( 25 ) Immunohistochemical application of these two molecules indicated that they could be sensitive markers for the differential diagnosis of early HCC from precancerous lesions.( 26 )
In addition to this diagnostic problem, the molecular mechanisms of early hepatocarcinogenesis are far from clear. Proliferation and progression toward malignancy of cancer cells have been found to be closely related to abnormalities in cell‐cycle regulators. As a proliferating nuclear antigen, Ki‐67 is present in cells that are replicating in the G1, S, G2, and M stages of the cell cycle.( 21 ) In this study, although AH and early HCC showed a far lower Ki‐67 LI than classical HCC, these lesions showed high expression of p27Kip1 The possible mechanisms remain to be elucidated, but previous studies of hepatocarcinogenesis have shown that genetic mutation of p27Kip1 is not a common event,( 27 ) so it is likely that p27Kip1 expression in AH and early HCC increases as a result of self‐reactive or epigenetic mechanisms. In very early stages of hepatocarcinogenesis, p27Kip1 could act as a negative regulator of the cell cycle to prevent cell proliferation, resulting in a low Ki‐67 LI. Real‐time quantitative RT‐PCR analysis further showed that the expression level of p27Kip1 mRNA was up‐regulated in the two early HCC examined and confirmed that p27Kip1 protein might be increased predominantly by transcriptional regulation rather than by genomic aberrations. However, Western blot analysis will be needed to confirm the overexpression of this protein in the future.
Previous studies have demonstrated that p27Kip1 is expressed at various levels in classical HCC, becoming significantly decreased in cases with biologically aggressive phenotypes featuring portal invasion, poor differentiation, large tumor size and intrahepatic metastasis.( 10 , 11 , 22 , 23 , 28 , 29 , 30 ) As observed in previous studies, our immunohistochemical investigation showed that the p27Kip1 LI in 36 poorly differentiated HCC was significantly lower than that in 74 moderately differentiated HCC. The p27Kip1 mRNA down‐regulation in comparison with matched non‐cancerous liver specimens was also evident in 10 of 12 poorly differentiated HCC. In cases of classical HCC with biologically aggressive phenotypes, it is possible that decreased p27Kip1 protein occurs as a result of failure in control of transcription. Recently, Timmerbeul et al.( 31 ) reported that low p27Kip1 transcript level was a good predictor of low p27Kip1 protein abundance in mouse models of lung and colon cancer, suggesting p27Kip1 mRNA misregulation is important during tumorigenesis. The discrepancy between high p27Kip1 LI and low levels of p27Kip1 mRNA in moderately differentiated HCC might be due to a time lag between down‐regulation and p27Kip1 degradation.
Decreased levels of p27Kip1 are caused, at least in part, by acceleration of degradation owing to Skp2.( 12 , 13 , 14 ) We therefore examined expression of the latter and revealed an inverse correlation with p27Kip1 expression in HCC, in line with reports for various malignancies.( 9 , 14 , 15 , 16 , 20 ) Prognostic significance of Skp2 overexpression has been described for gastric cancer,( 14 ) breast cancer,( 16 ) diffuse‐large B cell lymphoma( 20 ) and biliary tract cancers.( 32 , 33 ) Here increased Skp2 expression was observed more frequently in less differentiated tumors with microscopic portal vein invasion and Kaplan–Meier survival analysis showed a significant association with a poor prognosis. We demonstrated for the first time that Skp2 overexpression is associated with aggressive tumor behavior in HCC. However, Western blot analysis will be needed to confirm the overexpression of this protein in the future. Current evidence indicates that Skp2 is an oncogene, regulating not only the proliferation rate but also resistance to apoptosis, increasing the potential for invasion and motility in different cancer cells.( 34 , 35 , 36 ) However, the mechanism underlying overexpression of Skp2 in most cancers remains unknown.( 37 )
In the present study, immunoreactivity of Skp2 was localized primarily in the nuclei of tumor cells, but a cytoplasmic reaction without a nuclear reaction was also observed in one well differentiated HCC (6.3%), 17 moderately differentiated HCC (23.0%) and eight poorly differentiated HCC (24.0%). The percentage of cytoplasmic staining increased in accordance with the degree of the de‐differentiation of tumors, which is similar to Skp2 LI. Although Skp2 localizes to the nucleus fundamentally, a number of studies described the presence of cytoplasmic staining of Skp2 in various cancer types.( 38 , 39 ) Radke et al. identified a novel isoform of Skp2 that they named Skp2B, which differs from Skp2 only in the C‐terminal domain and, unlike Skp2, localizes to the cytoplasm and showed that Skp2B overexpression is observed in breast cancers.( 40 ) They also suggested that Skp2B overexpression may be associated with previous observations of cytoplasmic staining of Skp2.
The amount of p27Kip1 is high during G0 phase of the cell cycle but decreases rapidly upon re‐entry of cells into G1 phase. However, Skp2 is not expressed until the G1–S transition of the cell cycle, unequivocally later than the degradation of p27Kip1 apparent at G0–G1.( 41 ) The discrepancy between the temporal patterns of p27Kip1 and Skp2 expression suggest the existence of a Skp2‐independent pathway for degradation of p27Kip1, a novel ubiquitin ligase, KPC (Kip1 ubiquitylation‐promoting complex), consisting of KPC1 and KPC2, was shown to regulate the ubiquitin‐dependent degradation of p27Kip1 at G0 phase.( 42 ) In a preliminary study, we evaluated the KPC1 mRNA expression level in the same 47 HCC specimens using real‐time quantitative RT‐PCR and found the average ratio of tumor relative to matched non‐cancerous tissue (T : N ratio) to be 1.39 ± 0.24, 1.73 ± 0.40, 1.15 ± 0.13 and 1.17 ± 0.45 in early HCC, well differentiated HCC, moderately differentiated HCC and poorly differentiated HCC, respectively. The differences were not significant, however, suggesting that KPC1 does not play an important role in multistep hepatocarcinogenesis. Additional studies are now required to clarify the regulatory mechanisms involved in p27Kip1 degradation in HCC.
In conclusion, we here demonstrated high expression of p27Kip1 in AH and early HCC and relatively low expression of p27Kip1 in poorly differentiated HCC. These results suggest that p27Kip1 and related cell‐cycle regulators are most likely involved from early stages of multistep hepatocarcinogenesis. p27Kip1 might thus have potential as a diagnostic marker. Furthermore, our findings suggest that Skp2 contributes to the biological aggressiveness of HCC, possibly via degradation of p27Kip1.
Acknowledgments
We thank Dr Shugo Suzuki, Nagoya City University, and Drs Norimasa Koide and Rie Shibata, Keio University School of Medicine, for access to archival material. Furthermore, we would like to thank Yukie Yoshino, Satomi Saijo, Mizuho Kuroda and Aya Hashimoto for valuable technical assistance. This work was supported by Grants‐in‐Aid for Scientific Research (15790717, 17790919) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Current address: Shinichi Yachida, Gastrointestinal/Liver Division, Department of Pathology, Johns Hopkins Medical Institutions, 1550 Orleans Street, CRBII Rm 316, Baltimore MD 21231, USA
References
- 1. Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Human Pathol 1991; 22: 172–8. [DOI] [PubMed] [Google Scholar]
- 2. Sakamoto M, Hirohashi S. Natural history and prognosis of adenomatous hyperplasia and early hepatocellular carcinoma. Multi‐institutional analysis of 53 nodules followed up for more than 6 months and 141 patients with single early hepatocellular carcinoma treated by surgical resection or percutaneous ethanol injection. Jpn J Clin Oncol 1998; 28: 604–8. [DOI] [PubMed] [Google Scholar]
- 3. Oikawa T, Ojima H, Yamasaki S, Takayama T, Hirohashi S, Sakamoto M. Multistep and multicentric development of hepatocellular carcinoma: histological analysis of 980 resected nodules. J Hepatol 2005; 42: 225–9. [DOI] [PubMed] [Google Scholar]
- 4. Takayama T, Makuuchi M, Hirohashi S et al . Malignant transformation of adenomatous hyperplasia to hepatocellular carcinoma. Lancet 1990; 336: 1150–3. [DOI] [PubMed] [Google Scholar]
- 5. Morimitsu Y, Hsia CC, Kojiro M, Tabor E. Nodules of less‐differentiated tumor within or adjacent to hepaocellular carcinoma. reactive expression of transforming growth factor‐alpha and its receptor in the different areas of tumor. Human Pathol 1995; 26: 1126–32. [DOI] [PubMed] [Google Scholar]
- 6. Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo‐insufficient for tumor suppression. Nature 1998; 396: 177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin‐dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 1995; 9: 639–49. [DOI] [PubMed] [Google Scholar]
- 8. Polyak K, Lee MH, Erdjument‐Bromage H et al . Cloning of p27Kip1, a cyclin‐dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994; 78: 59–66. [DOI] [PubMed] [Google Scholar]
- 9. Shintani S, Li C, Mihara M, Hino S, Nakashiro K, Hamakawa H. Skp2 and Jab1 expression is associated with inverse expression of p27KIP1 and poor prognosis in oral squamous cell carcinomas. Oncology 2003; 65: 355–62. [DOI] [PubMed] [Google Scholar]
- 10. Ito Y, Matsuura N, Sakon M et al . Expression and prognostic roles of the G1‐S modulators in hepatocellular carcinoma: p27 independently predicts the recurrence. Hepatology 1999; 30: 90–9. [DOI] [PubMed] [Google Scholar]
- 11. Tannapfel A, Grund D, Katalinic A et al . Decreased expression of p27 protein is associated with advanced tumor stage in hepatocellular carcinoma. Int J Cancer 2000; 89: 350–5. [DOI] [PubMed] [Google Scholar]
- 12. Carrano AC, Eytan E, Hershko A, Pagano M. Skp2 is required for ubiquitin‐mediated degradation of Cdk inhibitor p27. Nat Cell Biol 1999; 1: 193–7. [DOI] [PubMed] [Google Scholar]
- 13. Sutterluty H, Chatelain E, Mart A et al . p45Skp2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol 1999; 1: 207–14. [DOI] [PubMed] [Google Scholar]
- 14. Masuda T, Inoue H, Sonoda H et al . Clinical and biological significance of S‐phase kinase‐associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res 2002; 62: 3819–25. [PubMed] [Google Scholar]
- 15. Hershko D, Bornstein G, Ben‐Izhak O et al . Inverse relation between levels of p27Kip1 and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer 2001; 91: 1745–51. [DOI] [PubMed] [Google Scholar]
- 16. Sonoda H, Inoue H, Ogawa K, Utsunomiya T, Masuda T, Mori M. Significance of Skp2 expression in primary breast cancer. Clin Cancer Res 2006; 12: 1215–20. [DOI] [PubMed] [Google Scholar]
- 17. Hirohashi S, Ishak KG, Kojiro M et al . Hepatocellular carcinoma. In: Hamilton SR, Aaltonen LA, eds. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press, 2000; 159–72. [Google Scholar]
- 18. Liver Cancer Study Group of Japan . General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Tokyo: Kanehara, 2003. [Google Scholar]
- 19. Goto A, Niki T, Moriyama S et al . Immunohistochemical study of Skp2 and Jab1, two key molecules in the degradation of P27, in lung adenocarcinoma. Pathol Int 2004; 54: 675–81. [DOI] [PubMed] [Google Scholar]
- 20. Seki R, Okamura T, Koga H et al . Prognostic significance of the F‐box protein Skp2 expression in diffuse large B‐cell lymphoma. Am J Hematol 2003; 73: 230–5. [DOI] [PubMed] [Google Scholar]
- 21. Yeh TS, Chen TC, Chen MF. Dedifferentiation of human hepatocellular carcinoma up‐regulates telomerase and Ki‐67 expression. Arch Surg 2000; 135: 1334–9. [DOI] [PubMed] [Google Scholar]
- 22. Fiorentino M, Altimari A, D’Errico A et al . Acquired expression of p27 is a favorable prognostic indicator in patients with hepatocellular carcinoma. Clin Cancer Res 2000; 6: 3966–72. [PubMed] [Google Scholar]
- 23. Armengol C, Boix L, Bachs O et al . p27Kip1 is an independent predictor of recurrence after surgical resection in patients with small hepatocellular carcinoma. J Hepatol 2003; 38: 591–7. [DOI] [PubMed] [Google Scholar]
- 24. Chuma M, Sakamoto M, Yamazaki K et al . Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology 2003; 37: 198–207. [DOI] [PubMed] [Google Scholar]
- 25. Shibata R, Du Mori T, W et al . Overexpression of cyclase‐associated protein 2 in multistage hepatocarcinogenesis. Clin Cancer Res 2006; 12: 5363–8. [DOI] [PubMed] [Google Scholar]
- 26. Sakamoto M. Pathology of early hepatocellular carcinoma. Hepatol Res; 37: S135–8. [DOI] [PubMed] [Google Scholar]
- 27. Chen TC, Ng KF, Lien JM, Jeng LB, Chen MF, Hsieh LL. Mutational analysis of the p27kip1 gene in hepatocellular carcinoma. Cancer Lett 2000; 153: 169–73. [DOI] [PubMed] [Google Scholar]
- 28. Qin LF, Ng IO. Expression of p27KIP1 and p21WAF1/CIP1 in primary hepatocellular carcinoma: clinicopathologic correlation and survival analysis. Hum Pathol 2001; 32: 778–84. [DOI] [PubMed] [Google Scholar]
- 29. Matsuda Y, Ichida T, Genda T, Yamagiwa S, Aoyagi Y, Asakura H. Loss of p16 contributes to p27 sequestration by cyclin D1‐cyclin‐dependent kinase 4 complexes and poor prognosis in hepatocellular carcinoma. Clin Cancer Res 2003; 9: 3389–96. [PubMed] [Google Scholar]
- 30. Hui AM, Sun L, Kanai Y, Sakamoto M, Hirohashi S. Reduced p27Kip1 expression in hepatocellular carcinomas. Cancer Lett 1998; 132: 67–73. [DOI] [PubMed] [Google Scholar]
- 31. Timmerbeul I, Garrett‐Engele CM, Kossatz U et al . Testing the importance of p27 degaradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc Natl Acad Sci USA 2006; 103: 14009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanada T, Yokoi S, Arii S, Yasui K, Imoto I, Inazawa J. Skp2 overexpression is a p27Kip1‐independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci 2004; 95: 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li SH, Li CF, Sung MT et al . Skp2 is an independent prognosticator of gallbladder carcinoma among p27 (Kip1)‐interacting cell cycle regulators: an immunohistochemical study of 62 cases by tissue microarray. Mod Pathol 2007; 20: 497–507. [DOI] [PubMed] [Google Scholar]
- 34. Yokoi S, Yasui K, Iizawa T, Takahashi T, Fujisawa T, Inazawa J. Down‐regulation of SKP2 induces apoptosis in lung‐cancer cells. Cancer Sci 2003; 94: 344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gstaiger M, Jordan R, Lim M et al . Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA 2001; 98: 5043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakayama K, Nagahama H, Minamishima YA et al . Targeted disruption of Skp2 results in accumulation of cyclin E and p27 (Kip1), polyploidy and centrosome overduplication. EMBO J 2000; 19: 2069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiappetta G, De Marco C, Quintiero A et al . Overexpression of the S‐phase kinase‐associated protein 2 in thyroid cancer. Endocr Relat Cancer 2007; 14: 405–20. [DOI] [PubMed] [Google Scholar]
- 38. Signoretti S, Di Marcotullio L, Richardson A et al . Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest 2002; 110: 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Drobnjak M, Melamed J, Taneja S et al . Altered expression of p27 and Skp2 proteins in prostate cancer of African‐American patients. Clin Cancer Res 2003; 9: 2613–9. [PubMed] [Google Scholar]
- 40. Radke S, Pirkmaier A, Germain D. Differential expression of the F‐box proteins Skp2 and Skp2B in breast cancer. Oncogene 2005; 24: 3448–58. [DOI] [PubMed] [Google Scholar]
- 41. Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S, Nakayama KI. Degradation of p27Kip1 at the G0–G1 transition mediated by a Skp2‐independent ubiquitination pathway. J Biol Chem 2001; 276: 48937–43. [DOI] [PubMed] [Google Scholar]
- 42. Kotoshiba S, Kamura T, Hara T, Ishida N, Nakayama KI. Molecular dissection of the interaction between p27 and Kip1 ubiquitylation‐promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J Biol Chem 2005; 280: 17694–700. [DOI] [PubMed] [Google Scholar]


