Abstract
Tumor cells can migrate and invade tissue by two modes of motility: mesenchymal and amoeboid. X‐ray or γ‐ray irradiation increases the invasiveness of tumor cells with mesenchymal motility through the induction of matrix metalloproteinases (MMP), and this increase is suppressed by MMP inhibitors (MMPI). However, the effects of X‐ray or γ‐ray irradiation on the invasiveness of tumor cells with amoeboid motility remain unclear. We investigated the effect of irradiation on amoeboid motility by using cells of the human pancreatic cancer line, MIAPaCa‐2, which exhibits both modes of motility. The X‐ray‐induced invasiveness of MIAPaCa‐2 cells was associated with the upregulation of MMP2 at both the RNA and protein levels and was inhibited by MMPI treatment. Amoeboid–mesenchymal transition was slightly induced after irradiation. The MMPI treatment caused mesenchymal–amoeboid transition without significant increase in invasiveness, while the ROCK inhibitor (ROCKI) stimulated amoeboid–mesenchymal transition and enhanced invasiveness under both non‐irradiated and irradiated conditions. This ROCKI‐induced transition was accompanied by the upregulation of MMP2 mRNA and protein. Exposure to both irradiation and ROCKI further enhanced MMP2 expression and had an additive effect on the invasiveness of MIAPaCa‐2 cells. Additionally, exposure to MMPI led to significant suppression of both radiation‐induced and the basal invasiveness of MIAPaCa‐2 cells. This suggests that ROCKI treatment, especially with concomitant X‐ray irradiation, can induce invasion of cancer cells and should be used only for certain types of cancer cells. Simultaneous use of inhibitors, ROCKI and MMPI may be effective in suppressing invasiveness under both X‐ray‐irradiated and non‐irradiated conditions. (Cancer Sci 2011; 102: 792–798)
Pancreatic cancer is one of the most aggressive diseases with an extremely low 5‐year survival rate.( 1 , 2 ) Most patients with pancreatic cancer have advanced disease at the time of diagnosis, and this disease is associated with a high metastatic potential, which is a major clinical problem.( 1 , 3 ) Chemotherapy and radiotherapy are used as adjuvants to surgery and for palliative purposes in cases where surgical resection is not feasible. However, it has been reported that there is no clear evidence to indicate that intraoperative radiotherapy is more effective than other therapies in treating pancreatic cancer in locally advanced and metastatic stages.( 4 ) Further characterization of pancreatic cancer cells exposed to anti‐tumor drugs and irradiation is required to develop methods for improving treatment strategies.
It remains to be determined whether the application of local radiotherapy affects the characteristics of subsequently appearing metastatic tumors. Several studies have shown that conventional X‐ray or γ‐ray irradiation itself induces the metastasis of pancreatic tumor cells both in vitro and in vivo. ( 1 , 3 , 5 , 6 , 7 ) These studies revealed that photon‐irradiated cells produce proteases, such as matrix metalloproteinases (MMP), which remodel the extracellular matrix (ECM) to generate paths for cell migration. These experiments showed that the use of protease inhibitors, including MMP inhibitors (MMPI), resulted in the suppression of the radiation‐induced invasiveness. However, the basal level of invasiveness of the cells used in these studies was not suppressed, suggesting that mechanisms underlying the basal and radiation‐induced invasiveness of the cells are possibly different.
Individual tumor cells have two different modes of movements: mesenchymal and amoeboid.( 7 , 8 , 9 , 10 , 11 , 12 , 13 ) Some cell lines derived from tumors show movement by both these modes. Cells moving in the mesenchymal mode are elongated, and their movement depends on the proteolytic activity of MMP, which permits penetration of the ECM. Cells in the amoeboid mode are round with bleb‐like protrusions and their movement is mediated by actomyosin contraction, which is regulated by Rho‐kinase (ROCK). Cells can shift between these two modes depending on the environmental conditions. MMPI and ROCK inhibitors (ROCKI) suppress the mesenchymal and amoeboid mode of invasiveness, respectively. Previous studies have shown that radiation can induce invasiveness of cells that produce proteases.( 1 , 3 , 5 , 6 , 7 ) This finding implies that the cells in these studies were phenotypically mesenchymal. The mesenchymal and amoeboid modes are inter‐convertible depending on the environmental conditions;( 7 ) this may limit the effectiveness of single therapeutic agents such as MMPI. To our knowledge, no study has been conduced to examine the invasiveness of cells with amoeboid movement after exposure to radiation.
In this study, we first analyzed the migration and invasiveness of cells of the pancreatic tumor lines, Panc‐1 and MIAPaCA‐2, under X‐ray‐irradiated and non‐irradiated conditions; our results showed that MIAPaCa‐2 cells have greater invasiveness than Panc‐1 cells under both these conditions. Furthermore, we found that the MIAPaCa‐2 cell line is morphologically heterogeneous, that is, the cells may be elongated or round and thereby exhibit the mesenchymal or amoeboid mode of movement, respectively. The ROCKI‐induced inhibition of amoeboid motility of MIAPaCa‐2 cells increased the invasiveness of these cells. Furthermore, simultaneous exposure to both X‐ray irradiation and ROCKI treatment had an additive effect on the invasiveness of the cells. Additionally, we demonstrate that the combination of MMPI and ROCKI suppresses both X‐ray‐induced and basal invasiveness of MIAPaCa‐2 cells.
Materials and Methods
Cell culture, reagents and irradiation. Cells of the human pancreatic cancer cell lines MIAPaCa‐2 and Panc‐1 were purchased from ATCC (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA). GM6001, MMP2 inhibitor III and MMP9 inhibitor I were purchased from Calbiochem‐Novabiochem International Inc. (La Jolla, CA, USA), and Y27632 was obtained from Wako Ltd (Osaka, Japan).
Cells were irradiated with 0, 2 or 4 Gy of X‐rays, as described previously,( 14 ) at the dose rate of approximately 1 Gy/min.
Migration assay. The migration of cells was examined using the Oris Cell Migration Assay kit (Platypus Tech, Fitchburg, WI, USA).( 15 ) Irradiated cells were incubated for 24 h and 5 × 104 cells per well were seeded, and migration of cells was performed on day 2 (Fig. 1). Cells were fixed and stained with propidium iodide (PI) solution on day 3 and counted using ImageJ software (National Institutes of Health, Bethesda, MD, USA).( 16 )
Figure 1.
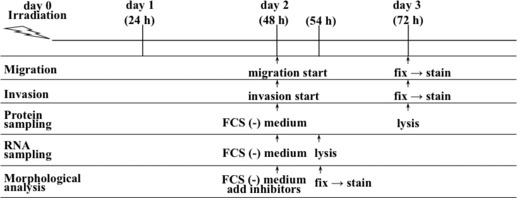
An outline of the experimental procedures.
The percentage of both viable and dead cells was determined with fluorescence‐activated cell sorting (FACS) by using a cell‐viability double‐staining kit (Dojindo Molecular Technologies Inc., Tokyo, Japan).( 17 ) We calculated the number of viable cells among the seeded cells by multiplying the number of seeded cells with the ratio of viable cells. We then determined the percentage of migrated cells.
Matrigel invasion assay. The invasiveness of cells was measured using transwell chambers containing a 6.5‐mm filter with a pore size of 8 μm (Corning, New York, NY, USA).( 18 ) On day 2, an aliquot of 120 μg matrigel (BD Biosciences, Redwood City, MA, USA) was added to the culture insert, and 1.5 × 105 cells suspended with DMEM with 0.35% BSA were then seeded onto it. DMEM with 10% FBS was added to the lower well, and the invasion assay was performed for 24 h (Fig. 1). On day 3, invasive cells that reached the undersurface of the transwell membrane were fixed with 70% ethanol and stained with PI. The number of invasive cells in three random fields was counted using a fluorescence microscope.
For the inhibitor studies, cells were pre‐treated with GM6001 (25 mM), Y27632 (10 mM), MMP2 inhibitor III (12 nM), MMP9 inhibitor I (5 nM), GM6001 (25 mM) plus Y27632 (10 mM) or MMP2 inhibitor III (12 nM) plus Y27632 (10 mM) and incubated for 6 h, and then used for the invasion assay. Inhibitors were also added to each culture inserts immediately after seeding the cells onto the matrigel. The number of invasive cells was adjusted by the percentage of viable cells determined by FACS.
Quantification of cells based on morphology. To quantify cells based on morphology, the medium was changed to serum‐free DMEM on day 2; the inhibitors GM6001, MMP2 inhibitor III, Y27632, or GM6001 plus Y27632 were added, and the cells were incubated for a further 6 h (Fig. 1). Two random fields from each well were photographed, and the total number of cells was counted using ImageJ. Cell morphology was classified as typical amoeboid type (spherical morphology) or mesenchymal type (spindle‐shaped or intermediate shape with protrusion), and the percentage of cells of each morphological type was determined (n = 3, 600–900 cells were analyzed for each group).
Immunoblotting. On day 2, the conditioned medium was replaced with serum‐free DMEM and the cells were incubated for a further 24 h (Fig. 1). On day 3, the cells and conditioned medium were lysed in ×2 Laemmli sample buffer. Protein (5 μg) was subjected to SDS‐PAGE and western blotting. Membranes were incubated with the primary antibody for human MMP2 (Daiichi Fine Chemical Co., Toyama, Japan) or MMP14 (Sigma–Aldrich Co., St. Louis, MO, USA). The membranes were then incubated with anti‐mouse IgG conjugated with horseradish peroxidase to detect MMP2 (Amersham Biosciences, Buckinghamshire, UK) or anti‐rabbit IgG conjugated with horseradish peroxidase to detect MMP14 (Amersham Biosciences). Immunoblots were detected by enhanced chemiluminescence and imaged using a Lumino image analyzer, LAS 4000 (Fujifilm, Tokyo, Japan).
Quantitative real‐time polymerase chain reaction. Quantitative real‐time polymerase chain reaction (qRT‐PCR) was performed on a LightCycler 480 with the Probes Master (Roche Diagnostics, Konzern‐Hauptsitz Grenzacherstrasse, Basel, Switzerland) as described previously.( 19 ) Nested PCR was used for the detection of MMP2. The Universal Probe Library (UPL) probes and primer sequences used are shown in Table S1.
Statistical analysis. Statistical analyses were performed using unpaired Student’s t‐test or Mann–Whitney U‐test. A P value of <0.05 was considered significant.
Results
Invasiveness of cells of the pancreatic cancer lines MIAPaCa‐2 and Panc‐1. Cells of the MIAPaCa‐2 and Panc‐1 lines were assayed for migration and invasiveness after irradiation. Under the non‐irradiated condition, the invasive rates of MIAPaCa‐2 cells were higher than that of Panc‐1 cells. X‐ray irradiation enhanced the migration of MIAPaCa‐2 cells and promoted the invasion of both cell lines (Fig. 2). The invasive rates of MIAPaCa‐2 cells were higher than those of Panc‐1 cells at 2 and 4 Gy of X‐ray irradiation. For both cell lines, the number of invasive cells reached a peak at a radiation dose of 4 Gy. We eliminated the cells killed by radiation from this calculation by adjusting the total number of seeded cells with the percentage of calcein‐fluorescence‐positive viable cells (Tables S2,S3). Therefore, we used MIAPaCa‐2 cells for further research on the basal and X‐ray‐induced invasiveness of the cells.
Figure 2.
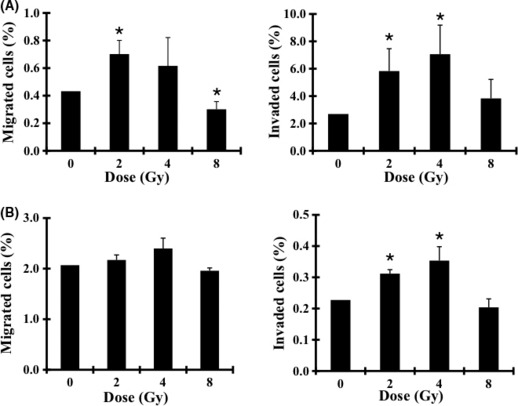
Effects of X‐ray irradiation on the migration and invasiveness of MIAPaCa‐2 (A) and Panc‐1 (B) cells. The percentage of migrated cells (left panel) and invasive cells (right panel) from days 2 to 3 are shown. Data are presented as mean ± SD of samples (n = 5 or 6). *P < 0.05 versus control.
Switch between mesenchymal and amoeboid modes. The MMPI, GM6001, diminished the X‐ray‐induced invasiveness to nearly that in the non‐irradiated condition, which suggests that the X‐ray‐induced invasiveness of MIAPaCa‐2 cells depended mainly on MMP activity towards degrading the ECM (Fig. 3A). The percentage of viable cells used to adjust the total number of seeded cells is shown in Table S4. The MIAPaca‐2 cell line is morphologically heterogeneous and is composed of both mesenchymal (elongated) type and amoeboid (round) type (Fig. 3B,C). X‐ray irradiation affected the amoeboid–mesenchymal transition to some extent, while GM6001 treatment definitely resulted in the mesenchymal–amoeboid transition (Fig. 3B,C). However, the cells with mesenchymal mode attaining amoeboid mode had little or no potential to penetrate the ECM.
Figure 3.
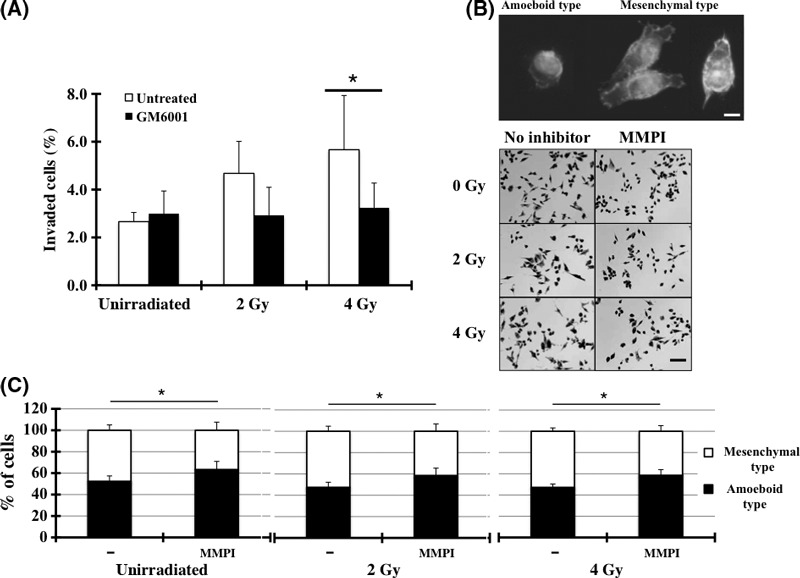
The effects of matrix metalloproteinase inhibitors (MMPI) on the invasiveness of MIAPaCa‐2 cells. (A) An invasion assay was performed with the addition of MMPI (GM6001). Data represent the mean ± SD of samples (n = 6). *P < 0.05 versus control. (B) Morphology of MIAPaCa‐2 cells. After incubation for 6 h with the inhibitor, the cells were photographed. Bar, 200 μm. The cells on the upper panels were stained with phalloidine and Hoechst 33258. (C) Cells with typical mesenchymal‐type or amoeboid‐type morphology shown in (B) were counted and the percentage of cells of each morphological type was calculated. *P < 0.05 versus control.
Effects of MMP2 on X‐ray‐induced invasiveness of MIAPaCa‐2 cells with mesenchymal motility. To identify the MMP that enhanced the X‐ray‐induced invasiveness of MIAPaCa‐2 cells with mesenchymal motility, we used MMP2‐ and MMP9‐specific protease inhibitors. Figure 4 shows that MMP2I attenuated the X‐ray‐induced invasiveness of MIAPaCa‐2 cells to some degree, while MMP9I did not. The percentage of viable cells used to adjust the total number of seeded cells is shown in Table S5.
Figure 4.
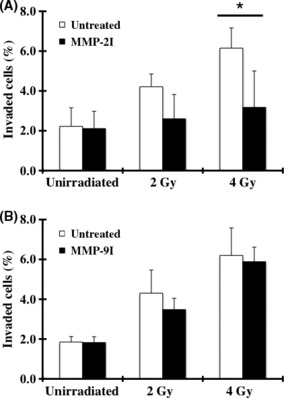
The effects of MMP2I and MMP9I on the invasiveness of MIAPaCa‐2 cells. (A) An invasion assay was performed with the addition of a MMP2‐specific inhibitor, MMP2 inhibitor III (A), or a MMP9‐specific inhibitor, MMP9 inhibitor I (B). Data are represented as mean ± SD of samples (n = 6). *P < 0.05.
We then investigated the expression of MMP2 protein in irradiated cells. Expression of protein with the molecular weight of the active form of MMP2 increased in the conditioned medium containing X‐ray‐irradiated MIAPaCa‐2 cells, and this increase was dose dependent when radiation doses of 0, 2 and 4 Gy were used, but the MMP2 expression level at 4 Gy was similar to that at 8 Gy (Fig. 5). Therefore the changes in active MMP2 levels were associated with the altered invasiveness of the irradiated cells, but no association with invasiveness was observed in the 8‐Gy‐irradiated cells, whose migration capability was suppressed. Furthermore, we analyzed the expression of MMP14, which mediates MMP2 activation.( 20 , 21 ) Levels of the active form of MMP14 were also elevated by irradiation in a dose‐dependent manner, indicating that this factor may mediate the rapid conversion of pro‐MMP2 to its active form in X‐ray‐irradiated cells (Fig. 5A,C). Furthermore, we detected that MMP2 enzymatic activity was enhanced upon irradiation (Fig. S1A).
Figure 5.

Expression analyses of MMP2 and MMP14. (A) The expression levels of MMP2 and MMP14 were determined by western blotting. Quantitative densitometric results are shown for MMP2 (B) and MMP14 (C), respectively. These data represent the mean ± SD of samples (n = 5 or 6). Quantification of mRNA for MMP2 (D) and MMP14 (E) by RT‐PCR. The results for the X‐ray‐irradiated samples are shown as arbitrary units calculated from the standard curve prepared using a serial dilution of the cDNA library. Data represents the mean ± SD (n = 3). *P < 0.05 versus control. ND, not detected.
To examine whether the expression of these MMP was regulated at the mRNA level, the amount of RNA coding for MMP2 or MMP14 was measured by the qRT‐PCR method. The MMP2 mRNA could not be detected in the non‐irradiated cells in our system, but its level clearly increased in X‐ray‐irradiated cells (Fig. 5D); this indicated that X‐ray irradiation enhanced MMP2 transcription and/or stabilized MMP2 mRNA. The mRNA of MMP14 was slightly increased at 2 Gy and reduced at 4 and 8 Gy (Fig. 5E); this may be sufficient to produce the MMP14 protein, which in turn activates MMP2.
Effects of ROCKI on the invasiveness and morphological transition of MIAPaCa‐2 cells. To suppress the invasiveness of MIAPaCa‐2 cells due to amoeboid motility, we used a ROCKI, namely, Y27632. Treatment of the cells with the ROCKI unexpectedly led to a substantial increase in the number of invasive cells (Fig. 6A). Cell morphological analysis showed a clear increase in the number of cells of the mesenchymal (elongated) type, with an associated reduction in the number of cells of the amoeboid (round) type, and both the elongated and round cells expressed filopodia‐like shapes (Fig. 6B–D, Fig. S2). We also observed that most of the invaded cells, which were treated with ROCKI, express filopodia‐like shapes (Fig. S3). The possible invasive potential of the round cells with filopodia could not be analyzed because we could not isolate and culture this type of cell from the MIAPaCa‐2 cells, which are morphologically heterogeneous.
Figure 6.

The effects of matrix metalloproteinase inhibitors (MMPI) and ROCK inhibitor (ROCKI) on the invasiveness of MIAPaCa‐2 cells. (A) An invasion assay was performed with the addition of ROCKI or ROCKI plus MMPI (GM6001). Data represent the mean ± SD of samples (n = 6). *P < 0.05 versus control. (B) Morphology of MIAPaCa‐2. After incubation for 6 h with the inhibitor(s), the cells were photographed. Bar, 200 μm. (C) Effects of ROCKI and ROCKI plus MMPI on cell morphology. The cells were stained with phalloidine and Hoechst 33258. (D) Cells with the typical mesenchymal‐type or amoeboid‐type morphology shown in Figure 6C were counted, and the percentage of cells of each morphological type were calculated. *P < 0.05 versus control.
Because ROCKI enhanced the amoeboid–mesenchymal transition and the invasion of cells, the MMP2 expression was analyzed. The data showed that ROCKI‐treated MIAPaCa‐2 cells exhibited upregulation of MMP2 mRNA, which was followed by the production of a protein with the molecular weight of the active form of MMP2 (Fig. 7). We also detected that ROCKI treatment enhanced MMP2 activity (Fig. S1B). These results indicate that ROCK activity modulates the transition of cells between amoeboid and mesenchymal motility, and induces MMP2 expression in these cells.
Figure 7.
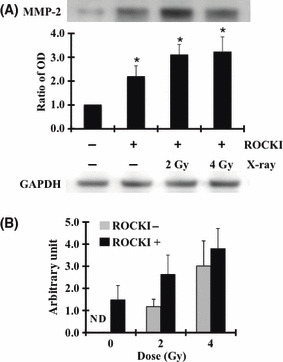
Induction of MMP2 expression by ROCK inhibitor (ROCKI). The expression of MMP2 was analyzed by western blotting (A). Quantitative densitometric results are shown. The data represent the mean ± SD of samples (n = 3). *P < 0.05 versus control. Quantification of MMP2 mRNA was performed using RT‐PCR (B). The data represent the mean ± SD of samples (n = 3). ND, not detected.
This ROCKI‐induced amoeboid–mesenchymal transition was also observed under X‐ray‐irradiated conditions. The number of the invasive cells increased, indicating the additive effects of ROCKI treatment and X‐ray irradiation (Fig. 6A).
Combined effects of MMP2I and ROCKI. We tested whether the invasiveness of MIAPaCa‐2 cells could be suppressed if the cells attained mesenchymal motility by treatment with ROCKI and whether the induced protease activity could be suppressed by treatment with MMPI. The simultaneous use of both MMPI and ROCKI significantly suppressed both the X‐ray‐induced and basal invasiveness of unirradiated MIAPaCa‐2 cells (Fig. 6A, Fig. S4).
Discussion
Studies have shown that the invasiveness of tumor cells is enhanced by X‐ray irradiation via induction of proteases, which degrade the ECM.( 5 , 6 , 22 , 23 ) Recent reports suggested that tumor cells can be classified into those that predominantly take on an elongated or mesenchymal morphology, a rounded or amoeboid morphology, or an intermediate (oval) type of morphology.( 7 , 8 , 9 , 10 , 11 , 12 , 13 ) Because the invasion of cells of the mesenchymal morphology depends on proteolysis, this type of invasion is expected to be suppressed by treatment of the cells with protease inhibitors. This was verified in the present study, wherein the inhibition of MMP activity by using its specific inhibitor decreased the number of invasive MIAPaCa‐2 cells. However, although MMPI treatment of the cells resulted in the mesenchymal–amoeboid switch, this transition did not enhance the invasiveness of MIAPaCa‐2 cells. Further investigation is required to understand the effect of this transition on the induction of ROCK activity required for amoeboid‐type invasion.
Invasion of cells with the amoeboid mode of motility is expected to be blocked by ROCKI. Y27632 blocks Rho‐mediated activation of myosin and suppresses the invasiveness of cells of several cancer lines.( 24 , 25 , 26 , 27 , 28 ) Wyckoff et al. ( 29 ) suggested that ROCKI may be related to the dramatic alteration in the localization of myosin light chains; instead of forming bundles around the cell cortex and behind the invasive edge, myosin light chains spread diffusely in the cytoplasm, except in certain localized areas around the cell margin. Furthermore, Xue et al. ( 30 ) showed that Y27632 and RhoC siRNA reduced the expression of MMP2 and MMP9 as well as the chemotactic migration of tumor cells in a dose‐dependent manner. In contrast, treatment of MIAPaCa‐2 cells with Y27632 stimulated the amoeboid–mesenchymal transition, induced MMP2, and, as a result, increased the number of invasive cells. Similar findings have been reported with astrocytoma treated with Y27632.( 24 )
Although several reports have indicated that MMP activation is involved in the altered invasiveness of X‐ray‐ or γ‐ray‐irradiated cells,( 5 , 6 , 22 , 23 ) these reports did not discuss the mode of cell motility. To our knowledge, this study presents, for the first time, evidence that ROCKI promotes MMP2 induction and enhances the invasiveness of cells with the ability to undergo the amoeboid–mesenchymal transition. MIAPaCa‐2 cells exhibited MMP2 expression on exposure to both irradiation and ROCKI. It has been reported that the transcription factors Sp1, Sp3 and AP2 are required for constitutive MMP2 expression.( 31 , 32 ) Several pathways may be associated with the radiation‐induced upregulation of MMP2 mRNA. Zhao et al. ( 33 ) reported that the upregulation of MMP2 is due to increased mRNA stability and is mediated by redox in rat tubule epithelial cells. Park et al. ( 34 ) showed that γ‐ray irradiation increased MMP2 promoter activity through Src‐dependent epidermal growth factor receptor activation, which triggers the p38/Akt and PI3K/Akt signaling pathways in PTEN‐mutant glioma cells. Our data showed that MMP2 expression is regulated at the mRNA level by ROCKI treatment as well as by photon irradiation; however, the mechanism underlying ROCKI‐induced activation of MMP2 expression is still unclear and requires further analysis.
The significance of MMP‐2 in the pathogenesis of pancreatic cancer has been recognized,( 35 , 36 , 37 ) but treatment with MMPI has not shown much benefit in the survival of pancreatic cancer patients.( 35 , 38 , 39 , 40 ) As we demonstrated the role of inter‐convertible mesenchymal and amoeboid modes in the invasiveness of MIAPaCa‐2 cells, which is modulated by inhibitors, this could provide an effective reduction in invasion, which is limited to the use of a single MMPI; the treatment with MMPI would be more effective with the use of ROCKI that suppress both X‐ray‐induced as well as basal invasiveness of cells.
Despite these findings, it remains unclear why some cells achieve invasive potential after exposure to irradiation or ROCKI treatment while others do not. In addition, the characteristics of the cells resistant to concomitant treatment with ROCKI and MMPI should be studied to help develop new therapeutic procedures for pancreatic tumors with invasive potential.
In conclusion, this is the first report indicating that the combination of X‐ray irradiation and the use of ROCKI enhance the invasiveness of pancreatic tumor cells with stimulation of amoeboid–mesenchymal transition, and that the simultaneous use of inhibitors of both amoeboid and mesenchymal movement of cells clearly suppresses the invasiveness of cells under both unirradiated and photon‐irradiated conditions.
Disclosure Statement
The authors declare that they have no competing financial interest.
Supporting information
Fig. S1. Effect of MMP2 activity by X‐ray irradiation and/or ROCKI.
Fig. S2. Morphological change of MIAPaCa‐1 cells after treatment with Y27632.
Fig. S3. Morphology of invaded cells of ROCKI‐treated cells.
Fig. S4. Effect of MMP2I and ROCKI on the invasiveness of MIAPaCa‐2.
Table S1. Primer sequences and probe numbers.
Table S2. Percentage of viable and dead cells used for migration and invasion assays.
Table S3. Percentage of viable and dead cells used for migration and invasion assays.
Table S4. Percentage of viable and dead cells used for invasion assays with inhibitor.
Table S5. Percentage of viable and dead cells used for invasion assays with inhibitor.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
This work was supported in part by a Grant‐in‐Aid for Scientific Research (22591394) from the Japan Society for the Promotion of Science. We thank Drs Yuichi Michikawa and Kenichi Ishikawa for helpful discussions and advice, and Naoko Shiomi and Katsuko Noshiro for technical assistance.
References
- 1. Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol 2009; 6: 412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lau WY, Lai ECH. Development and controversies of adjuvant therapy for pancreatic cancer. Hepatobiliary Pancreat Dis Int 2008; 7: 121–5. [PubMed] [Google Scholar]
- 3. Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer 2003; 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruano‐Ravina A, Ortega RA, Guedea F. Intraoperative radiotherapy in pancreatic cancer: a systemic review. Radiother Oncol 2008; 87: 318–25. [DOI] [PubMed] [Google Scholar]
- 5. Qian LW, Mizumoto K, Urashima T et al. Radiation‐induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor; CGS27023. Clin Cancer Res 2002; 8: 1223–7. [PubMed] [Google Scholar]
- 6. Ogata T, Teshima T, Kagawa K et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005; 65: 113–20. [PubMed] [Google Scholar]
- 7. Wolf K, Mazo I, Leung H et al. Compensation mechanism in tumor cell migration: mesenchymal‐amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 2003; 160: 267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanz‐Morero V, Gadea G, Ahn J et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135: 510–23. [DOI] [PubMed] [Google Scholar]
- 9. Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol 2008; 10: 127–37. [DOI] [PubMed] [Google Scholar]
- 10. Yamazaki D, Kurisu S, Takenawa T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene 2009; 28: 1570–83. [DOI] [PubMed] [Google Scholar]
- 11. Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev 2009; 28: 65–76. [DOI] [PubMed] [Google Scholar]
- 12. Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010; 188: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mishima T, Naotsuka M, Horita Y, Sato M, Ohashi K, Mizuno K. LIM‐kinase is critical for the mesenchymal‐to‐amoeboid cell morphological transition in 3D matrices. Biochem Biophys Res Commun 2010; 392: 577–81. [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto Y, Iwakawa M, Furusawa Y et al. Gene expression analysis in human malignant melanoma cell lines exposed to carbon beams. Int J Radiat Biol 2008; 84: 299–314. [DOI] [PubMed] [Google Scholar]
- 15. Park JH, Han HJ. Caveolin‐1 plays important role in EGF‐induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol 2009; 297: C935–44. [DOI] [PubMed] [Google Scholar]
- 16. Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 2004; 11: 36–42. [Google Scholar]
- 17. Kaneshiro ES, Wyder MA, Wu YP, Cushion MT. Reliability of calcein acetoxy methyl ester and ethidium homodimer or propidium iodide for viability assessment of microbes. J Microbiol Methods 1993; 17: 1–16. [Google Scholar]
- 18. Albini AY, Iwamoto H, Kleinman KG et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987; 47: 3239–45. [PubMed] [Google Scholar]
- 19. Imadome K, Iwakawa M, Nojiri K et al. Upregulation of stress‐response genes with cell cycle arrest induced by carbon ion irradiation in multiple murine tumors models. Cancer Biol Ther 2008; 7: 208–17. [DOI] [PubMed] [Google Scholar]
- 20. Sato H, Takino T, Miyamori H. Roles of membrane‐type matrix metalloproteinase‐1 in tumor invasion and metastasis. Cancer Sci 2005; 96: 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol 2007; 26: 587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akino Y, Teshima T, Kihara A et al. Carbon‐ion beam irradiation effectively suppresses migration and invasion of human non‐small‐cell lung cancer cells. Int J Radiat Oncol Biol Phys 2009; 75: 475–81. [DOI] [PubMed] [Google Scholar]
- 23. Goetze K, Scholz M, Taucher‐Scholz G, Mueller‐Klieser W. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int J Radiat Biol 2007; 83: 889–96. [DOI] [PubMed] [Google Scholar]
- 24. Salhia B, Rutten F, Nakada M et al. Inhibition of Rho‐kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res 2005; 65: 8792–800. [DOI] [PubMed] [Google Scholar]
- 25. Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho‐associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 2009; 69: 8742–51. [DOI] [PubMed] [Google Scholar]
- 26. Deng L, Li G, Li R, Liu Q, He Q, Zhang J. Rho‐kinase inhibitor, fasudil, suppresses glioblastoma cell line progression in vitro and in vivo. Cancer Biol Ther 2010; 9: 875–84. [DOI] [PubMed] [Google Scholar]
- 27. Micuda S, Rösel D, Ryska A, Brabek J. ROCK inhibitors as emerging therapeutic candidates for sarcomas. Curr Cancer Drug Targets 2010; 10: 127–34. [DOI] [PubMed] [Google Scholar]
- 28. Sun XZ, Li ZF, Liu Y, Fang P, Li MX. Inhibition of cGMP phosphodiesterase 5 suppresses matrix metalloproteinase‐2 production in pulmonary artery smooth muscles cells. Clin Exp Pharmacol Physiol 2010; 37: 362–7. [DOI] [PubMed] [Google Scholar]
- 29. Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK‐ and myosin‐dependent matrix deformation enables protease‐independent tumor‐cell invasion in vivo. Curr Biol 2006; 16: 1515–23. [DOI] [PubMed] [Google Scholar]
- 30. Xue F, Takahara T, Yata Y et al. Blockade of Rho/Rho‐associated coiled coil‐forming kinase signaling can prevent progression of hepatocellular carcinoma in matrix metalloproteinase‐dependent manner. Hepatol Res 2008; 38: 810–7. [DOI] [PubMed] [Google Scholar]
- 31. Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP‐2 are required for constitutive matrix metalloproteinase‐2 gene expression in astroglioma cells. J Biol Chem 1999; 274: 29130–7. [DOI] [PubMed] [Google Scholar]
- 32. Pan MR, Hung WC. Nonsteroidal anti‐inflammatory drugs inhibit matrix metalloproteinase‐2 via suppression of the ERK/Sp1‐mediated transcription. J Biol Chem 2002; 277: 32775–80. [DOI] [PubMed] [Google Scholar]
- 33. Zhao W, Goswami PC, Robbins ME. Radiation‐induced up‐regulation of Mmp2 involves increased mRNA stability, redox modulation, and MAPK activation. Radiat Res 2004; 161: 418–29. [DOI] [PubMed] [Google Scholar]
- 34. Park CM, Park MJ, Kwak HJ et al. Ionizing radiation enhances matrix metalloproteinase‐2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor‐mediated p38/Akt and phosphatidylinositol 3‐kinase/Akt signaling pathways. Cancer Res 2006; 66: 8511–9. [DOI] [PubMed] [Google Scholar]
- 35. Jones L, Ghaneh P, Humphreys M, Neoptolemos JP. The matrix metalloproteinases and their inhibitors in the treatment of pancreatic cancer. Ann NY Acad Sci 1999; 880: 288–307. [DOI] [PubMed] [Google Scholar]
- 36. Koshiba T, Hosotani R, Wada M et al. Involvement of MMP2 activity in invasion and metastases of pancreatic cancer. Cancer 1998; 82: 642–50. [DOI] [PubMed] [Google Scholar]
- 37. Bramhall SR, Stamp GWH, Dunn J, Lemoine NR, Neoptolemos JP. Expression on collagenease (MMP2), stromelysin (MMP3), and tissue inhibitor of the metalloproteinases (TIMP1) in pancreatic and ampullary disease. Br J Cancer 1996; 73: 972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kruger A, Soeltl R, Sopov I et al. Hydroxamate‐type matrix metalloproteinase inhibitor batismastat promotes liver metastasis. Cancer Res 2001; 61: 1272–5. [PubMed] [Google Scholar]
- 39. Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JAC. Marimastat as first‐line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol 2001; 19: 3447–55. [DOI] [PubMed] [Google Scholar]
- 40. Moore MJ, Hamm P, Eisenberg P, Dagenais M, Hagan K, Fields A. A comparison between gemcitabine and the matrix metalloproteinase inhibitor BAY 12‐9566 in patients with advanced pancreatic cancer. Proc Am Soc Clin Oncol 2000; 19: 240a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of MMP2 activity by X‐ray irradiation and/or ROCKI.
Fig. S2. Morphological change of MIAPaCa‐1 cells after treatment with Y27632.
Fig. S3. Morphology of invaded cells of ROCKI‐treated cells.
Fig. S4. Effect of MMP2I and ROCKI on the invasiveness of MIAPaCa‐2.
Table S1. Primer sequences and probe numbers.
Table S2. Percentage of viable and dead cells used for migration and invasion assays.
Table S3. Percentage of viable and dead cells used for migration and invasion assays.
Table S4. Percentage of viable and dead cells used for invasion assays with inhibitor.
Table S5. Percentage of viable and dead cells used for invasion assays with inhibitor.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


