Abstract
Secretory leukocyte peptidase inhibitor (SLPI) belongs to the whey acidic protein four‐disulfide core family of proteins, and has antimicrobial and antiprotease functions. SLPI is produced by the epithelial cells lining the respiratory, digestive, and reproductive tracts. Gene‐targeting experiments in mice indicated that one function of SLPI is to protect proepithelin from elastase cleavage in wound healing. In addition to its antiprotease function, SLPI has an anti‐inflammatory function through the modulation of nuclear factor‐κB acting intracellularly, especially in macrophages. SLPI is also produced in cancer tissues, but its role in cancer is not well understood. SLPI genes are often upregulated under tumorigenic conditions. We found a negligible number of tumors in the lungs of SLPI knockout mice 20 or 40 weeks after administration of urethane, an interesting experimental model for investigating the function of SLPI in cancer. This review discusses the normal function of SLPI and its possible roles in cancer tissues. (Cancer Sci 2008; 99: 849–855)
Secretory leukocyte peptidase inhibitor (SLPI), previously known as secretory leukoprotease (or leukocyte protease) inhibitor or antileukoproteinase, was originally isolated from the secretions of patients with chronic obstructive pulmonary disease in the 1970s and consists of 107 amino acids (11.7 kDa) organized in a duplicated, boomerang‐shaped whey acidic protein four‐disulfide core (WFDC) domain (Fig. 1).( 1 , 2 ) SLPI is secreted from mucosal epithelial cells lining the respiratory, digestive, and reproductive tracts, as well as from neutrophils and macrophages.( 3 , 4 ) It is highly expressed in the normal lung. According to the microarray data deposited in the National Centre for Biotechnology Information Gene Expression Omnibus (GEO; GSE1643), SLPI mRNA is among the most abundant transcripts, including those of surfactant proteins.( 5 ) The lung epithelial lining fluid contains approximately 10 µM SLPI protein.( 1 ) However, the SLPI protein is measured using lining fluid from respiratory, digestive, and genital organs, whereas the SLPI mRNA transcripts are determined using whole organs. In this context, the most recent reference of the relative expression of the SLPI mRNA transcript will come from GSE3526 (comparison of gene expression profiles across the normal human body) deposited by Dr RB Roth (Neurocrine Biosciences, Inc.) in 2005. The relative SLPI mRNA expression is high in trachea, bronchus, oral mucosa, salivary gland pharyngeal mucosa, esophagus, lung, and vagina (Table 1).
Figure 1.
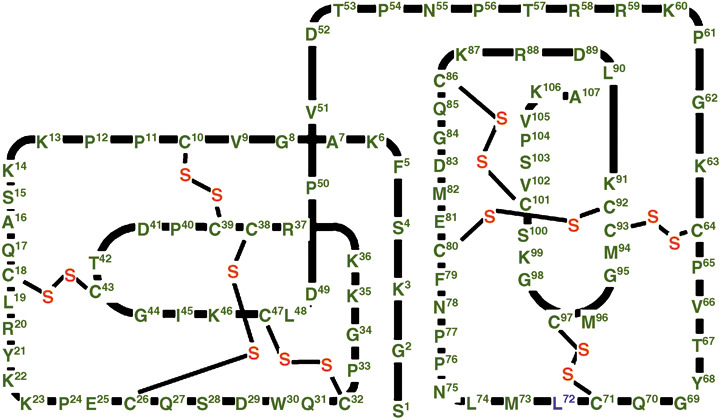
Schematic representation of the polypeptide chain arrangement of human secretory leukocyte peptidase inhibitor (SLPI). SLPI consists of 107 amino acids with a boomerang‐shaped structure composed of two topologically superimposable domains: an N‐terminal domain consisting of Ser1–Pro54 and a C‐terminal domain consisting of Asn55–Ala107. Four disulfide bridges are present in each domain (indicated by thin lines).
Table 1.
Relative expression of secretory leukocyte peptidase inhibitor (SLPI) mRNA in human tissue
| Organ | Relative expression (%) |
|---|---|
| Trachea | 100 |
| Bronchus | 85 |
| Oral mucosa | 51 |
| Salivary gland | 46 |
| Pharyngeal mucosa | 29 |
| Esophagus | 20 |
| Lung | 11 |
| Vagina | 7 |
| Mammary gland | 4 |
| Tonsil | 4 |
| Adipose tissue | 3 |
| Bone marrow | 2 |
| Liver | 2 |
| Prostata grand | 1 |
| Colon cecum | 1 |
| Thyroid gland | 1 |
| Testis | 1 |
| Kidney medulla | 1 |
| Adrenal cortex | 1 |
| Skeletal muscle | 1 |
| Kidney cortex | 0 |
| Ovary | 0 |
| Lymph node | 0 |
| Spleen | 0 |
Adopted from the microarray data from GSE3526 that analyzed representative normal tissue from 10 postmortem donors using Affymetrix U133 plus 2.0. The average value of the expression of the SLPI mRNA transcripts from three to five donors were sorted and shown as relative expression (%).
In addition, SLPI is expressed in cancer cells originating from organs that normally express SLPI. SLPI is upregulated in non‐small cell lung cancer (NSCLC), and cancers of the ovary, cervix, and pancreas, but not in those of the breast, kidney, intestinal tract, or nasopharynx.( 6 ) For example, SLPI is one of the six genes in pancreas cancer identified as being highly expressed by U133 oligonucleotide array, cDNA microarray, and serial analysis of gene expression (SAGE).( 7 ) The function of SLPI expression in cancer tissues is not clear. As cancer tissues are sites of inflammation, consisting of cancer cells and mesenchymal stromal cells, SLPI may have a role in the formation of cancer tissues. Interestingly, the function of SLPI is not restricted to that of a protease inhibitor as originally defined. Recent reports have described distinct roles in immune and inflammatory modulation.( 2 , 8 ) We attempted to establish urethane‐based chemical carcinogenesis in the lungs of SLPI knockout mice, and found that lung carcinogenesis is suppressed in the absence of SLPI. The present review article provides an overview of the current understanding of the function of SLPI and discusses the role of SLPI in lung carcinogenesis.
Secretory leukocyte peptidase inhibitor as a whey acidic protein motif protein: Cloning, chromosome location, and regulation of tissue expression
To further investigate the function of SLPI in the lungs, we first cloned mouse SLPI mRNA from the lung poly A+ RNA of C57BL/6 mice using degenerate primers based on human SLPI gene sequences, a region that is highly homologous with porcine SLPI.( 9 ) The full‐length mouse SLPI cDNA had 66% nucleotide homology and 58% amino acid homology with human SLPI. The tissue distribution of the SLPI mRNA transcripts in the mouse revealed high levels of expression in the lungs, spleen, small intestine, and epididymis, and low levels of expression in the liver and seminal vesicles.( 9 ) We have identified a lung cell‐specific region in the promoter of the human SLPI gene.( 10 ) We examined the regulation of SLPI expression in the lungs of mice with pneumonia, and found that the SLPI transcript levels were upregulated by three‐fold 10 h after nasal inoculation with Streptococcus pneumoniae.( 9 )
Secretory leukocyte peptidase inhibitor is located on chromosome 20q12–13.1 in humans and on chromosome 2H in mice with a similar exon–intron configuration.( 11 ) Although physiologically unique and designated as a protease inhibitor, SLPI has the structural characteristics of a WFDC domain. Chromosome 20q13 was recently recognized as the WFDC locus, containing the genes encoding 14 WFDC‐type protease inhibitors.( 12 ) The WFDC‐encoded proteins are thought to play roles in innate immunity and in regulating endogenous kallikreins, and are expressed predominantly in the epididymis, testis, and trachea. More recently, the WFDC locus was reported to have undergone rapid divergence in the primate lineage.( 13 ) WFDC‐type protease inhibitors also differ considerably between human and mouse. For example, elafin, another airway WFDC‐type protease inhibitor, has no ortholog in the mouse. To search for new WFDC‐type protease inhibitors, we cloned the mouse SWAM1 and SWAM2 genes and showed that both gene products are antibacterial.( 14 ) In summary, SLPI is secreted in the lining fluid of the airways, and works as an antiprotease and has unknown innate immune functions.
Complicated function of SLPI
Protease inhibitor. SLPI was originally discovered as a protein with antiprotease activity, and this is its major function. Although it consists of two similar domains, the antiprotease active site of SLPI is Leu72‐Met73 in a loop (residues 67–74) on the C‐terminal domain.( 2 ) SLPI inhibits elastase, cathepsin G, trypsin, chymotrypsin, chymase, and tryptase. However, its physiological target is neutrophil elastase, because SLPI accounts for approximately 90% of all elastase inhibitors in human bronchial secretions, and it has high affinity for neutrophil elastase.( 1 ) Experiments in SLPI knockout mice clarified the balance of SLPI and elastase in a model of wound healing. Ashcroft et al. reported high elastase activity, large amounts of active transforming growth factor (TGF)‐β in the wound, and impaired healing in SLPI knockout mice.( 15 ) Zhu et al. analyzed this mechanism further and reported that SLPI protects proepithelin (also called progranulin) from cleavage by neutrophil elastase.( 16 ) In SLPI knockout mice, proepithelin is cleaved into epithelins, which recruit more neutrophils and suppress epithelial cell growth. Several recent reports have described SLPI as being inactivated by proteolytic cleavage by cathepsins and bacterial proteases. At sites of inflammation, the balance between the processes involved in augmenting the innate immune reaction and tissue repair and regeneration is controlled by the relative amounts of neutrophil elastase and its inhibitor SLPI (Fig. 2; Table 2). Considering normal wound healing in individuals with a deficiency in α‐1‐antitrypsin, another antiprotease for neutrophil elastase, the function of SLPI in tissue repair and maintenance appears to be highly specialized.
Figure 2.
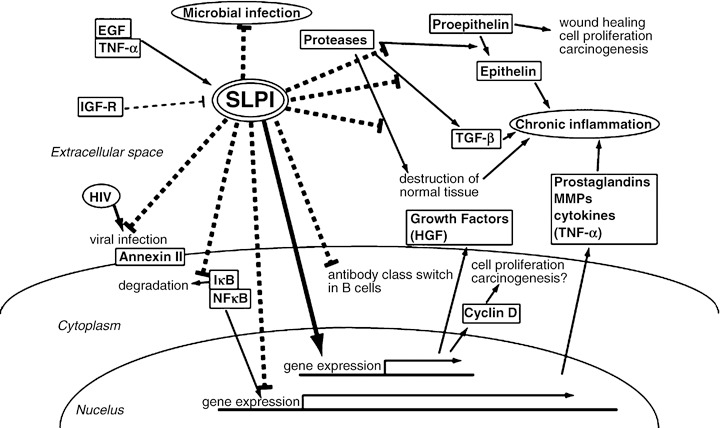
A schema of the various reported biological functions of secretory leukocyte peptidase inhibitor (SLPI). A major physiological role of SLPI is thought to be antineutrophil elastase protection at sites of inflammation. In addition, recent studies have revealed that SLPI has several biological properties, some of which are independent of its protease inhibitor activity.
Table 2.
Biological functions of secretory leukocyte peptidase inhibitor (SLPI)
| Effect on pathway | Target | Mechanism of regulation |
|---|---|---|
| Control of inflammation | ||
| Suppression of epithelin( 16 ) | Epithelin | Dependent of antiprotease activity |
| Decrease TNF‐α, MCP‐1, IL‐6 expression( 25 ) | TNF‐α | Dependent of antiprotease activity |
| MCP‐1 | ||
| IL‐6 | ||
| Increase HGF expression( 29 ) | HGF | Dependent of antiprotease activity |
| Suppression of PGE2, MMP1, MMP9( 27 ) | PGE2 | Independent of antiprotease activity |
| MMP1 | ||
| MMP9 | ||
| Decrease TGF‐β activity( 15 , 30 ) | TGF‐β | Regulating gene expression? |
| Dependent of antiprotease activity? | ||
| Increase TGF‐β, IL‐10( 26 ) | TGF‐β | Unknown mechanism |
| IL‐10 | ||
| Suppression of class switch( 24 ) | AID? | Inhibition of AID induction |
| Suppression of NF‐κB activity( 8 , 23 ) | IκBβ | Inhibition of IκB‐β degradation? |
| NF‐κB | Inhibition of NF‐κB and DNA binding? | |
| Cell proliferation | ||
| Increase cyclin D expression( 30 ) | Cyclin D | Unknown mechanism |
| Anti‐infection | ||
| Anti‐HIV‐infection( 20 ) | Annexin II | Binding to annexin II |
| Antimicrobial infection( 17 ) | Unknown | Unknown |
| Independent of antiprotease activity | ||
AID, activation‐induced cytidine deaminase; HGF, hepatocyte growth factor; HIV, human immunodeficiency virus; IL, interleukin; MCP, monocyte chemoattractant protein‐1; MMP, matrix metalloprotease; NF, nuclear factor; PGE, prostaglandin E; TGF, transforming growth factor; TNF, tumor necrosis factor.
Antimicrobial and anti‐human immunodeficiency virus (HIV) capacity. Hiemstra et al. discovered an SLPI‐like peptide from equine neutrophils with antimicrobial capacity, although they reported that the antimicrobial activity of SLPI was less than those of lysozyme or defensins.( 17 ) They also found that the residues with antimicrobial activity were located in the N‐terminal domain, in contrast to the antiprotease activity. Although the precise mechanism underlying the antimicrobial capacity of SLPI remains unclear, the possible involvement of the cationic nature of the WFDC domains is suggested by previous findings that eNap‐2, a cationic polypeptide with features characteristic of the WFDC family, displays antibacterial activity.( 18 , 19 )
Ma et al. reported that SLPI prevents HIV transmission. They demonstrated that SLPI binds to the membrane of human macrophages through a phospholipid binding protein, annexin II, which is a cellular cofactor that acts as a phosphatidylserine‐binding moiety.( 20 ) Annexin II supports macrophage HIV‐1 infection and SLPI disrupts the interaction (Fig. 2). These antimicrobial and anti‐HIV capacities of SLPI may be effective because of its high concentration in the airway lining fluid.
Immunomodulatory and anti‐inflammatory functions. In an early clinical study of the administration of recombinant SLPI to patients with cystic fibrosis, McElvaney et al. reported that there were reductions in interleukin (IL)‐8 and neutrophil elastase in the bronchoalveolar lavage fluid from these patients after inhalation of aerosolized SLPI.( 21 ) Subsequent studies showed that SLPI exerts its immunomodulatory effects through attenuation of nuclear factor (NF)‐κB. We constructed SLPI knockout mice, and examined the changes in immune‐related factors after lipopolysaccharide (LPS) administration.( 22 ) In SLPI −/– mice after LPS administration, the survival rate was lower than that of wild‐type (WT) controls, and high levels of serum IL‐6, but not IL‐1β, tumor necrosis factor (TNF)‐α, or NO2 − were noted. High mobilty group B1 (HMGB1) in macrophages was increased in SLPI −/– mice. In SLPI −/– mice, IκB‐β was suppressed after LPS administration. In contrast, the DNA‐binding activities of NF‐κB and CCAAT/enhancer binding protein (C/EPP)‐β were stronger in SLPI −/– macrophages.( 22 ) We also reported that splenic B cells from SLPI −/– mice had higher proliferation rates and produced higher levels of Ig M. One of the mechanisms for the regulation of NF‐κB activation by SLPI was assumed to be that SLPI elevates the protein, not mRNA, level of IκB‐β, a NF‐κB inhibitor.( 23 ) Recently, Taggart et al. reported that on incubation with the U937 monocytic cell line or peripheral blood monocytes, SLPI enters the cells and is rapidly localized to the cytoplasm and nucleus where it affects NF‐κB activation by direct binding to NF‐κB sites in a site‐specific manner( 8 ) (Fig. 2). This was confirmed recently by Xu et al. who showed that SLPI from tonsillar epithelial cells restrained class switching by inhibiting activation‐induced cytidine deaminase (AID) induction in B cells, and again they found SLPI in the nucleus and cytoplasm.( 24 )
Control of inflammation and tissue repair. There have been many reports of the attenuation of inflammation by SLPI. It has been reported that the production of TNF‐α, monocyte chemoattractant protein‐1 (MCP‐1), and IL‐6 in macrophages is downregulated,( 25 ) whereas the expression of the anti‐inflammatory cytokines IL‐10 and TGF‐β is upregulated.( 26 ) Ashcroft et al. reported high TGF‐β activity in SLPI‐deficient mice,( 15 ) although some inconsistencies, especially about TGF‐β and TNF‐α, were noted due to the experimental systems used (Table 2). SLPI decreased the production of cyclooxygenase (COX)‐2, PGE2, matrix metalloprotease (MMP)‐1, and MMP‐9 with the result of suppressed tissue injury.( 27 ) Mouse SLPI was purified from mouse fibroblasts as a scatter factor‐ or hepatocyte growth factor (HGF)‐inducing factor,( 28 ) and so we examined HGF induction by SLPI. Our results indicated that SLPI induced HGF from human fibroblasts in a time‐ and dose‐dependent manner.( 29 ) Moreover, HGF was induced by the C‐terminal domain of SLPI, but not by the N‐terminal half of SLPI or by defensin (Fig. 3). Interestingly, α‐1‐antitrypsin also induced HGF, but not ONO‐5046, an elastase inhibitor. Zhang et al. reported that cyclin D1 gene expression was elevated when SLPI was expressed.( 30 ) Cyclin D1 may be associated with cell proliferation during tissue repair.( 30 ) Therefore, SLPI acts as a multifunctional defense factor: as an antiprotease to promote wound healing, by controlling pro‐inflammatory factors by suppression of the action of NF‐κB, suppression of COX‐2 and PGE2, and by induction of HGF, a tissue‐regenerating factor, from mesenchymal cells (Fig. 2).
Figure 3.
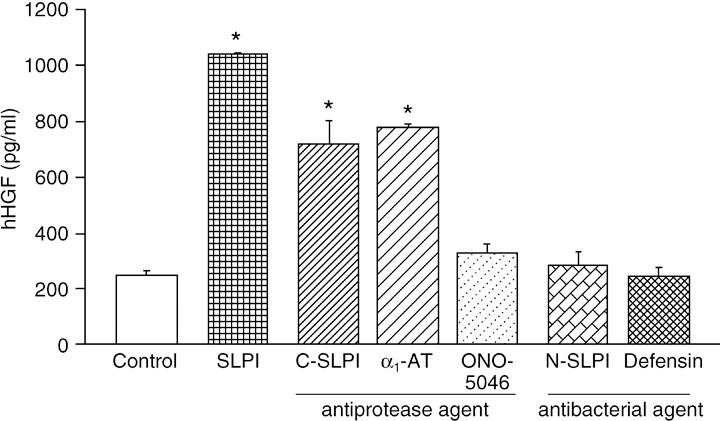
Effects of secretory leukocyte peptidase inhibitor (SLPI) on hepatocyte growth factor (HGF) production by human lung fibroblasts.( 29 ) Human lung fibroblast CCD‐11Lu cells were cultured for 16 h under the following conditions: control, no addition; SLPI, 10 µM full‐length SLPI; C‐SLPI, 10 µM C‐terminal domain of SLPI; α1‐AT, 50 µg/mL human α1‐antitrypsin; ONO‐5046, 10 µM synthetic neutrophil elastase inhibitor; N‐SLPI, 10 µM N‐terminal domain of SLPI; defensin, 2.5 µg/mL human neutrophil peptides (HNP)‐1. The concentrations of human HGF (hHGF) in the culture medium were determined by enzyme‐linked immunosorbent assay. The results are shown as the mean ± SEM (n = 3 per data point). Asterisks indicate significant differences at the 95% confidence level compared with control cells.
Secretory leukocyte peptidase inhibitor and cancer
Overexpression and downregulation of SLPI. Based on the tissue distribution of SLPI expression, neoplasms of the respiratory, digestive, and reproductive tracts should express SLPI transcripts and protein. Bouchard et al. reported in their review( 6 ) that SLPI expression is highly upregulated in pancreatic, papillary thyroid, uterine cervix, endometrial, and ovarian cancer; by contrast, SLPI is underexpressed in nasopharyngeal carcinoma, bladder tumors, and some breast carcinoma, although overexpression of this protein correlates with more‐invasive forms of breast carcinoma. Although the underlying mechanisms have not been fully elucidated, Devoogdt et al. suggested that, at least in female cancers, the amplification of chromosome 20q (20q12 to q13.2) encoding the SLPI gene may result in increased SLPI expression.( 31 , 32 )
Secretory leukocyte peptidase inhibitor in lung cancer. Immunostaining with anti‐SLPI antibody indicates that lung cancer tissue contains SLPI protein. Ameshima et al. reported that the SLPI concentration in serum is higher in patients with lung cancer than in healthy controls (71.5 ± 2.4 vs 50.5 ± 1.5 ng/mL, P < 0.005), higher in advanced cases of NSCLC (stages III and IV), and decreased after effective treatment,( 33 ) indicating that the serum SLPI level reflects the tumor volume. However, in the gene‐expression profiling data for lung cancer (NCBI GEO DataSet GSE3141), the levels of SLPI transcripts in lung adenocarcinoma or squamous cell carcinoma are not always upregulated.( 34 ) The average SLPI to β‐actin expression ratio in the normal lung is 0.47 ± 0.17 (mean ± SD)( 5 ) whereas 24 and 23% of adenocarcinoma and squamous cell carcinoma samples, respectively, expressed more than the range of SD in normal lung samples, and 43 and 40%, respectively, expressed less than the SD range (Fig. 4). Several factors such as inflammatory cytokines and steroid hormones have been reported to affect SLPI gene expression.( 31 ) The scattering result of SLPI transcripts in lung tumors may be due to the various clinical settings of the tumor samples.
Figure 4.
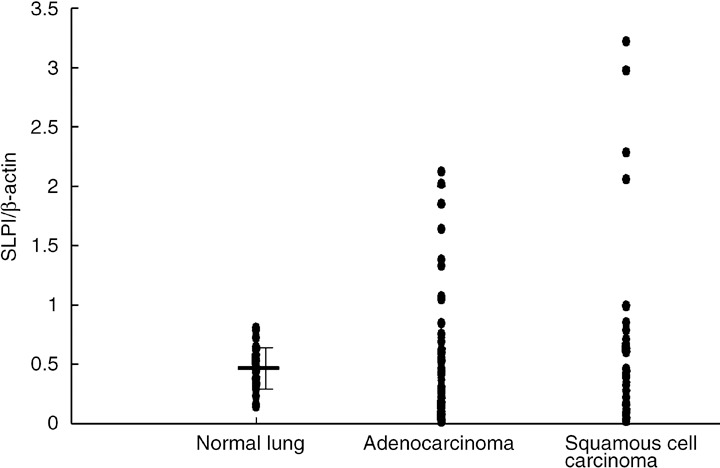
Comparison of secretory leukocyte peptidase inhibitor (SLPI) expression in normal tissue, lung adenocarcinoma, and squamous cell carcinoma. The data were extracted from the NCBI GEO DataSets GSE1643 (normal lung, 40 samples,( 5 ) and GSE3141 lung adenocarcinoma, 58 samples; squamous cell carcinoma, 53 samples).( 34 ) The probe ID in the Affymetrix platforms were 2030211_at (SLPI) and AFFX‐HSAC07/X00351–5_at (β‐actin). The relative expression ratios of SLPI to β‐actin were evaluated.
As SLPI is a secretory protein and is expressed at levels similar to those of highly expressed proteins, such as surfactants, the promoter regions of the SLPI gene can be utilized for lung cancer‐specific expression of transgenes by intratumoral injection. We applied this idea in a preclinical experimental system of a replication‐competent adenovirus vector for NSCLC.( 35 ) A large xenograft (approximately 600 mm3) of H358 cells in nude mice was effectively reduced in size with a single intratumoral dose of combined AdSLPI.E1AdB (adenovirus aimed for high E1A gene expression under the SLPI promoter in lung cancer) and AdCMV.NK4 (NK4; N‐terminal truncated HGF that antagonizes HGF, vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF)( 36 )) indicating the advantages of utilizing the promoter of the highly expressed SLPI gene.
Upregulation of SLPI in the gene expression profiles under tumorigenic conditions: Microarray analysis and SLPI transfection. As SLPI functions physiologically as an anti‐inflammatory agent and plays a role in tissue regeneration, the SLPI gene is often amplified under conditions related to tumorigenesis. When phorbol‐12‐myristate‐13‐acetate (TPA) was applied to mouse skin to produce chemical carcinogenesis, the SLPI expression in the treated skin was upregulated 6 h after TPA induction.( 37 ) When low‐malignant Lewis lung carcinoma 3LL‐S cells were inoculated subcutaneously, and high‐malignant 3LL‐S‐sc cells were isolated from the subcutaneous tumor, the subtraction procedure used to identify genes modulated during the subcutaneous growth of 3LL‐S cells identified mouse SLPI.( 38 ) Transfection of the mouse or human SLPI gene into 3LL‐S cells enhanced tumor growth, but tumor formation by these transfectants was not related to in vitro cell proliferation. SLPI, HSP‐70, and CXCL‐1 were identified among 106 genes expressed differentially >2.5‐fold between poorly metastatic and highly metastatic variants of human breast cancer cell lines, GI101A and GILM2, respectively.( 39 ) Viatour et al. reported that glycogen synthase kinase (GSK) 3‐mediated B cell lymphoma (BCL)‐3 phosphorylation modulates its degradation and upregulates the expression of SLPI, CXCL‐1, interferon activated gene (IFI)205, and cytochrome P4SO 1B1 (CYP1B1).( 40 )
In addition, SLPI was identified as a potential regulatory gene in the microarray expression profile at markedly higher levels (>2 log difference) in non‐metastatic 3LL M‐27 lung carcinoma cells compared with liver‐metastatic 3LL H‐59 lung carcinoma cells.( 41 ) Wang et al. transfected the SLPI gene into H‐59, and showed that SLPI from transfected H‐59 cells suppressed TNF‐α production from a macrophage cell line in vitro by regulation of NF‐κB, and inhibited TNF‐α induction as well as E‐selectin in response to infiltrating tumor cells, and ultimately suppressed experimental liver metastasis in vivo.( 41 ) Interestingly, Devoogdt et al. reported that the tumor‐promoting effect of TNF‐α involves the induction of SLPI.( 42 ) They used cancer cell lines derived from subcutaneous nodules of 3LL‐S cells in WT mice (3LL‐S‐sc WT) and TNF‐α knockout mice (3LL‐S‐sc KO). Although the tumor growth of 3LL‐S‐sc WT cells was higher than that of 3LL‐S‐sc KO cells, SLPI was expressed in larger amounts in 3LL‐S‐sc WT cells( 42 ) suggesting that endogenous TNF‐α contributed to SLPI protein production at the tumor site. Interestingly, 3LL‐S cells transfected with SLPI small interfering RNA (siRNA) showed no tumor formation in WT mice.( 42 )
Finally, Sugino et al. reported that SLPI promotes blood‐borne metastasis via an invasion‐independent pathway.( 43 ) They transfected the SLPI gene into a poorly metastatic clone of the MCH66 mouse mammary tumor cell line. Although SLPI‐transfected cells showed a less‐invasive nature in a Matrigel assay, in vivo inoculation of SLPI‐transfected cells into the mammary fat pads resulted in greater lung and lymph node metastasis. Histological examination revealed that SLPI‐transfected tumors formed a well‐developed CD31‐positive sinusoidal vasculature surrounding the tumor nests, where tumor cells are supposed to enter the circulation without vascular invasion.( 43 ) In fact, these tumors showed intravascular growth and the formation of metastatic nodules in the lung. Based on these results, the authors proposed the functional role of SLPI in the invasion‐independent metastatic potential of cancer cells.( 43 )
In summary, how the SLPI expression of cancer cells affects metastatic potential remains to be fully defined; specifically, the higher SLPI expression in higher‐metastatic breast cancer cell lines( 41 , 43 ) and, in contrast, the higher SLPI expression in non‐metastatic lung cancer cells.( 39 ) The discordance among previous reports may depend on the experimental conditions used, such as cell line.
Carcinogenesis is suppressed in the SLPI knockout mouse
Urethane carcinogenesis. To investigate whether SLPI induces carcinogenesis or restrains the growth of cancer tissue, we induced chemical carcinogenesis using urethane. When urethane was administered intraperitoneally to WT mice at a dose of 1 mg/g bodyweight, urethane‐induced tumors in the lung were visible at 20 or 40 weeks. The induced tumors were lung adenocarcinoma‐like histologically, and the tumor cells showed strong staining with anti‐SLPI antibody. When we administered urethane to SLPI knockout mice, negligible tumor formation was detected at 20 or 40 weeks (Fig. 5). We confirmed that urethane‐metabolizing CYP2e1 is expressed in both WT and SLPI −/– mice.
Figure 5.
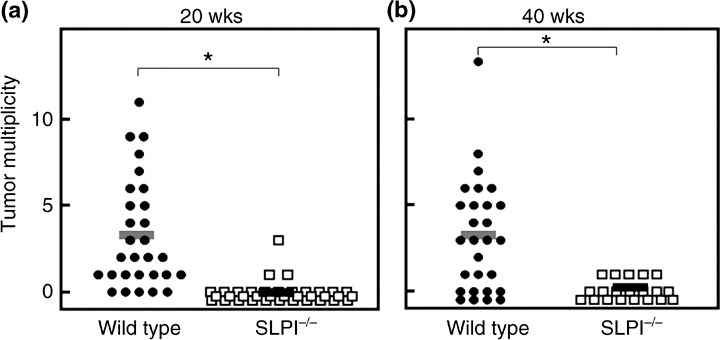
Decreased susceptibility to urethane‐induced lung tumors in secretory leukocyte peptidase inhibitor (SLPI)‐deficient mice. Wild‐type or SLPI −/– mice were injected intraperitoneally with urethane (1 mg/g bodyweight). The animals were killed (a) 20 or (b) 40 weeks later, and the number of macroscopic external pulmonary nodules was counted. Each bar represents the mean of the group: (a) wild‐type, n = 29 mice; SLPI −/–, n = 36 mice; (b) wild‐type, n = 28 mice; SLPI −/–, n = 19 mice. Asterisks indicate significant differences at the 95% confidence level between wild‐type and SLPI −/– mice.
Working hypothesis and further analyses. The functions of SLPI include: (i) its action as an extracellular protease inhibitor; (ii) penetration of the cytoplasm and nucleus in monocytes and macrophages to elicit anti‐inflammatory effects through suppression of NF‐κB function; and (iii) the induction of HGF from fibroblasts (Fig. 2). We have not succeeded in analyzing completely the suppression of urethane‐induced tumor formation in SLPI −/– mice. Even without SLPI function, some microseeds of tumor cells are formed, as determined by the low number of tumors in SLPI −/– mice at 20 or 40 weeks. The effects of SLPI in the tumor cells and in the surrounding stromal cells must be considered separately. When both tumor cells and environmental stromal cells contain SLPI, urethane‐induced tumors are formed. Conversely, when neither tumor cells nor environmental stromal cells contain SLPI, tumor formation is suppressed. In this context, the observation that 3LL‐S cells transfected with SLPI siRNA did not form tumors, as reported by Devoogdt et al.,( 42 ) indicated that the SLPI in tumor cells, but not that in the environmental stromal cells, is essential for tumor formation. Whether 3LL cells transfected with the SLPI gene inoculated into SLPI −/– mice (i.e. tumor cells are SLPI+ and environmental stromal cells are SLPI−) can form tumors is another essential question. In this regard, 3LL cells inoculated into SLPI −/– mice grew in the same manner as in WT mice, at least with subcutaneous inoculation (unpublished observation, Zaini and Nukiwa, 2007).
Gene expression in the lungs was examined in microarray analyses of WT and SLPI −/– mice, and interesting genes upregulated in the lungs of SLPI −/– mice are currently under investigation. SLPI binds to a number of proteins, such as proepithelin and annexin II. In a yeast two‐hybrid assay, we detected 18 partner candidate proteins, including both proepithelin and annexin II.( 44 ) The direct protein–protein interaction of SLPI would be another route for investigating SLPI function in tumorigenesis.
Finally, we noticed that some lung adenocarcinoma cell lines with epidermal growth factor receptor (EGFR) kinase‐activating mutations express and secrete large amounts of SLPI. The production of SLPI is suppressed by gefitinib, an inhibitor of EGFR kinase, but the effect is not complete. The role of SLPI in the EGFR signaling pathway in SLPI‐induced tumorigenesis should be examined in future studies.
Perspectives
Although located at a very unstable locus in chromosome 20q, SLPI is conserved in mammals, and abundant SLPI is produced in the respiratory and reproductive tracts. These observations are the background to the unique function of SLPI in cell physiology. In studies to clarify the function of SLPI, we noticed an unexpected phenotype in SLPI −/– mice: suppression of urethane‐induced carcinogenesis in the lung. There is accumulating evidence that a paucity of SLPI in cancer cells suppresses tumor formation. The control of elastase activity in the treatment of cancer is not generally accepted. However, Sato et al. used ONO‐5046, a synthetic elastase inhibitor, to suppress the growth of EBC‐1 lung cancer.( 45 ) However, SLPI has the opposite effect. SLPI expression is upregulated in tumor formation, and the absence of SLPI production does not promote tumor formation. Because urethane‐induced carcinogenesis in the lung is suppressed in SLPI −/– mouse, further analysis of SLPI function in relation to the tumor‐forming capacity may lead to the development of a novel treatment modality.
Acknowledgments
We thank Dr Takashi Tsuruo for encouragement regarding this review. The authors also thank our colleagues Drs Tatsuya Abe, Yuriko Mori, Akira Nakamura, Toshiyuki Takai, Koichi Hagiwara, and Daizo Koinuma for their efforts in the construction and analysis of SLPI knockout mice.
References
- 1. Abe T, Kobayashi N, Yoshimura K et al . Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Invest 1991; 87: 2207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weldon S, McGarry N, Taggart CC, McElvaney NG. The role of secretory leucoprotease inhibitor in the resolution of inflammatory responses. Biochem Soc Trans 2007; 35: 273–6. [DOI] [PubMed] [Google Scholar]
- 3. Doumas S, Kolokotronis A, Stefanopoulos P. Anti‐inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun 2005; 73: 1271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 1997; 88: 417–26. [DOI] [PubMed] [Google Scholar]
- 5. Gruber MP, Coldren CD, Woolum MD et al . Human lung project: evaluating variance of gene expression in the human lung. Am J Respir Cell Mol Biol 2006; 35: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey‐acidic‐protein motifs and cancer. Lancet Oncol 2006; 7: 167–74. [DOI] [PubMed] [Google Scholar]
- 7. Iacobuzio‐Donahue CA, Ashfaq R, Maitra A et al . Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res 2003; 63: 8614–22. [PubMed] [Google Scholar]
- 8. Taggart CC, Cryan SA, Weldon S et al . Secretory leucoprotease inhibitor binds to NF‐κB binding sites in monocytes and inhibits p65 binding. J Exp Med 2005; 202: 1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abe T, Tominaga Y, Kikuchi T et al . Bacterial pneumonia causes augmented expression of the secretory leukoprotease inhibitor gene in the murine lung. Am J Respir Crit Care Med 1997; 156: 1235–40. [DOI] [PubMed] [Google Scholar]
- 10. Kikuchi T, Abe T, Satoh K et al . Cis‐acting region associated with lung cell‐specific expression of the secretory leukoprotease inhibitor gene. Am J Respir Cell Mol Biol 1997; 17: 361–7. [DOI] [PubMed] [Google Scholar]
- 11. Kikuchi T, Abe T, Hoshi S et al . Structure of the murine secretory leukoprotease inhibitor (Slpi) gene and chromosomal localization of the human and murine SLPI genes. Am J Respir Cell Mol Biol 1998; 19: 875–80. [DOI] [PubMed] [Google Scholar]
- 12. Clauss A, Lilja H, Lundwall A. A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein. Biochem J 2002; 368: 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurle B, Swanson W, Green ED. Comparative sequence analyses reveal rapid and divergent evolutionary changes of the WFDC locus in the primate lineage. Genome Res 2007; 17: 276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagiwara K, Kikuchi T, Endo Y et al . Mouse SWAM1 and SWAM2 are antibacterial proteins composed of a single whey acidic protein motif. J Immunol 2003; 170: 1973–9. [DOI] [PubMed] [Google Scholar]
- 15. Ashcroft GS, Lei K, Jin W et al . Secretory leukocyte protease inhibitor mediates non‐redundant functions necessary for normal wound healing. Nat Med 2000; 6: 1147–53. [DOI] [PubMed] [Google Scholar]
- 16. Zhu J, Nathan C, Jin W et al . Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 2002; 111: 867–78. [DOI] [PubMed] [Google Scholar]
- 17. Hiemstra PS, Maassen RJ, Stolk J, Heinzel‐Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun 1996; 64: 4520–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couto MA, Harwig SS, Cullor JS, Hughes JP, Lehrer RI. eNAP‐2, a novel cysteine‐rich bactericidal peptide from equine leukocytes. Infect Immun 1992; 60: 5042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couto MA, Harwig SS, Lehrer RI. Selective inhibition of microbial serine proteases by eNAP‐2, an antimicrobial peptide from equine neutrophils. Infect Immun 1993; 61: 2991–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma G, Greenwell‐Wild T, Lei K et al . Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV‐1 infection. J Exp Med 2004; 200: 1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McElvaney NG, Nakamura H, Birrer P et al . Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin‐8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest 1992; 90: 1296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura A, Mori Y, Hagiwara K et al . Increased susceptibility to LPS‐induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)‐deficient mice. J Exp Med 2003; 197: 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lentsch AB, Jordan JA, Czermak BJ et al . Inhibition of NF‐κB activation and augmentation of IκBβ by secretory leukocyte protease inhibitor during lung inflammation. Am J Pathol 1999; 154: 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu W, He B, Chiu A et al . Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol 2007; 8: 294–303. [DOI] [PubMed] [Google Scholar]
- 25. Greene CM, McElvaney NG, O’Neill SJ, Taggart CC. Secretory leucoprotease inhibitor impairs Toll‐like receptor 2‐ and 4‐mediated responses in monocytic cells. Infect Immun 2004; 72: 3684–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sano C, Shimizu T, Sato K, Kawauchi H, Tomioka H. Effects of secretory leucocyte protease inhibitor on the production of the anti‐inflammatory cytokines, IL‐10 and transforming growth factor‐beta (TGF‐β), by lipopolysaccharide‐stimulated macrophages. Clin Exp Immunol 2000; 121: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, DeWitt DL, McNeely TB, Wahl SM, Wahl LM. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase‐2, prostaglandin E2, and matrix metalloproteinases. J Clin Invest 1997; 99: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosen EM, Knesel J, Goldberg ID et al . Scatter factor modulates the metastatic phenotype of the EMT6 mouse mammary tumor. Int J Cancer 1994; 57: 706–14. [DOI] [PubMed] [Google Scholar]
- 29. Kikuchi T, Abe T, Yaekashiwa M et al . Secretory leukoprotease inhibitor augments hepatocyte growth factor production in human lung fibroblasts. Am J Respir Cell Mol Biol 2000; 23: 364–70. [DOI] [PubMed] [Google Scholar]
- 30. Zhang D, Simmen RC, Michel FJ, Zhao G, Vale‐Cruz D, Simmen FA. Secretory leukocyte protease inhibitor mediates proliferation of human endometrial epithelial cells by positive and negative regulation of growth‐associated genes. J Biol Chem 2002; 277: 29 999–30 009. [DOI] [PubMed] [Google Scholar]
- 31. Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P. Secretory leukocyte protease inhibitor in cancer development. Ann NY Acad Sci 2004; 1028: 380–9. [DOI] [PubMed] [Google Scholar]
- 32. Israeli O, Goldring‐Aviram A, Rienstein S et al . In silico chromosomal clustering of genes displaying altered expression patterns in ovarian cancer. Cancer Genet Cytogenet 2005; 160: 35–42. [DOI] [PubMed] [Google Scholar]
- 33. Ameshima S, Ishizaki T, Demura Y, Imamura Y, Miyamori I, Mitsuhashi H. Increased secretory leukoprotease inhibitor in patients with nonsmall cell lung carcinoma. Cancer 2000; 89: 1448–56. [DOI] [PubMed] [Google Scholar]
- 34. Bild AH, Yao G, Chang JT et al . Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006; 439: 353–7. [DOI] [PubMed] [Google Scholar]
- 35. Maemondo M, Saijo Y, Narumi K et al . Gene therapy with secretory leukoprotease inhibitor promoter‐controlled replication‐competent adenovirus for non‐small cell lung cancer. Cancer Res 2004; 64: 4611–20. [DOI] [PubMed] [Google Scholar]
- 36. Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four‐kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res 2000; 60: 6737–43. [PubMed] [Google Scholar]
- 37. Schlingemann J, Hess J, Wrobel G et al . Profile of gene expression induced by the tumour promotor TPA in murine epithelial cells. Int J Cancer 2003; 104: 699–708. [DOI] [PubMed] [Google Scholar]
- 38. Devoogdt N, Hassanzadeh Ghassabeh G, Zhang J, Brys L, De Baetselier P, Revets H. Secretory leukocyte protease inhibitor promotes the tumorigenic and metastatic potential of cancer cells. Proc Natl Acad Sci USA 2003; 100: 5778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kluger HM, Chelouche Lev D, Kluger Y et al . Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res 2005; 65: 5578–87. [DOI] [PubMed] [Google Scholar]
- 40. Viatour P, Dejardin E, Warnier M et al . GSK3‐mediated BCL‐3 phosphorylation modulates its degradation and its oncogenicity. Mol Cell 2004; 16: 35–45. [DOI] [PubMed] [Google Scholar]
- 41. Wang N, Thuraisingam T, Fallavollita L, Ding A, Radzioch D, Brodt P. The secretory leukocyte protease inhibitor is a type 1 insulin‐like growth factor receptor‐regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res 2006; 66: 3062–70. [DOI] [PubMed] [Google Scholar]
- 42. Devoogdt N, Revets H, Kindt A, Liu YQ, De Baetselier P, Ghassabeh GH. The tumor‐promoting effect of TNF‐α involves the induction of secretory leukocyte protease inhibitor. J Immunol 2006; 177: 8046–52. [DOI] [PubMed] [Google Scholar]
- 43. Sugino T, Yamaguchi T, Ogura G et al . The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood‐borne metastasis via an invasion‐independent pathway. J Pathol 2007; 212: 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nukiwa T, Suzuki T. Effect of tobacco smoke in the intracellular trafficking of type II alveolar epithelial cells. Smoking Res Foundation Annu Res Report 2004; 10: 409–14. [Google Scholar]
- 45. Sato T, Takahashi S, Mizumoto T et al . Neutrophil elastase and cancer. Surg Oncol 2006; 15: 217–22. [DOI] [PubMed] [Google Scholar]


