Abstract
The purpose of the present study was to develop a new method of chemoembolization to improve the therapeutic effectiveness and safety profile of cancer treatment. A chemoembolization approach was designed for human solid tumors using resorbable calcium‐phosphate ceramic microspheres loaded with an agent anti‐angiogenic to tumor vasculature in vivo. The human uterine sarcoma cell line FU‐MMT‐3 was used in this study because this tumor is aggressive and also exhibits a poor response to radiotherapy or any chemotherapy currently used. The calcium‐phosphate ceramic microspheres loaded with TNP‐470, an anti‐angiogenic agent, were injected into FU‐MMT‐3 xenografts in nude mice three times per week for 8 weeks. The treatment using TNP‐470‐loaded microspheres suppressed tumor growth, compared to treatment with TNP‐470 alone, microspheres alone, and the control. The mean tumor weight after treatment using TNP‐470‐loaded microspheres was significantly lower than that after treatment with microspheres alone. These ceramic microspheres were remarkably embolized in tumor microvessels as well as in the feeding arteries and a significant reduction of intratumoral vascularity was also demonstrated following treatment with TNP‐470‐loaded microspheres. Severe loss of body weight was not observed in any mice treated with the TNP‐470‐loaded microspheres, compared to treatment with TNP‐470 alone. These results suggest that targeting tumor vasculature in human uterine sarcoma using calcium‐phosphate microspheres might be more effective and safer than the treatment that employs anti‐angiogenic agent alone. This new chemoembolization method incorporating an anti‐angiogenic agent may contribute to the effective treatment of locally advanced or recurrent solid tumors.
(Cancer Sci 2010; 101: 984–990)
Sarcomas, including carcinosarcomas, are uncommon, but are the most aggressive neoplasms among the known uterine malignancies.( 1 , 2 ) It is well known that these tumors have poor responses to radiotherapy or any of the chemotherapeutic agents with substantial toxic effects that are currently in use.( 1 , 2 , 3 , 4 ) The overall 5‐year survival rate in all stages of uterine sarcomas is under 40%, which is significantly lower than that in other uterine cancers.( 3 ) The standard treatments for these tumors, except for the early stages, have not yet been determined; thus, new therapeutic strategies must be immediately investigated. Recent studies, including that of the authors, have shown that rapid growth and early metastasis in these tumors might be associated with high angiogenic properties, in comparison to other uterine malignancies.( 4 , 5 , 6 )
Radiotherapy is one of the most effective treatments for cancers. For deep‐seated cancer, however, external irradiation provides only small doses and often causes damage to healthy tissue. Thus, chemoembolization approaches that use various biomaterials might be effective for treating these solid tumors, including cases that have already received radiotherapy.( 7 ) Many biomaterials are used as substitutes for human hard‐tissues such as bone or cartilage; these biomaterials have also recently been studied as carriers for drug delivery( 8 ). β‐Tricalcium phosphate (TCP) is a major compound of calcium‐phosphate and is known to be a resorbable ceramic. Recent studies have shown that TCP is an ideal biomaterial that dissolves gradually without any cytotoxic and allergic reaction and elicits no immune response.( 9 , 10 , 11 ) Calcium‐phosphate ceramic biomaterials have been synthesized for the purpose of clinical use in our (M.A., T.O., H.Y., and N.K) previous studies.( 12 , 13 , 14 ) In the present study, we used resorbable hollow TCP microspheres, which were successfully refined in our laboratory, as a new biomaterial for chemoembolization.
Recently, anti‐angiogenesis therapies have been introduced into the clinical field, representing a turning point in cancer therapy.( 15 ) However, an array of unexpected side effects of anti‐vascular endothelial growth factor (VEGF)/VEGF‐R treatments is now seen in clinical practice.( 16 ) Therefore, particulate drugs transported by carrier systems to tumor vasculature are expected to improve the therapeutic effectiveness and the safety profile of anti‐angiogenic therapy. These types of anti‐angiogenic treatments are thus expected for uterine sarcomas; however, such therapies have not yet been fully evaluated. Our previous studies have shown the effectiveness of anti‐angiogenic therapy using TNP‐470 for highly aggressive human uterine carcinosarcoma in vitro and in vivo.( 17 , 18 , 19 ) TNP‐470 is a low‐molecular‐weight synthetic analogue of fumagillin, a natural compound secreted by the Aspergillus fumigatus,( 20 ) and inhibits angiogenesis via endothelial cell cycle arrest in the late G1 phase.( 21 ) Methionine aminopeptidase‐2 (MetAP‐2) has been identified as a molecular target.( 22 ) The antitumor effect of TNP‐470 has been shown in various human malignancies in vitro and in vivo, including our own studies.( 23 , 24 , 25 , 26 , 27 )
To establish a new chemoembolization approach for aggressive solid tumors, we designed a novel biomaterial composed of resorbable hollow ceramic microspheres loaded with TNP‐470, an anti‐angiogenic agent for tumor vasculature. We then evaluated the usefulness and safety of this new anti‐angiogenic chemoembolization therapy using a human uterine sarcoma xenograft model that was previously established in our laboratory.( 28 )
Materials and Methods
Preparation of calcium‐phosphate ceramic microspheres. The calcium‐phosphate ceramic microspheres were synthesized in our laboratory at Meiji University, as previously reported.( 29 , 30 ) Briefly, the starting solution with a Ca/P ratio of 1:50 was prepared by mixing Ca(NO3)2, (NH4)2HPO4, and HNO3 so that their concentrations were 0.60 mol/dm, 0.40 mol/dm3, and 0.10 mol/dm3, respectively. The upper‐ and lower‐furnace temperatures were fixed at 850 and 300°C, respectively. The solution was sprayed into the heating zone using an ultrasonic vibrator with a frequency of 2.4 MHz and then the sprayed droplets were dried and pyrolyzed to form calcium‐phosphate microspheres. The resulting microspheres were washed with pure water, and freeze‐dried to prepare the “washed powder” for this examination. The resulting calcium‐phosphate microspheres were composed of b‐TCP (∼50 mass%) and Ca‐def HAp (∼50 mass%) biphases on the basis of X‐ray diffraction measurements. These b‐TCP and Ca‐def HAp are known as biodegradable ceramics. The Ca/P molar ratio, as determined by X‐ray fluorescence spectrometry, was 1:49, which agreed well with the nominal composition of the starting solution. The ultrastructure of the particle morphologies of the washed powder is shown in Figure 1(a). The powders were composed of microspheres with diameters of about 1 μm, determined using scanning electron microscopy (SEM), and the transparent appearance seen using transmission electron microscopy (TEM) suggests the hollow structure of the microsphere particles (Fig. 1a).
Figure 1.
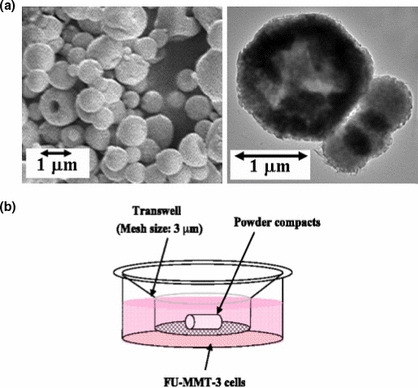
Particle morphologies of calcium‐phosphate microsphere and illustration of in vitro testing of the microsphere loaded with TNP‐470. (a) SEM (left), TEM (right) observations. (b) TNP‐470‐loaded microsphere with FU‐MMT‐3 cells using Transwell membrane inserts in vitro. First, 5 × 105 FU‐MMT‐3 cells were seeded on a 12‐well plate and cultured for 1 day, then the five different powder compacts were set on the membrane inserts of a Transwell polycarbonate membrane dipped in DMEM/F12 and cultured at 37°C under an atmosphere containing 5% CO2 for 1 and 3 days.
In vitro drug release. The release behavior of the TNP‐470 agent from the resulting TNP‐470‐loaded calcium‐phosphate microspheres was examined as follows. The TNP‐470‐loaded microspheres (0.05 g) were immersed into physiological saline (1 cm3) at 37°C for 0.5, 1, 3, 5, 10, 24, 48, 72, and 120 h during shaking at the rate of 100 strokes/min. After the desired immersion periods, released TNP‐470 agent was separated by centrifuging the above suspension at 1000 rpm for 5 min. The amount of TNP‐470 in the supernatant was determined by high performance liquid chromatography (HPLC) (UV/VIS Detector SSC‐5410; Senshu Scientific, Tokyo, Japan). The column used was ODS‐1251‐N (4.6 × 250 mm), and the mobile phase was the solution of pure water/acetonitrile = 30/70 [v/v]. The wavelength of the HPLC detector was 217 nm in the UV region.
Evaluation of antitumor effects. TNP‐470 was dissolved in ethanol to concentrations of 0, 100, 500, and 1000 μg/mL. TNP‐470 was loaded by adding the microspheres (0.05 g) into the above‐mentioned ethanol solution (1 cm3) and freeze‐drying followed. The TNP‐470‐loaded microspheres were used as sample powders in vitro and in vivo in the present study. In vitro testing of four types of calcium‐phosphate microspheres loaded with or without TNP‐470 was conducted using a polystyrene plate as a control. The examined samples were as follows: (i) microspheres (washed powder) alone; (ii) 100 μg/mL TNP‐470 alone; (iii) 500 μg/mL TNP‐470 alone; (iv) 1000 μg/mL TNP‐470 alone; (v) microspheres loaded with 100 μg/mL TNP‐470; (vi) microspheres loaded with 500 μg/mL TNP‐470; and (vii) microspheres loaded with 1000 μg/mL TNP‐470. The human uterine sarcoma cell line FU‐MMT‐3, previously established by our laboratory, was used in this study.( 28 ) First, 5 × 105 FU‐MMT‐3 cells were seeded on a 12‐well plate and cultured for 1 day, then the five different powder compacts were set on the membrane inserts of a Transwell polycarbonate membrane (Corning, Corning, NY, USA) dipped in DMEM/F12 (10% FBS) and cultured at 37°C under an atmosphere containing 5% CO2 for 1 and 3 days, as illustrated in Figure 1(b). Cell proliferation was examined by counting with an erythrocytometer and the morphology was observed with a phase‐contrast microscope. The antitumor proliferation activity of the FU‐MMT‐3 xenografts was then examined in vivo. Ceramic microspheres loaded with 1000 μg/mL TNP‐470 were suspended in physiological saline (1 cm3) and then injected (0.4 cm3).
Cell line and nude mice. A human uterine sarcoma cell line, FU‐MMT‐3, previously established by our laboratory from a patient with uterine carcinosarcoma, was used in the present study because this tumor is one of the most malignant neoplasms of human solid tumors, and it exhibits a poor response to radiotherapy and currently used chemotherapeutic agents.( 1 , 2 , 3 , 4 , 5 ) FU‐MMT‐3 also shows a highly progressive activity both in vitro and in vivo. This cell line is chiefly composed of myogenic sarcoma cells; its immuno‐phenotype, tumorigenicity, and cytogenetic characteristics have already been reported.( 28 ) Female BALB/cA Jcl‐nu athymic nude mice were obtained from Clea (Tokyo, Japan), and 5–6‐week‐old mice (body weight, 20 g) were used in the experiments. All animals were kept in isolation rooms at a controlled temperature and were caged in groups of five or fewer and had free access to standard animal chow and water ad libitum according to the instructions of the Institute of Experimental Animal Science, Fukuoka University Medical School.
Chemicals. TNP‐470 was kindly donated by Takeda Chemical Industries (Osaka, Japan). Its structure and characteristics have been previously described.( 19 ) TNP‐470 was suspended in a vehicle of 0.5% ethanol plus 5% gum arabic in saline.
Injection of TNP‐470‐loaded microspheres. On the basis of in vitro results, the in vivo evaluation was conducted using the microspheres prepared from the 1000 μg/mL TNP‐470 solution. First, the mice were injected subcutaneously with 2 × 105 FU‐MMT‐3 cells in 0.2 mL DMEM in the right auxiliary region of the flank. Mice bearing resultant tumors measuring 5–10 mm in diameter on day 14 were randomly separated into four groups as follows: (i) TCP‐microsphere (25 mg) injection alone (n = 5); (ii) TNP‐470 injection alone (n = 8); (iii) TCP microspheres loaded with 1000‐ppm TNP‐470 suspended in physiological saline (0.4 cm3) (n = 5); and (iv) non‐treatment control (n = 12: injection of 0.5% ethanol plus 5% gum arabic in saline). These therapies were administered three times per week for 8 weeks. The materials were subcutaneously injected around the xenografts, under the guidance of trans‐dermal color Doppler ultrasound (SSD‐4000; Aloka, Tokyo, Japan) with a 7.5‐MHz curved array transducer (UST‐987‐7.5; Aloka.) while searching for the feeding arteries, as previously described.( 18 ) Briefly, a 30‐gauge microinjection needle (0.25 mm in diameter) was advanced toward the feeding arteries into the bottom of the xenografts while monitoring the feeding arteries by color Doppler ultrasound. The solution was slowly injected into these vessels; where feeding arteries could not be fully visualized, injection was made close to the vessels. The mice were anesthetized with ether before injection. Tumor growth was monitored by measuring the weekly volume twice, calculated as V = a × b 2/2 (a = length; b = width). An autopsy was performed on all mice sacrificed soon after completion of the therapies or after death during the course of therapies, and both the size and weight of the excised tumors were measured. Mean, SD, median, and SE of the tumor size during the different therapies and those of the tumor weight after completion of the therapies in each group were calculated.
Immunohistochemical evaluation of tumor vascularity. Frozen sections from each tumor were reacted with each primary antibody for 1 h at room temperature in our pathology laboratory. The attached antibodies were visualized by the labeled streptavidin–biotin (LSAB) method (Zymed, San Francisco, CA, USA). The monoclonal antibody used was anti‐CD31 (an endothelial marker, 1:100; BD Pharmingen, San Diego, CA, USA) for endothelial cells in the tumor vessels. The negative controls consisted of an omission of the primary antibody. Microvessel density (MVD) was measured in all tumors treated using the present therapy. Intratumoral microvessels were highlighted by anti‐CD31 immunostaining in frozen sections in each tumor. The MVD quantitation in the highest vascularization (hot spots) was examined in each tumor in an identical manner to our previous study of uterine sarcomas.( 5 )
Statistical analysis. The in vivo data were expressed as the mean ± SD. The Mann–Whitney U‐test (non‐parametric) was used to compare tumor growth, or tumor weight, between the three treatment groups and the control. The unpaired t‐test (parametric) was used to compare MVD between the three treatment groups and the control. These statistical analyses were done using the software package StatView 5.0 (SAS Institute, Cary, NC, USA) for Macintosh. The results were judged to be statistically significant for P‐values less than 0.05 for each respective statistical test.
Results
Release profile of TNP‐470 from microspheres in vitro. The drug release profile of the TNP‐470‐loaded TCP microspheres was examined over a period of 25 h. Seventy‐five percent of the total TNP‐470 from the microspheres was rapidly released within 30 min, and the remaining 25% was slowly released up to 25 h following immersion. After 25 h of immersion, TNP‐470 was not detected in the supernatant (Fig. 2a).
Figure 2.
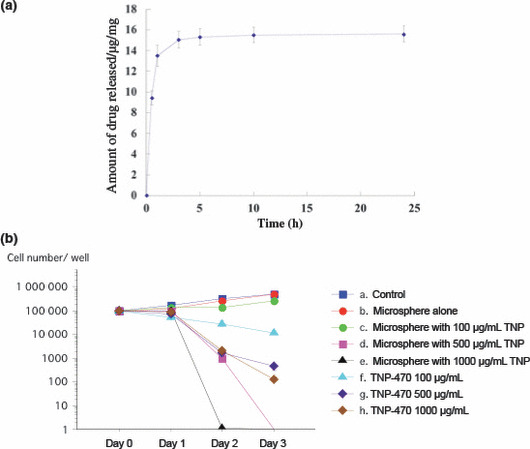
Results of in vitro testing of calcium‐phosphate microspheres loaded with TNP‐470. (a) Seventy‐five percent of the TNP‐470 was released rapidly from microspheres and the remaining 25% was released continuously until 25 h after immersion (two‐step release). (b) Results of four types of calcium‐phosphate microspheres loaded with or without TNP‐470. The microspheres loaded with 500 or 1000 μg/mL TNP‐470 significantly inhibited the FU‐MMT‐3 cells, compared to microspheres alone and 500 or 1000 μg/mL TNP‐470 alone.
Inhibitory effects in vitro. The powder compacts were prepared using microspheres loaded with various contents of TNP‐470, then in vitro assays were performed. The FU‐MMT‐3 cells were seeded on 12‐well plates and cultured for 24 h. The powder compacts were then set on the membrane inserts of a Transwell and cultured for up to 72 h; the results are shown in Figure 2(b). The powder compacts of TCP microspheres alone did not inhibit the proliferation of FU‐MMT‐3 cells. However, the powder compacts of TCP microspheres prepared using the 100 μg/mL TNP‐470 solution inhibited the proliferation of FU‐MMT‐3 cells, compared to powder compacts with microspheres alone, and control. The microspheres loaded with 500 or 1000 μg/mL TNP‐470 significantly inhibited the FU‐MMT‐3 cells, compared to microspheres alone (t‐test; P = 0.0004, P = 0.0001) and 500 or 1000 μg/mL TNP‐470 alone (P = 0.004, P = 0.001).
Suppression of tumor growth in vivo. The weekly changes in the mean tumor volume in xenografts during the course of therapies are shown in Figure 3(a). Treatment with microspheres alone significantly inhibited the growth of the FU‐MMT‐3 xenografts, compared to the control (Mann–Whitney U‐test; P = 0.048). Moreover, a significantly enhanced effect on tumor growth suppression was obtained by treatment with TNP‐470‐loaded microspheres, compared to treatment with TNP‐470 alone (P = 0.046) and microspheres alone (P = 0.025).
Figure 3.
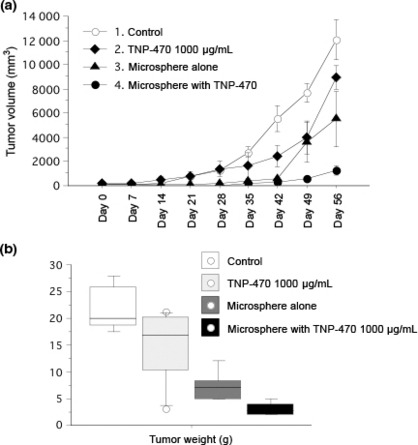
Effects on growth and weight of the FU‐MMT‐3 xenografts. (a) Each curve represents the mean ± SD of the tumor volume (cm3) in each group. Treatment with microspheres alone significantly inhibited the growth of the FU‐MMT‐3 xenografts compared to control. Moreover, a significantly enhanced effect on tumor growth suppression was obtained with treatment of TNP‐470‐loaded microspheres, compared to treatments with 1000 μg/mL TNP‐470 alone and microsphere alone. (b) The mean tumor weight in the FU‐MMT‐3 xenografts resected after treatments. The mean tumor weight after treatment with TCP microspheres or TCP + 1000 μg/mL TNP‐470 was significantly smaller compared to control. The mean tumor weight after treatment of TCP + 1000 μg/mL TNP‐470 was significantly smaller compared to treatment with 1000 μg/mL TCP alone.
The mean weight of xenografts after treatment with either microspheres alone (7.2. ± 2.9 g; range, 5.0–12.0 g) or TNP‐470 alone (14.8 ± 7.1 g; range, 3.1–21.2 g) was less than that of the control (21.9 ± 4.4 g; range, 17.4–28.0 g) (Mann–Whitney U‐test vs microspheres alone, P = 0.009; vs TNP‐470 alone, P = 0.253). An enhanced effect on weight reduction in the xenografts was shown with treatment of TNP‐470‐loaded microspheres (3.1 ± 1.2 g; range, 2.0–5.0 g), compared to either microspheres alone (P = 0.016) or TNP‐470 alone (P = 0.018) (Fig. 3b). Dissemination or direct invasion of the tumor into the abdominal cavity was only observed in the control (33.3%; 4 of 12 mice).
CD31 staining for tumor microvessels. TCP microspheres were embolized in the feeding arteries of the xenografts in both treatment groups, with or without TNP‐470. Fibrosis with granuloma formation and lymphocytic infiltration was observed in the lumen of the feeding arteries; no injury caused by microspheres was found in the vessel walls (Fig. 4a). Moreover, the TCP microspheres were shown as basophilic amorphous materials in H&E staining, and were remarkably embolized in tumor microvessels in all mice of the TCP‐microsphere treatment groups (Fig. 4b–d). The destruction of tumor microvessels and areas of coagulative necrosis were seen in tumors treated with TCP microspheres, with or without TNP‐470 (Fig. 4e).
Figure 4.
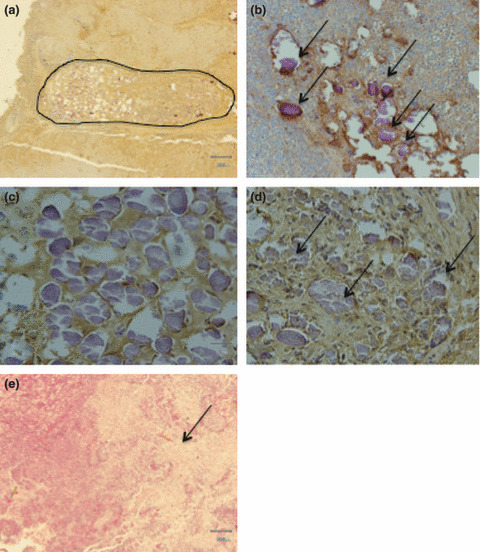
Histopathologic findings of the FU‐MMT‐3 xenografts. A feeding artery (encircled) was entirely embolized by β‐tricalcium phosphate (TCP) ceramic microspheres. (a) Fibrosis with granuloma formation and lymphocytic infiltration was observed in the lumen of the feeding arteries; no injury caused by microspheres was found in the vessel walls (×40). (b) TCP microspheres were remarkably embolized (arrows) in various‐sized microvessels (×200). (c) TCP microspheres showing uniform basophilia (×400). (d) Aggregates of TCP microspheres (arrows) in tumor microvessels (×400). (e) Coagulative necrosis (arrows) is extensively seen in treatments with microspheres loaded with or without TNP‐470 (×40).
The mean MVD of all treatment groups (Fig. 5b–d) were significantly lower compared to the control (61.3 ± 10.7; unpaired t‐test, P < 0.05) (Fig. 5a). The mean MVD in TNP‐470‐loaded TCP microspheres (15.0 ± 4.6) (Fig. 5d) was significantly lower than those of the TNP‐470 treatment alone (30.8 ± 13.7) (Fig. 5b) (unpaired t‐test; P = 0.00005), and TCP‐microsphere treatment alone (23.8 ± 3.7) (Fig. 5c) (unpaired t‐test; P = 0.0008). Thus, the reduction of MVD was significantly enhanced by treatment with TNP‐470‐loaded TCP microspheres (Fig. 5e).
Figure 5.
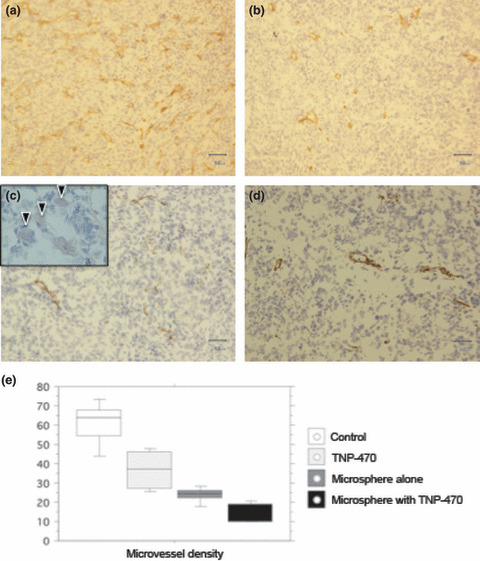
Microvessel densities of the FU‐MMT‐3 xenografts by CD31‐stainning (brown color). (a) The control group (no treatment) (×200). (b) Treatment with TNP‐470 alone (×200). (c) Treatment with TCP microspheres alone (×200). Basophilic TCP microspheres (arrow heads) were seen in tumor microvessel (×400) (inset in 5c). (d) Treatment with TNP‐470‐loaded TCP microspheres (×200). The mean microvessel densities (MVD) of all treatment groups were significantly lower compared to control. (e) The mean MVD in TNP‐470‐loaded TCP microspheres was significantly lower than those of the TNP‐470 treatment alone and TCP‐microsphere treatment alone.
Toxicity. Severe body weight loss was not observed in any mice treated with TNP‐470‐loaded TCP microspheres compared to treatment with TNP‐470 alone. The mean body weight at 56 days for treatment with TNP‐470 alone was 16.9 ± 2.55 g, which was significantly lower than that for treatment with TNP‐470‐loaded microspheres (25.17 ± 0.68) (P = 0.01) (Fig. 6).
Figure 6.
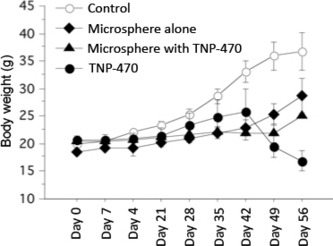
Body weights of mice during various treatments. Each curve represents the mean ± SD of mice body weight (g) in each group. The mean body weight on day 56 of the mice treated with TNP‐470 alone was significantly lower compared to the group treated with TNP‐470‐loaded microspheres.
Discussion
Many forms of human malignancy, particularly solid tumors, have historically presented significant challenges to conventional chemotherapy, and despite the advancement in therapeutic cocktails, the outcome of chemotherapy remains unsatisfactory. Uterine sarcomas including carcinosarcoma (malignant mixed müllerian tumors) are aggressive tumors with highly angiogenic features, which represent a major therapeutic challenge, as all existing therapeutic approaches fail to increase patient survival.( 1 , 2 , 3 , 4 , 5 ) Our previous studies have shown the efficacy of anti‐angiogenesis treatments using TNP‐470 for this tumor in vitro and in vivo, compared to the other anti‐angiogenic agents, such as thalidomide, and FR118487.( 17 , 18 , 19 ) To advance this therapeutic approach, we designed a new chemoembolization technique using a biomaterial of resorbable hollow ceramic microspheres loaded with TNP‐470 to suppress the growth of human uterine sarcoma, one of the most aggressive solid tumors. TNP‐470 blocks endothelial cell cycle progression in the late G1 phase to activate p53 in endothelial cells, thus leading to an increase in cyclin‐dependent kinase inhibitor p21CIP/WAF expression and subsequent growth arrest.( 21 ) The molecular target of TNP‐470 was found to be MetAP‐2, an intracellular enzyme necessary for the process of protein myristolation, thus preventing the translocation of membrane proteins to the cell surface.( 22 ) Moreover, TNP‐470 suppresses the production of angiogenic factors, such as basic fibroblast growth factor (bFGF) and VEGF in vitro.( 17 , 25 ) TNP‐470 not only inhibits VEGF‐induced endothelial function but also the proliferation of many types of tumor cells.( 23 , 24 , 25 , 26 , 27 ) A recent drug delivery study showed that the conjugation of TNP‐470 with water‐soluble polymers tends to both prolong the half‐life and facilitate the accumulation of the agent in tissues involving angiogenesis without weight loss or neurotoxicity.( 31 , 32 )
Drug delivery systems (DDS) could potentially be used for active or passive targeting of tumor tissues. The former refers to the development of monoclonal antibodies directed against tumor‐related molecules. However, the application of a DDS using monoclonal antibodies is restricted to tumors expressing high levels of related antigens. Passive targeting is based on the enhanced permeability and retention (EPR) effect, which is based on the pathophysiological characteristics of solid tumor tissues, including hypervascularity, incomplete vascular architecture, secretion of vascular permeability factors stimulating extravasation within cancer tissue, and absence of effective lymphatic drainage from tumors that impedes the efficient clearance of macromolecules accumulated in solid tumor tissues.( 33 ) However, DDS using anti‐angiogenic agents have not yet been fully studied. Thus, anti‐angiogenesis drug delivery developed in the present study is expected to suppress tumor growth in two steps, by not only directly embolizing the tumor vessels, but also releasing angiogenesis inhibitor from the microsphere carrier to neighboring endothelial and tumor cells. Resorbable ceramic microparticles have been recently developed as a new embolic material. The size of these microparticles range between 100 and 250 μm and their effectiveness and safety have been proved. Excellent results have been reported for their use in microcatheter superselective renal artery embolization in rabbits and also following clinical therapy for human meningioma.( 34 , 35 ) Because the microparticles are spherical in shape, no complications such as hemorrhage or microcatheter clogging have occurred. These studies described their excellent biocompatibility and good visibility during injection control to produce occlusion of the distal arteriocapillary bed.( 34 , 35 )
TCP is also major compound of calcium‐phosphate and is known to be a resorbable ceramic. Recent studies have shown that TCP is an ideal biomaterial, especially in substitution for bone, because TCP dissolves gradually without any cytotoxic and allergic reaction and elicits no immune response.( 9 , 10 , 11 ) Thus, TCP microspheres were used in the present drug delivery study both as a drug carrier and an embolization material. TNP‐470 exhibited a two‐step sustainable release, in vitro, from the internal space and external surface of the hollow microspheres. Although the powder compacts of TCP microspheres alone did not inhibit the proliferation of FU‐MMT‐3 cells in vitro (Fig. 2b), treatment with microspheres alone significantly inhibited the growth of the FU‐MMT‐3 xenografts, compared to the control in vivo (Fig. 3). It is suggested that although TCP microspheres alone do not inhibit sarcoma cell proliferation, a direct antitumor effect of this material by embolization occurred in vivo, because histopathological investigations revealed that microspheres were remarkably embolized in the feeding arteries of the xenografts, as well as in many tumor microvessels (Fig. 4). This strong embolization effect exhibited by TCP microspheres alone is potentially useful for cancer treatment. Further studies using other animal experiments are needed, such as embolization of renal artery in rabbits, or embolization of hepatic artery in pigs, and some liver cancer models, to confirm the characteristics and embolic properties of different‐sized TCP microspheres.
In the present study, the diameter of the microspheres was approximately 1 μm, and no evidence of blood injury, such as remarkable hemorrhage or hematoma, was found in any of the treated mice. Thus, this new DDS using TCP ceramic microspheres might be a useful approach to chemoembolization for treatment of deep‐seated cancers, including advanced or recurrent tumors in the abdomen, as well as urogenital tumors that have already received radiotherapy. This new approach should be followed by clinical studies to assess their treatment effectiveness for aggressive solid tumors.
Histopathologically, microspheres were remarkably embolized in the feeding arteries in the peripheral areas of the xenografts, as well as in many tumor microvessels in vivo. The destruction of tumor vessels and areas of coagulative necrosis were seen in both groups treated with microspheres, with or without TNP‐470, compared to treatment with TNP‐470 alone, suggesting a physical effect of embolization by the TCP microspheres. Growth of the xenografts was significantly reduced in treatment with TNP‐470‐loaded microspheres compared to treatment with unloaded microspheres or TNP‐470 alone. The synergistic effect of the anti‐angiogenic ceramic‐microsphere delivery might be thus shown in vivo. It is suggested that TNP‐470 is successfully released from hollow TCP microspheres after embolization at the tumor site. Thus, physiochemical effects may be involved in a two‐step inhibitory process of tumor angiogenesis. Recently, it has been suggested that embolization causes ischemic changes in parts of cancer tissues, which induces angiogenic growth factors, such as VEGF and bFGF.( 36 ) As TNP‐470 suppresses VEGF,( 16 ) our DDS targeting tumor vasculature may supplement the effects of embolization through release of an anti‐angiogenic agent. Further studies of this chemoembolization using other anti‐angiogenic agents, such as bevacizumab (Avastin) or monoclonal antibodies for VEGF‐Rs, will be conducted in vivo.
In previous studies using TNP‐470, body weight loss of mice was frequently observed in vivo.( 25 , 26 ) In the present study, TNP‐470 loaded on microspheres was able to be gently released, so that no remarkable side effects, including body weight loss, were observed compared to treatment with TNP‐470 alone. This result may support the safety of our DDS as a chemoembolization approach using hollow ceramic microspheres.
In conclusion, this study showed that calcium‐phosphate ceramic microspheres loaded with TNP‐470 inhibited the growth of human uterine sarcoma in vivo by physiochemical action. This new chemoembolization method incorporating an anti‐angiogenic agent may contribute to more effective treatment of locally advanced or recurrent solid tumors.
Acknowledgments
This research was supported in part by the “Academic Frontier” project (2006–2010) from the Ministry of Education, Culture, Sports, Science and Technology of Japan granted to the “Development of high‐performance biomaterials supporting highly advanced medical technology and their application to medical devices” proposal. This research was also supported by funds (no. 071001) from the Central Research Institute of Fukuoka University, Fukuoka, Japan, granted to the “Establishment of Drug Delivery System Using Bioresorbable Ceramics Particle for Anti‐Angiogenic Cancer Therapy” proposal.
References
- 1. Salazar OM, Bonfiglio TA, Patten SF et al. Uterine sarcomas: Natural history, treatment and prognosis. Cancer 1978; 42: 1152–60. [DOI] [PubMed] [Google Scholar]
- 2. Spanos WJ Jr, Wharton JT, Gomez L, Fletcher GH, Oswald MJ. Malignant mixed Müllerian tumors of the uterus. Cancer 1984; 15: 53. Review. [DOI] [PubMed] [Google Scholar]
- 3. Acharya S, Hensley ML, Montag AC, Flemming GF. Rate uterine cancers. Review. Lancet Oncol 2005; 6: 961–71. [DOI] [PubMed] [Google Scholar]
- 4. Rose PG, Piver MS, Tsukada Y, Lau T. Patterns of metastasis in uterine sarcoma. An autopsy study. Cancer 1989; 63: 935–8. [DOI] [PubMed] [Google Scholar]
- 5. Emoto M, Iwasaki H, Ishiguro M et al. Angiogenesis in carcinosarcomas of the uterus: Differences in the microvessel density and expression of vascular endothelial growth factor between the epithelial and mesenchymal elements. Hum Pathol 1999; 30: 1232–41. [DOI] [PubMed] [Google Scholar]
- 6. Emoto M, Charnock‐Jones DS, Licence D et al. Localisation of the VEGF and angiopoietin genes in uterine carcinosarcoma. Gynecol Oncol 2004; 95: 474–82. [DOI] [PubMed] [Google Scholar]
- 7. Kawashita M, Shineha R, Kim HM et al. Preparation of ceramic microspheres for in situ radiotherapy of deep‐seated cancers. Biomaterials 2003; 24: 2955–63. [DOI] [PubMed] [Google Scholar]
- 8. Petersen LK, Narashimhan B. Combinational design of biomaterials for drug delivery: opportunities and challenges. Expert Opin Drug Deliv 2008; 5: 837–46. [DOI] [PubMed] [Google Scholar]
- 9. Yuan H, Kurashina K, de Bruijn JD, Li Y, de Groot K, Zhang X. A preliminary study on osteoinduction of two kinds of calcium‐phosphate ceramics. Biomaterials 1999; 20: 1799–806. [DOI] [PubMed] [Google Scholar]
- 10. Nagayama M, Takeuchi H, Doi Y. Comparison of carbonate apatite and b‐tricalcium phosphate (resorbable calcium phosphates) implanted subcutaneously into the back of rats. Dent Mater J 2006; 25: 219–25. [DOI] [PubMed] [Google Scholar]
- 11. Victor SP, Sampath Kumar TS. BCP ceramics microspheres as drug delivery carriers: synthesis, characterization, and doxycycline release. J Mater Sci Mater Med 2008; 19: 283–90. [DOI] [PubMed] [Google Scholar]
- 12. Kawata M, Uchida H, Itatani K, Okada I, Koda S, Aizawa M. Development of porous ceramics with well‐controlled porosities and pore sizes from apatite fibers and their evaluations. J Mater Sci Mater Med 2004; 15: 817–23. [DOI] [PubMed] [Google Scholar]
- 13. Aizawa M, Porter AE, Best SM, Bonfield M. Ultrastructural observation of single‐crystal apatite fibres. Biomaterials 2005; 26: 3427–33. [DOI] [PubMed] [Google Scholar]
- 14. Morisue H, Matsumoto M, Chiba K et al. Novel apatite fiber scaffolds can promote three‐dimensional proliferation of osteoblasts in rodent bone regeneration models. J Biomed Mater Res A. 2009; 90: 811–8. [DOI] [PubMed] [Google Scholar]
- 15. Willett CG, Boucher Y, di Tomaso E et al. Direct evidence that the VEGF‐specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roodhart JM, Langenberg MH, Witteveen E, Voest EE. The molecular basis of class side effects due to treatment with inhibitors of the VEGF/VEGFR pathway. Curr Clin Pharmacol 2008; 3: 132–43. Review. [DOI] [PubMed] [Google Scholar]
- 17. Miura S, Emoto M, Matsuo Y, Kawarabayashi T, Saku K. Carcinosarcoma‐induced endothelial cells tube formation through KDR/Flk‐1 is blocked by TNP‐470. Cancer Lett 2004; 203: 45–50. [DOI] [PubMed] [Google Scholar]
- 18. Emoto M, Ishiguro M, Iwasaki H, Kikuchi M, Kawarabayashi T. Effect of Angiogenesis Inhibitor TNP‐470 on the Growth, Blood Flow, and Microvessel Density in Xenografts of Human Uterine Carcinosarcoma in Nude Mice. Gynecol Oncol 2003; 89: 88–94. [DOI] [PubMed] [Google Scholar]
- 19. Emoto M, Tachibana K, Iwasaki H, Kawarabayashi T. Antitumor effect of TNP‐470, an angiogenesis inhibitor, combined with ultrasound irradiation for human uterine sarcoma xenografts evaluated using contrast color Doppler ultrasound. Cancer Sci 2007; 98: 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ingber D, Fujita T, Kishimoto S et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990; 348: 555–7. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Griffith EC, Sage J, Jacks T, Liu JO. Cell cycle inhibition by the anti‐angiogenic agent TNP‐470 is mediated by p53 and p21WAF1/CIP1. Proc Natl Acad Sci U S A 2000; 97: 6427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM. The anti‐angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP‐2. Proc Natl Acad Sci U S A 1997; 94: 6099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yanase T, Tamura M, Fujita K, Kodama S, Tanaka K. Inhibitory effect of angiogenesis inhibitor TNP‐470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res 1993; 53: 2566–70. [PubMed] [Google Scholar]
- 24. Yoshida T, Kaneko Y, Tsukamoto A, Han K, Ichinose M, Kimura S. Suppression of hepatoma growth and angiogenesis by a fumagillin derivative TNP 470. Cancer Res 1998; 58: 3751–6. [PubMed] [Google Scholar]
- 25. Inoue K, Chikazawa M, Fukata S, Yoshikawa C, Shuin T. Frequent administration of angiogenesis inhibitor TNP‐470 (AGM‐1470) at an optimal biological dose inhibits tumor growth and metastatic human transitional cell carcinoma in the urinary bladder. Clin Cancer Res 2002; 8: 2389–98. [PubMed] [Google Scholar]
- 26. Inoue K, Chikazawa M, Fukata S, Yoshikawa C, Shuin T. Docetaxel enhances the therapeutic effect of the angiogenesis inhibitor TNP‐470 (AGM‐1470) in metastatic human transitional cell carcinoma. Clin Cancer Res 2003; 9: 886–99. [PubMed] [Google Scholar]
- 27. Nahari D, Satchi‐Fainaro R, Chen M et al. Tumor cytotoxicity and endothelial Rac inhibition induced by TNP‐470 in anaplastic thyroid cancer. Mol Cancer Ther 2007; 6: 1329–37. [DOI] [PubMed] [Google Scholar]
- 28. Emoto M, Iwasaki H, Oshima K, Kikuchi M, Kaneko Y, Kawarabayashi T. Characteristics of rhabdomyosarcoma cell lines derived from uterine carcinosarcomas. Virchow Archiv 1997; 431: 249–56. [DOI] [PubMed] [Google Scholar]
- 29. Aizawa M, Hanazawa T, Itatani K, Howell FS, Kishioka A. Characterization of hydroxyapatite powders prepared by ultrasonic spray‐pyrolysis technique. J Mater Sci 1999; 34: 2865–73. [Google Scholar]
- 30. Ohno T, Aizawa M. Effect of the concentrations of the starling solution on the syntheses and powder properties of hollow tricalcium‐phosphate microspheres by ultrasonic spray‐pyrolysis. Key Engineer Mater 2006; 309: 235–8. [Google Scholar]
- 31. Yasukawa T, Kimura H, Tabata Y et al. Targeted delivery of anti‐angiogenic agent TNP‐470 using water‐soluble polymer in the treatment of choroidal neovascularization. Invest Ophthalmol Vis Sci 1999; 40: 2690–6. [PubMed] [Google Scholar]
- 32. Satchi‐Fainaro R, Puder M, Davies JW et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP‐470. Nat Med 2004; 10: 255–61. [DOI] [PubMed] [Google Scholar]
- 33. Matsumura Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies (Review). Adv Drug Deliv Rev 2008; 22: 60. [DOI] [PubMed] [Google Scholar]
- 34. Kubo M, Kuwayama N, Hirashima Y, Takaku A, Ogawa T, Endo S. Hydroxyapatite ceramics as a particulate embolic material: report of the physical properties of the hydroxyapatite particles and the animal study. AJNR 2003; 24: 1540–4. [PMC free article] [PubMed] [Google Scholar]
- 35. Kubo M, Kuwayama N, Hirashima Y, Takaku A, Ogawa T, Endo S. Hydroxyapatite ceramics as a particulate embolic material: report of the clinical experience. AJNR 2003; 24: 1545–7. [PMC free article] [PubMed] [Google Scholar]
- 36. Sergio A, Cristofori C, Cardin R et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastro 2008; 103: 914–21. [DOI] [PubMed] [Google Scholar]


