Abstract
The transporter protein genes and lipids in human ovarian carcinoma‐derived KF28 cells with anticancer‐drug‐sensitive properties were compared with those in resistant cells, taxol‐resistant KF28TX, cisplatin‐resistant KFr13, and taxol‐ and cisplatin‐resistant KFr13TX, to identify the molecules required for anticancer‐drug resistance. In accordance with previous reports, taxol and cisplatin resistance was closely correlated with expression of the multidrug resistance 1 and bile acid export pump, and multidrug resistance‐associated protein 2 genes, respectively. In addition, we found a distinct difference in glycosphingolipids between the sensitive and resistant cells. Although GlcCer was the major glycolipid (83.0%) in sensitive cells, GalCer, LacCer and, particularly, Gb3Cer were characteristically increased in all resistant cells, irrespective of whether the resistance was to taxol or cisplatin, and comprised 65–84% of total glycosphingolipids. GM3, which was present at 0.04 µg/mg dry weight in the sensitive cells, showed a twofold increase in the taxol‐resistant cells, but was absent in the cisplatin‐resistant cells. The altered glycolipid composition was proven to be due to enhanced or suppressed expression of the respective sugar transferase genes. In addition, the ceramide moiety of ceramide monohexoside in the sensitive cells constituted 83% of non‐hydroxy fatty acids, but that in the resistant cells comprised 67–74% of α‐hydroxy fatty acids. Thus, cells containing Gb3Cer with α‐hydroxy fatty acids were found to survive selectively in the presence of taxol and cisplatin, and modification of the glycolipid structure was revealed to occur in association with anticancer‐drug resistance. (Cancer Sci 2006; 97: 1321–1326)
Abbreviations:
- ABC
ATP‐binding cassette
- BSEP
bile salt export pump
- CDH
ceramide dihexoside
- CMH
ceramide monohexoside
- CTH
ceramide trihexoside
- FABMS
fast atom bombardment mass spectrometry
- GAPDH
glyceraldehyde 3‐phosphate dehydrogenase
- GLC
gas‐liquid chromatography
- Hex
hexose
- MDR
multidrug resistance
- MRP
multidrug resistance‐associated protein
- PBS
phosphate‐buffered saline
- PVP
polyvinylpyrolidone
- RT‐PCR
reverse transcription‐polymerase chain reaction
- TLC
thin‐layer chromatography. The glycolipid nomenclature is based on the recommendations of the IUPAC‐IUB Commission on Biochemical Nomenclature.( 35 )
The chemosensitivity or chemoresistance of cancer cells toward anticancer drugs is closely related to the rates of entry and extrusion of drugs into and out of cells through transporter molecules, and with the signal transduction cascade leading to apoptosis in response to anticancer drugs.( 1 ) In particular, ABC transporters, such as MDR or MRP transporters, have been revealed to be closely implicated in the chemoresistance of cancer cells.( 2 , 3 ) The chemoresistance mediated by ABC transporters is mainly due to decreased cellular accumulation of anticancer drugs due to extrusion of the drugs out of the cells, and the correlation between several types of drugs and the expression of various transporter genes has been extensively studied to optimize chemotherapies, as well as to characterize the genetic mechanism underlying the acquisition of resistance during chemotherapy.( 4 )
Chemosensitivity is relevant to the ceramide‐mediated apoptosis induced by anticancer drugs. Stimuli, including anticancer drugs and γ‐irradiation, have been reported to activate sphingomyelinase to generate ceramide, which triggers apoptosis as a second messenger.( 5 , 6 , 7 , 8 ) When sphingolipids in anticancer drug‐sensitive and ‐resistant cancer cells were compared, glucosylceramide was found to increase characteristically in the resistant cells, suggesting that glucosylation of ceramide plays a role in the detoxification pathway for the removal of ceramide.( 9 , 10 ) Furthermore, hyperglucosylation of ceramide through transfection of the ceramide glucosyltransferase gene into human breast cancer cells has been successfully used to convert adriamycin‐sensitive cells into resistant ones, whose drug‐resistance is not associated with p‐glycoprotein or Bcl‐2.( 11 ) However, an experiment involving inhibitors of glucosylceramide synthase revealed that the drug‐sensitive property achieved was not only due to inhibition of ceramide glucosyltransferase,( 12 ) but that ceramide derived from sphingomyelin itself is not a second messenger but rather the precursor of both an apoptosis second messenger, GD3, and an apoptotic protector, GalCer.( 13 ) In the case of ovarian carcinoma‐derived cells, paclitaxel‐resistant cells showed an increased concentration of GM3, but no changes in the amounts of ceramide, neutral glycosphingolipids and sphingomyelin, in comparison to those in the original cells.( 14 )
Thus, although the glycosphingolipid composition of cancer cells changes in association with the acquisition of chemoresistance toward anticancer drugs, the mode of alteration of sphingolipids in cells surviving in the presence of anticancer drugs is not identical for all types of cells and kinds of drugs. To clarify the differences in lipid constituents between drug‐sensitive and ‐resistant cells, we determined the lipid compositions of ovarian carcinoma‐derived cells with resistance properties toward cisplatin and taxol, both of which are versatile anticancer drugs.
Materials and Methods
Materials. Sphingolipids from various sources were purified in our laboratory: GlcCer, LacCer, Gb3Cer, Gb4Cer and GM3 from human erythrocytes, and GalCer and sphingomyelin from bovine brain. Their N‐stearoyl derivatives were prepared by deacylation with sphingolipid ceramide N‐deacylase (Pseudomonas sp. TK4), followed by reacylation with stearoyl chloride.( 14 ) Ceramides were prepared by treatment of sphingomyelin with Clostridium perfringens phospholipase C, which was kindly donated from Dr M. Kitamura, National Institute of Infectious Diseases, Tokyo. A monoclonal anti‐GM3 antibody (M2590) was obtained from Seikagaku (Tokyo, Japan) and an antisphingomyelin antibody (VJ‐41) was established in our laboratory.( 15 ) Fatty acids and fatty acid methyl esters were purchased from Supelco (Bellefonte, PA, USA). Cisplatin and taxol were obtained from Bristol‐Myers Squibb (Tokyo, Japan) and Sigma Aldrich (St Louis, MO, USA), respectively.
Cell lines. The cell lines KF28, KF28TX, KFr13 and KFr13TX were kindly donated by Professor Y. Kikuchi, National Defense Medical College, Saitama, Japan. KF28 was the original human ovarian carcinoma‐derived cell line, and KF28TX and KFr13 were the taxol‐ and cisplatin‐resistant lines, which were established by repeated exposure of the parent KF28 cells to taxol and cisplatin, respectively. KFr13TX was the cell line derived from KFr13 cells through exposure to taxol. Cells were cultured in Dulbecco's modified Eagle's medium, supplemented with 10% fetal calf serum, 100 U/mL penicillin and 0.1 mg/mL streptomycin, in a humidified incubator at 37°C under a 5% CO2 atmosphere.
Separation and quantitation of lipids. After lyophilization of cells, total lipids were extracted from the lyophilized powders with chloroform/methanol/water (20:10:1, 10:20:1 and 1:1, by vol.). Then the concentrations of cholesterol were determined by GLC with 5α‐cholestane as an internal standard and of lipid‐bound phosphorus by Bartlett's method.( 16 ) Cholesterol ester, ceramides and phospholipids were developed on TLC plates with N‐hexane/diethyl ether/acetic acid (80:30:2, by vol.), chloroform/methanol/acetic acid (94:1:5, by vol.) and chloroform/methanol/water (60:35:8, by vol.), respectively. Their concentrations were determined by TLC‐densitometry at the analytical wavelength of 500 nm after visualization with cupric acetate‐phosphoric acid reagent using cholesterol oleate, N‐stearoyl sphingosine, palmitoyl oleoyl phosphatidyl ethanolamine and choline, and N‐stearoyl sphingosylphosphorylcholine as standards, respectively. Then, the lipid extracts were fractionated into neutral and acidic lipids on a DEAE‐Sephadex column (A‐25, acetate form; Pharmacia, Uppsala, Sweden). The neutral glycolipids were separated from the unabsorbed neutral lipid fraction by acetylation, separation of the acetylated derivatives, deacetylation and desalting, whereas the gangliosides were prepared from the absorbed acidic lipid fraction by cleavage of the ester‐containing lipids, followed by dialysis.( 16 )
The gangliosides and neutral glycolipids thus obtained were developed on TLC plates with chloroform/methanol/0.5% CaCl2 in water (55:45:10, by vol.) and chloroform/methanol/water (60:35:8, by vol.), and then visualized with resorcinol‐HCl and orcinol‐H2SO4 reagents, respectively. The density of spots was determined at the analytical wavelengths of 580 nm for resorcinol‐HCl‐positive spots and 420 nm for orcinol‐H2SO4‐positive spots, respectively, using a dual‐wavelength TLC densitometer (CS‐9000; Shimadzu, Kyoto, Japan). Standard glycolipids, N‐stearoyl derivatives of GalCer, LacCer, Gb3Cer and GM3 (0.1–1.5 µg), were developed on the same TLC plates for preparation of standard curves for quantitation.
TLC‐immunostaining. The total lipid extracts were applied to plastic‐coated TLC plates, which were then developed as above. Each plate was incubated with a blocking buffer (1% PVP and 1% ovalbumin in PBS) at 4°C overnight and then with anticarbohydrate antibodies in 3% PVP in PBS at 37°C for 2 h. Afterwards, the plates were washed five times with 0.1% Tween 20 in PBS, and the antibodies bound to the TLC plates were detected using peroxidase‐conjugated antimouse immunoglobulin G and M antibodies (Cappel Laboratories, Cochranville, PA, USA), diluted 1:1000 (by vol.) with 3% PVP in PBS, and with enzyme substrates H2O2 and 4‐chloro‐1‐naphthol, as described previously.( 15 , 16 , 17 , 18 ) The density of spots was also determined using 10–100 ng of GM3 and sphingomyelin as standards for quantitation with a TLC‐densitometer as described above, the limit of detection being 5 ng.
Structural analysis of glycolipids. The individual glycolipids were purified using a silica gel (Iatrobeads 6RS8060; Iatron Laboratory, Tokyo, Japan) column by gradient elution with chloroform/isopropyl alcohol/water (85:15:0.2 and 40:60:2, by vol.). The purified glycolipids were identified by negative ion FABMS (JMS‐700TKM; JEOL, Tokyo, Japan) with triethanolamine as a matrix solvent, and by GLC‐mass spectrometry as the trimethylsilyl derivatives of carbohydrates, the methyl esters of fatty acids and the N‐acetyl O‐trimethylsilyl derivatives of long chain bases.( 16 ) Also, linkage analysis of carbohydrate residues was performed by permethylation of glycolipids, acetolysis, reduction and acetylation to generate partially methylated aldohexitol acetates, which were analyzed by GLC‐mass spectrometry on an ECNSS‐M column (QP‐5050 A; Shimadzu, Kyoto, Japan).( 16 )
RT‐PCR analysis. Total RNA extracted from the cell lines by the acid guanidine thiocyanate‐phenol‐chloroform (AGPC) method,( 20 ) was reverse‐transcribed to cDNA with reverse transcriptase (M‐MuLV; Takara, Kyoto, Japan) and random primers, and then subjected to PCR under the following conditions: MDR1 (GenBank Accession Number AF016535), sense primer, CCCATCATTGCAATAGCAGG, antisense primer, GTTCAAACTTCTGCTCCTGA, 35 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 40 s; MRP1 (NM004996), sense primer, ATGTCACGTGGAATACCAGC, antisense primer, GAAGACTGAACTCCCTTCCT; MRP2 (NM000392), sense primer, CTGCCTCTTCAGAATCTTAG, antisense primer, ATAACCCAAGTTGCAGGCT; and BSEP (AF136523), sense primer, GCTCCACACTTGTCCTGGAT, antisense primer, CCAAACAAGGAGAGGCATTG, 35 cycles of 95°C for 15 s, 56°C for 30 s, and 72°C for 30 s; ceramide glucosyltransferase (NM003358), sense primer, CAAAACTCTGGCTCATATTC, antisense primer, ATATTGTCATGGATTCGCGG, 35 cycles of 95°C for 15 s, 52°C for 30 s, and 72°C for 40 s; ceramide galactosyltransferase (NM003360), sense primer, CTCTCTGAAGGCAGAGACATCGCC, antisense primer, CATCCACAGGCTGGACCCATGAAC, 35 cycles of 95°C for 15 s, 64°C for 30 s, and 72°C for 30 s; and LacCer sialyltransferase (AB018356), sense primer, ATTTGAGCACAGGTATAGC, antisense primer, GATGTCAAAGGCAGTCTCT, 35 cycles of 95°C for 15 s, 52°C for 30 s, and 72°C for 40 s. The primers for GAPDH were used as a control. The resulting PCR products were electrophoresed on a 1.5% agarose gel, stained with ethidium bromide, and then examined under a UV transilluminator.
Results
Taxol‐ and cisplatin‐sensitive and ‐resistant cells. As previously reported,( 19 ) KF28TX and KFr13 cells were derived from KF28 cells based on their resistance to taxol and cisplatin, respectively, and KFr13TX cells were established from KFr13 cells based on their resistance to taxol. The IC50 of taxol for KF28, KF28TX, KFr13 and KFr13TX are 4.65, 53.30, 2.61 and 12.70 µM, respectively, indicating that the relative resistances of KF28TX and KFr13TX cells to taxol are 11.5‐ and 5.0‐fold that of the original KF28 cells. Also, the IC50 of cisplatin for KF28, KF28TX, KFr13 and KFr13TX are 0.18, 0.14, 0.85 and 0.53 µM, respectively, indicating that KFr13 and KFr13TX cells retain 4.7‐ and 2.9‐times higher resistance to cisplatin in comparison to the original KF28 cells.
When the transporter protein genes were examined by RT‐PCR, expression of the MDR1 and BSEP genes was characteristically observed in taxol‐resistant KF28TX and KFr13TX cells, showing their involvement in the resistance to taxol, and the MRP2 gene was expressed in the cisplatin‐sensitive KFr13 and KFr13TX cells at relatively higher levels than in KF28 and KF28TX cells (Fig. 1), showing its involvement in the resistance to cisplatin, as reported previously.( 1 )
Figure 1.
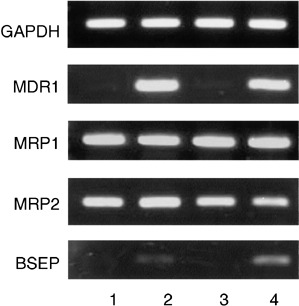
RT‐PCR analysis of transporter protein genes in human ovarian carcinoma‐derived cells that are sensitive and resistant to taxol and cisplatin. 1, KFr13; 2, KFr13TX; 3, KF28; 4, KF28TX.
Lipids in drug‐sensitive and ‐resistant cells. Cholesterol, phospholipids and glycolipids were the major lipid classes in the cells, and all of them were present in higher amounts in the drug‐resistant cells than in the drug‐sensitive KF28 cells (Table 1). A great difference in lipids between the drug‐sensitive and ‐resistant cells was observed in the glycosphingolipid compositions. As shown in Fig. 2, although CMH was the major neutral glycolipid in the original KF28 cells, CTH was the major one in all of the taxol‐ and cisplatin‐resistant cells, indicating that expression of CTH is associated with the anticancer drug‐resistant property, irrespective of whether the resistance was to taxol or cisplatin. The structure of CTH was characterized as that of globotriaosyl ceramide, Gb3Cer, from the following evidence. Its negative ion FABMS spectrum exhibited a molecular ion with palmitic acid, M‐H−, at m/z 1022, and fragment ions due to sequential cleavage at glycosidic linkages (M‐Hex)− at m/z 860, (Hex‐Cer‐H)− at m/z 698 and (Cer‐H)− at m/z 536, and permethylation analysis yielded 2,3,6‐tri‐O‐methyl‐1,4,5‐tri‐O‐acetylglucitol, 2,3,6‐tri‐O‐methyl‐1,4,5‐tri‐O‐acetylgalactitol and 2,3,4,6‐tetra‐O‐methyl‐1,5‐di‐O‐acetylgalactitol in the ratio of 1:1:1. In the same way, CDH was characterized as LacCer.
Table 1.
Lipid compositions in human ovarian carcinoma‐derived cells that are sensitive and resistant to taxol and cisplatin
| Lipid | KF28 | KF28TX | KFr13 | KFr13TX |
|---|---|---|---|---|
| Cholesterol | 4.34 ± 1.51 | 5.51 ± 1.39 | 5.75 ± 1.17 | 5.57 ± 1.11 |
| PE | 2.37 ± 0.09 | 3.19 ± 0.23 | 2.93 ± 0.23 | 2.81 ± 0.16 |
| PG | 0.37 ± 0.06 | 0.53 ± 0.06 | 0.67 ± 0.03 | 0.56 ± 0.02 |
| PC/PS | 1.94 ± 0.28 | 2.67 ± 0.38 | 2.62 ± 0.41 | 2.73 ± 0.41 |
| SM | 0.50 ± 0.07 | 0.62 ± 0.07 | 0.68 ± 0.07 | 0.68 ± 0.09 |
| Ceramides | 0.15 ± 0.02 | 0.17 ± 0.01 | 0.17 ± 0.02 | 0.18 ± 0.01 |
| GlcCer | 0.20 ± 0.01 | 0.18 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.02 |
| GalCer | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 |
| CDH | Trace | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.27 ± 0.02 |
| CTH | Trace | 0.32 ± 0.01 | 0.44 ± 0.01 | 0.52 ± 0.02 |
| GM3 | 0.04 ± 0.01 | 0.09 ± 0.01 | Trace | Trace |
Values are mean ± SD of four different experiments, shown as µg/mg dried cells. PC, phosphatidyl choline; PE, phosphatidyl ethanolamine; PG, phosphatidyl glycerol; PS, phosphatidyl serine; SM, sphingomyelin.
Figure 2.
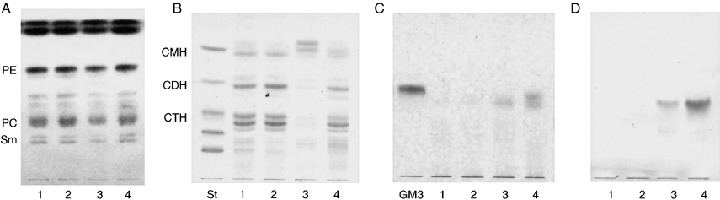
Total lipids (A), and neutral (B) and acidic (C, D) glycolipids in human ovarian carcinoma‐derived cells that are sensitive and resistant to taxol and cisplatin. The lipids, corresponding to 0.5 mg of dry weight, were developed on TLC plates with chloroform/methanol/water (60:35:8, by vol.) for A and B, and chloroform/methanol/0.5% CaCl2 in water (55:45:10, by vol.) for C and D, and were detected with (A) cupric acetate‐phosphoric acid, (B) orcinol‐H2SO4, (C) resorcinol‐HCl, and (D) anti‐GM3 antibodies. St, glycolipid mixture, GalCer, LacCer, Gb3Cer, Gb4Cer and Forssman glycolipid from the top; 1, KFr13; 2, KFr13TX; 3, KF28; 4, KF28TX.
In addition, CMH was found to comprise galactose and glucose by GLC in the following ratios, 5.2% and 94.8% for KF28, 16% and 84% for KF28TX, 39% and 61% for KFr13, and 40% and 60% for KFr13TX cells, respectively, showing enhanced synthesis of GalCer in the drug‐resistant cells, particularly in the cisplatin‐resistant ones. As to acidic glycosphingolipids, the original KF28 cells contained 0.04 µg GM3 per mg of dried cells, and double this amount was found in the taxol‐resistant KF28TX cells; however, GM3 was undetectable in the cisplatin‐resistant KFr13 and KFr13TX cells, even with the TLC‐immunostaining procedure involving anti‐GM3 antibodies. This indicates that the synthesis of GM3 was accelerated in the taxol‐resistant cells and lost in the cisplatin‐resistant cells (Fig. 2).
To clarify the genetic background of the changes in glycosphingolipid composition, the expression of glycosyltransferase genes was examined by RT‐PCR (Fig. 3). Although the glucosyltransferase gene for the synthesis of GlcCer was strongly expressed in all cells, expression of the ceramide galactosyltransferase gene for the synthesis of GalCer in the resistant KF28TX, KFr13 and KFr13TX cells was greater than that in the sensitive KF28 cells. Also, in accordance with the results of TLC‐immunostaining of GM3 (Fig. 2), the LacCer sialyltransferase gene for the synthesis of GM3 was expressed in KF28 and KF28TX cells more highly than in KFr13 and KFr13TX cells. These findings indicate that the increases in GalCer in the KFr13, KFr13TX and KF28TX cells, and in GM3 in the KF28 and KF28TX cells, are regulated via transcription through enhanced expression of the ceramide galactosyltransferase and LacCer sialyltransferase genes, respectively.
Figure 3.
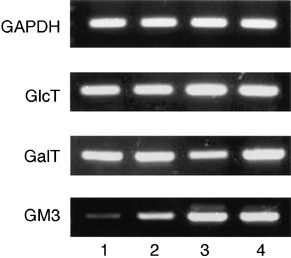
RT‐PCR analysis of glycosyltransferase genes in human ovarian carcinoma‐derived cells that are sensitive and resistant to taxol and cisplatin. 1, KFr13; 2, KFr13TX; 3, KF28; 4, KF28TX; GlcT, ceramide glucosyltransferase; GalT, ceramide galactosyltransferase; GM3, LacCer sialyltransferase.
Molecular species of glycosphingolipids in drug‐sensitive and ‐resistant cells. As shown in Fig. 2B, CMH was separated into three bands on TLC, and the relative intensities of the upper two bands were greater for KF28 cells, while a band that migrated to a position lower than that of standard N‐stearoyl GalCer was more prominent for KFr13, KFr13TX and KF28TX cells. To characterize its molecular structure, CMH was purified from individual cells by silica gel column chromatography and then analyzed by negative ion FABMS. This revealed distinct differences in the ceramide structure between the drug‐sensitive and drug‐resistant cells. As shown in Fig. 4, although the molecular ions at m/z 698 and m/z 810, corresponding to N‐palmitoyl and N‐lignoceroyl sphingosyl hexose, respectively, were abundant in the sensitive KF28 cells (Fig. 4A), the ions at m/z 714 and m/z 826 appeared as the abundant ions in all of the resistant cells (Fig. 4B,C), indicating that the lower bands of CMH in the resistant cells represent oxygen‐added molecules.
Figure 4.
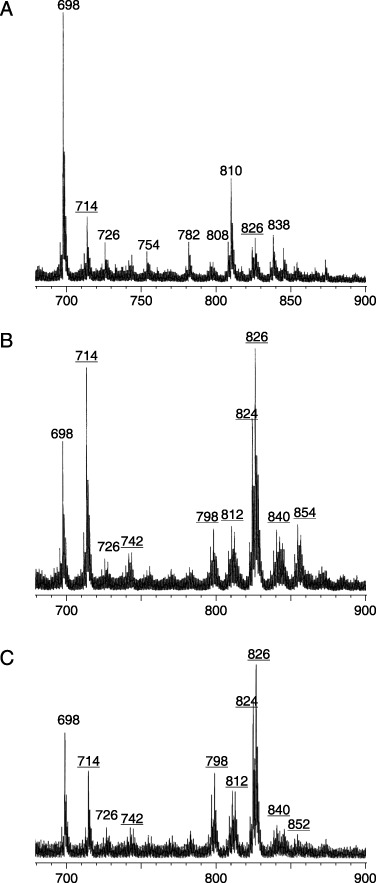
Negative ion FABMS spectra of CMH in (A) KF28, (B) KF28TX, and (C) KFr13 cells. CMH was purified by Iatrobeads column chromatography and analyzed by negative ion FABMS with triethanolamine as the matrix, and the molecular ion regions are represented. The underlined ions are from α‐hydroxy fatty acid‐containing molecules.
Further structural analysis by GLC‐mass spectrometry demonstrated that the addition of oxygen in the resistant cells occurs at the α‐position of the fatty acid moieties. As shown in Table 2, palmitic (C16:0) and linoceric (C24:0) acids comprised 52.5% of the fatty acids of CMH in KF28 cells, whereas their α‐hydroxy derivatives, 16 h:0, 24 h:0 and 24 h:1, were the dominant molecular species of CMH in the resistant KF28TX (53.2%), KFr13 (46.0%), and KFr13TX (45.3%) cells. α‐Hydroxy fatty acids were not only abundant in CMH of the resistant cells, but were also in LacCer, Gb3Cer and GM3, all of which were characteristically increased in the resistant cells (Table 1). Thus, cells whose glycolipids predominantly comprised α‐hydroxy fatty acids were found to selectively survive in the presence of taxol and cisplatin.
Table 2.
Non‐hydroxy fatty acids (NFA) and α‐hydroxy fatty acids (HFA) of glycosphingolipids in human ovarian carcinoma‐derived cells that are sensitive and resistant to taxol and cisplatin
| Cells | Ceramide monohexoside | Ceramide dihexoside | Ceramide trihexoside | GM3 | ||||
|---|---|---|---|---|---|---|---|---|
| NFA | HFA | NFA | HFA | NFA | HFA | NFA | HFA | |
| KF28 | 82.8 | 17.2 | – | – | – | – | 88.5 | 11.5 |
| KF28TX | 25.1 | 74.9 | 27.2 | 72.8 | 50.4 | 49.6 | 38.0 | 62.0 |
| KFr13 | 32.5 | 67.5 | 36.7 | 63.3 | 59.2 | 40.8 | – | – |
| KFr13TX | 32.2 | 67.8 | 31.5 | 68.5 | 58.7 | 41.3 | – | – |
Values are shown as percentage of total fatty acids.
Discussion
The establishment of cell lines with anticancer‐drug resistance followed by comparison of the resistance‐related molecules between sensitive and resistant cell lines is an effective procedure for clarifying the molecular basis of anticancer‐drug resistance. The ovarian carcinoma‐derived KF28 cells and taxol‐ and cisplatin‐resistant cell lines that were used in this study have well‐characterized anticancer drug resistance. The KF28TX and KFr13TX cells, and KFr13 and KFr13TX cells exhibiting reproducible taxol‐ and cisplatin‐resistance, respectively.( 19 ) Comparison of transporter protein genes in the sensitive and resistant cells indicated that the MDR1 and BSEP, and MRP2 genes in the taxol‐ and cisplatin‐resistant cells, respectively, were expressed more highly than those in the sensitive cells, suggesting that the extrusion of taxol and cisplatin via the respective transporter proteins is promoted in KF28TX and KFr13TX, and KFr13 and KFr13TX cells, respectively, to decrease the intracellular accumulation of drugs, as reported previously.( 1 , 19 )
The common observation regarding the differences in membrane lipids between drug‐sensitive and drug‐resistant cells was related to glycosphingolipids, whose structures associated with anticancer drug‐resistance are not always identical in various cancer cells.( 21 ) In multidrug resistant melanoma, and epidermoid and breast carcinoma cells, but not in their sensitive cells, GlcCer was found to be consistently present and was thought to attenuate the ceramide‐mediated apoptotic signals, resulting in the acquisition of drug resistance.( 9 , 10 ) However, another study found that ceramides synthesized de novo in or added exogenously to human leukemic cells were readily converted to GlcCer, but those induced by anticancer drugs were not converted to GlcCer by ceramide glucosyltransferase.( 22 ) In that case, ceramides were demonstrated not to be the immediate messengers for apoptosis, but to be the precursors of both apoptosis mediator GD3 and apoptosis protector GalCer.( 13 , 23 ) This indicates that the glycosylation of ceramides is a crucial step during the apoptotic program induced by anticancer drugs. The distinctive differences in glycosphingolipids between drug‐sensitive and drug‐resistant cells found in the present study are also thought to be implicated in the survival of ovarian carcinoma‐derived cells cultured in the presence of anticancer drugs.
In the present study, glycosphingolipids with longer carbohydrate chains and α‐hydroxy fatty acids were characteristically increased in the resistant cells, irrespective of whether the resistance was to taxol or cisplatin, and it is suggested that these molecules are required for survival against anticancer drugs. The α‐hydroxy groups of fatty acids were distributed in the hydrogen bonding region of the lipid bilayer, which is an essential region for formation of the membrane structure and for the holding of intrinsic proteins.( 24 , 25 ) It was recently proposed that the hydrogen‐donor groups in glycosphingolipids, sphingomyelin and cholesterol, together with functional membrane proteins, cluster in a raft structure in the membrane to regulate the activities of proteins and to mediate biological signals.( 26 , 27 , 28 ) The increased concentration of α‐hydroxy fatty acid‐containing glycolipids might be involved in the regulation of transporter proteins in this raft structure.
As to the carbohydrate moieties of glycolipids, the correlation of the carbohydrate structure and anticancer drug‐resistance has not been clarified yet. Our previous finding that modification of the carbohydrate structure in ovarian carcinoma‐derived cells on transfection of the fucosyltransferase gene resulted in acquisition of resistance to anticancer drugs( 29 ) indicated the possible involvement of carbohydrates in the anticancer‐drug resistance. In that report, we suggested that a loss of sulfo‐ and sialoglycolipids, which are the differentiation‐associated antigens,( 30 ) was brought about by sulfo‐ and sialyltransferases competing with fucosyltransferase. Clinically, well‐differentiated types of carcinomas are generally susceptible to anticancer drugs in comparison to poorly differentiated types. As the fucosylated galactose at position 2 could not be sulfated, the glycolipid composition including the precursors and products was dramatically altered on gene manipulation of fucosyltransferase. As the carbohydrate structures of glycolipids were produced by the sequential addition of carbohydrate, the metabolic rates at the individual steps determined the glycolipid composition. In fact, a 20‐ to 30‐fold increase in the fucosyltransferase activity resulted in dramatic alteration of the glycolipid composition, including not only fucosylated products, but also precursor glycolipids.( 29 ) Accordingly, the overall profile of the carbohydrate structures of glycolipids seems to be important for clarifying the functional significance of glycolipids. As reported in the present study, the common observation for glycolipids in the cells with resistance to taxol and cisplatin, in comparison to those in the sensitive cells, was increased concentrations of LacCer and Gb3Cer, which was the major glycolipid in all resistant cells. The metabolic shift to LacCer, followed by Gb3Cer, resulted in the loss of negatively charged GM3, which comprised 10% of total glycolipids in taxol‐resistant KF28TX cells, but was negligible in the cisplatin‐resistant KFr13 and KFr13TX cells. This is thought to be implicated in the anticancer‐drug resistance through modification of the activities of transporter proteins.
In contrast, Gb3Cer has been characterized as serological antigen Pk in human erythrocytes, and Burkitt's lymphoma‐ and B cell lineage‐associated antigens.( 31 , 32 ) Although cross‐linking of Gb3Cer by anti‐immunoglobulin M antibodies and Shiga‐like toxin from the Escherichia coli 0157 strain was able to induce apoptosis, the signals on cross‐linking of antigens were completely different from those with anticancer drugs.( 33 , 34 )
Based on the hypothesis that the modification of ceramides with carbohydrates results in a decrease in ceramide‐mediated apoptosis for resistant cells, the syntheses of LacCer and Gb3Cer were effective for acquisition of the resistance property. However, there has been a report that the carbohydrate moieties of glycolipids were involved in the resistance property. Although GlcCer‐containing lymphoblasts from a patient with Gaucher's disease readily underwent the apoptosis program with anthracycline daunorubicin, GalCer‐containing lymphoblasts in Krabbe's disease were revealed to be resistant to the drug.
Overall, the physicochemical properties of glycolipids including both ceramides and carbohydrates are thought to be involved in the anticancer drug‐sensitive and ‐resistant properties of cancers. Our findings might provide useful clues for elucidating the molecular mechanism of anticancer‐drug resistance.
References
- 1. Huang Y, Sadee W. Membrane transporters and channels in chemoresistance and ‐sensitivity of tumor cells. Cancer Lett 2006; 239: 168–82. [DOI] [PubMed] [Google Scholar]
- 2. Mahadevan D, Shirahatti N. Strategies for targeting the multidrug resistance‐1 (MDR1)/P‐gp transporter in human malignancies. Curr Cancer Drug Targets 2005; 5: 445–55. [DOI] [PubMed] [Google Scholar]
- 3. Chintamani Singh JP, Mittal MK, Saxena S, Bansal A, Bhatia A, Kulshreshtha P. Role of p‐glycoprotein expression in predicting response to neoadjuvant chemotherapy in breast cancer: A prospective clinical study. World J Surg Oncol 2005; 3: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shtil AA, Azare J. Redundancy of biological regulation as the basis of emergence of multidrug resistance. Int Rev Cytol 2005; 246: 1–29. [DOI] [PubMed] [Google Scholar]
- 5. Jaffrezou JP, Levade T, Bettaieb A et al. Daunorubicin‐induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J 1996; 15: 2417–24. [PMC free article] [PubMed] [Google Scholar]
- 6. Santana P, Pena LA, Haimovitz‐Friedman A et al. Acid sphingomyelinase‐deficient human lymphoblasts and mice are defective in radiation‐induced apoptosis. Cell 1996; 86: 189–99. [DOI] [PubMed] [Google Scholar]
- 7. Cifone MG, Roncaioli P, De Maria R et al. Multiple pathways originate at the Fas/APO‐1 (CD95) receptor. Sequential involvement of phosphatidylcholine‐specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J 1995; 14: 5859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tepper CG, Jayadev S, Liu B et al. Role for ceramide as an endogenous mediator of Fas‐induced cytotoxicity. Proc Natl Acad Sci USA 1995; 92: 8443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lavie Y, Cao H, Bursten SL, Giuliano AE, Cabot MC. Accumulation of glucosylceramides in multidrug‐resistant cancer cells. J Biol Chem 1996; 271: 19 530–6. [DOI] [PubMed] [Google Scholar]
- 10. Lucci A, Cho WI, Han TY, Giuliano AE, Morton DL, Cabot MC. Glucosylceramide: a marker for multiple‐drug resistant cancers. Anticancer Res 1998; 18: 475–80. [PubMed] [Google Scholar]
- 11. Liu YY, Han TY, Giuliano AE, Cabot MC. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem 1999; 274: 1140–6. [DOI] [PubMed] [Google Scholar]
- 12. Norris‐Cervetto E, Callaghan R, Platt FM, Dwek RA, Butters TD. Inhibition of glucosylceramide synthase does not reverse drug resistance in cancer cells. J Biol Chem 2004; 279: 40 412–8. [DOI] [PubMed] [Google Scholar]
- 13. Grazide S, Terrisse AD, Lerouge S, Laurent G, Jaffrezou JP. Cytoprotective effect of glucosylceramide synthase inhibition against daunorubicin‐induced apoptosis in human leukemic cell lines. J Biol Chem 2004; 279: 18 256–61. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki H, Ito M, Kimura T, Iwamori Y, Iwamori M. Inhibitory activity of sulphoglycolipid derivatives towards pancreatic trypsin. Glycoconj J 2000; 17: 787–93. [DOI] [PubMed] [Google Scholar]
- 15. Iwamori M, Hirota K, Utsuki T et al. Sensitive method for the determination of pulmonary surfactant phospholipid/sphingomyelin ratio in human amniotic fluids for the diagnosis of respiratory distress syndrome by thin‐layer chromatography‐immunostaining. Anal Biochem 1996; 238: 29–33. [DOI] [PubMed] [Google Scholar]
- 16. Iwamori M, Shimomura J, Tsuyuhara S, Mogi M, Ishizaki S, Nagai Y. Differential reactivities of fucosyl GM1 and GM1 gangliosides on rat erythrocyte membrane revealed by analysis with anti‐fucosyl GM1 and GM1 antisera. J Biochem (Tokyo) 1983; 94: 1–10. [DOI] [PubMed] [Google Scholar]
- 17. Iwamori M, Iwamori Y. Changes in the glycolipid composition and characteristic activation of GM3 synthase in the thymus of mouse after administration of dexamethasone. Glycoconj J 2005; 22: 119–26. [DOI] [PubMed] [Google Scholar]
- 18. Iwamori M, Domino SE. Tissue‐specific loss of fucosylated glycolipids in mice with targeted deletion of alpha (1,2) fucosyltransferase genes. Biochem J 2004; 380: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto K, Kikuchi Y, Kudoh K, Nagata I. Modulation of cisplatin sensitivity by taxol in cisplatin‐sensitive and ‐resistant human ovarian carcinoma cell lines. J Cancer Res Clin Oncol 2000; 126: 168–72. [DOI] [PubMed] [Google Scholar]
- 20. Chomczynski P, Sacchi N. Single‐step method of RNA isolation by acid guanidinium thiocyanate‐phenol‐chloroform extraction. Anal Biochem 1987; 162: 156–9. [DOI] [PubMed] [Google Scholar]
- 21. Prinetti A, Millimaggi D, D’Ascenzo S et al. Lack of ceramide generation and altered sphingolipid composition are associated with drug resistance in human ovarian carcinoma cells. Biochem J 2006; 395: 311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tepper AD, Diks SH, Van Blitterswijk WJ, Borst J. Glucosylceramide synthase does not attenuate the ceramide pool accumulating during apoptosis induced by CD95 or anti‐cancer regimens. J Biol Chem 2000; 275: 34 810–7. [DOI] [PubMed] [Google Scholar]
- 23. Kang Y, Kang SK, Lee YC et al. Transcriptional regulation of the human GD3 synthase gene expression in Fas‐induced Jurkat T cells: a critical role of transcription factor NF‐κB in regulated expression. Glycobiology 2006; 16: 375–89. [DOI] [PubMed] [Google Scholar]
- 24. Crain RC, Zilversmit DB. Two nonspecific phospholipid exchange proteins from beef liver. 2. Use in studying the asymmetry and transbilayer movement of phosphatidylcholine, phosphatidylethanolamine, and sphingomyelin in intact rat erythrocytes. Biochemistry 1980; 19: 1440–7. [DOI] [PubMed] [Google Scholar]
- 25. Nilsson OS, Dallner G. Transverse asymmetry of phospholipids in subcellular membranes of rat liver. Biochim Biophys Acta 1977; 464: 453–8. [DOI] [PubMed] [Google Scholar]
- 26. Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–72. [DOI] [PubMed] [Google Scholar]
- 27. Brown DA, Rose JK. Sorting of GPI‐anchored proteins to glycolipid‐enriched membrane subdomains during transport to the apical cell surface. Cell 1992; 68: 533–44. [DOI] [PubMed] [Google Scholar]
- 28. Klausner RD, Rouault TA, Harford JB. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 1993; 72: 19–28. [DOI] [PubMed] [Google Scholar]
- 29. Iwamori M, Tanaka K, Kubushiro K et al. Alterations in the glycolipid composition and cellular properties of ovarian carcinoma‐derived RMG‐1 cells on transfection of the alpha1,2‐fucosyltransferase gene. Cancer Sci 2005; 96: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubushiro K, Tsukazaki K, Tanaka J et al. Human uterine endometrial adenocarcinoma: characteristic acquisition of synthetic potentials for II3SO3‐LacCer and ganglio series sulfoglycosphingolipids after transfer of the cancer cells to culture. Cancer Res 1992; 52: 803–9. [PubMed] [Google Scholar]
- 31. Marcus DM, Kundu SK, Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol 1981; 18: 63–71. [PubMed] [Google Scholar]
- 32. Wiels J, Fellous M, Tursz T. Monoclonal antibody against a Burkitt lymphoma‐associated antigen. Proc Natl Acad Sci USA 1981; 78: 6485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gregory CD, Dive C, Henderson S et al. Activation of Epstein–Barr virus latent genes protects human B cells from death by apoptosis. Nature 1991; 349: 612–4. [DOI] [PubMed] [Google Scholar]
- 34. O’Brien AO, Lively TA, Chen ME, Rothman SW, Formal SB. Escherichia coli O157: H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet 1983; 1: 702. [DOI] [PubMed] [Google Scholar]
- 35. IUPAC‐IUB Commission on Biochemical Nomenclature . The Nomenclature of Lipids. Eur J Biochem 1977; 79: 11–21. [DOI] [PubMed] [Google Scholar]


