Abstract
We studied whether total gastrectomy for gastric cancer would affect the pharmacokinetics of 5‐fluorouracil (5‐FU) and its degradation products, such as dihydrouracil (FUH2) and α‐fluoro‐β‐alanine (FBAL), after oral administration of the fluorouracil derivative S‐1, composed of tegafur, 5‐chloro‐2,4‐dihydroxypyridine (CDHP; a dihydropyrimidine dehydrogenase inhibitor) and potassium oxonate. Blood and urine samples were obtained, both preoperatively and at least 2 weeks postoperatively, from six patients with advanced gastric cancers who were undergoing total gastrectomy. Plasma levels of tegafur, 5‐FU, CDHP, potassium oxonate, FUH2 and FBAL were measured prior to and at 1, 2, 4, 6 and 10 h after oral administration of 40 mg/m2 S‐1. The total amounts of 5‐FU, FUH2 and FBAL excreted into urine during the 24‐h period after S‐1 administration were also measured. Total gastrectomy significantly increased the maximum concentration and the area under the curve until 10 h after administration (AUC1–10h) of plasma 5‐FU. The plasma AUC1–10h of CDHP was significantly higher than the preoperative value. In terms of clinical efficacy, the higher AUC1–10h of 5‐FU after total gastrectomy may be beneficial to S‐1 administered as adjuvant chemotherapy, and might be caused by the higher postoperative AUC1–10h of CDHP relative to preoperative values. However, the dose of S‐1 for patients who have undergone total gastrectomy might be diminished to avoid severe adverse events and to continue the treatment for a long period. (Cancer Sci 2007; 98: 1604–1608)
The oral dihydropyrimidine dehydrogenase (DPD) inhibitor fluoropyrimidine S‐1 has been the first‐choice anticancer agent in Japan for advanced gastric cancer since 1999.( 1 ) S‐1 contains tegafur (FT; a masked compound of 5‐fluorouracil [5‐FU]) and two modulators of 5‐FU (Fig. 1). One modulator is 5‐chloro‐2,4‐dihydroxypyridine (CDHP), which is a reversible competitive inhibitor of DPD. As DPD is an enzyme that degrades 5‐FU, CDHP with FT is expected to yield prolonged and high 5‐FU concentrations in blood and tumor tissues. The other modulator, potassium oxonate (OXO), is a reversible competitive inhibitor of orotate phosphoribosyltransferase (OPRT). OPRT is an enzyme for 5‐FU phosphoribosylation, to metabolize 5‐FU into an active form. Interestingly, OXO concentrates selectively in gastrointestinal tissues after oral administration and suppresses gastrointestinal toxicity by inhibiting OPRT without decreasing the anticancer effect of 5‐FU.( 2 )
Figure 1.
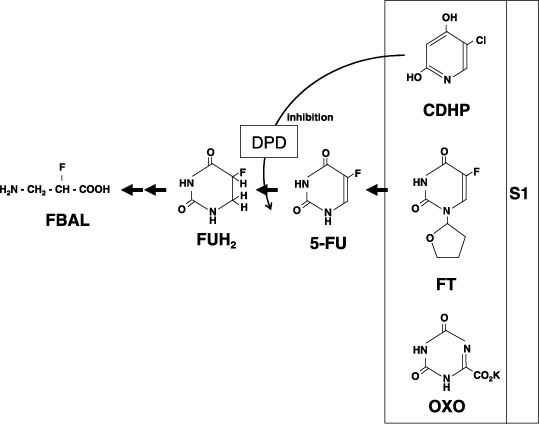
Chemical structure and catabolism of a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine S‐1.
In the catabolic pathway, 5‐FU is degraded by DPD into dihydrofluorouracil (FUH2), which is degraded rapidly into the main 5‐FU metabolite of α‐fluoro‐β‐alanine (FBAL; Fig. 1). FBAL has been demonstrated to cause cardiotoxicity and neurotoxicity.( 3 , 4 )
S‐1 has been administered as adjuvant chemotherapy after gastrectomy for patients with resectable gastric cancer.( 5 ) These days, combination therapies such as S‐1 with cisplatin, taxane or irinotecan have been investigated vigorously and found to be promising for patients with recurrent gastric cancer after gastrectomy as well as for patients with unresectable tumors.( 6 , 7 , 8 , 9 ) A Japanese nationwide postmarketing survey of S‐1 in 3808 patients with advanced gastric cancer found that two‐thirds of patients receiving S‐1 had a history of gastrectomy.( 1 ) The absorptive change of an orally administered 5‐FU masked compound and its modulator, such as a DPD inhibitor, may occur due to rapid intestinal transit, resulting in the alteration of 5‐FU and FBAL pharmacokinetics associated with the modification of the anticancer effect and toxicity. There have been few investigations into alternative pharmacokinetics regarding the S‐1 component and its degradation by gastrectomy. We therefore undertook the present study.
Patients and Methods
Patients. Patients between 20 and 80 years of age with a histological diagnosis of advanced gastric cancer, and who intended to undergo total gastrectomy with Roux‐en‐Y reconstruction, were eligible. In the present study, single administrations of S‐1 at 1 day before operation and at 1 day after operation were required to observe the pharmacokinetics of S‐1 components and its degradation. However, continuous administration of S‐1 was not necessary after operation, and adjuvant therapy was used anyway. In our Department, S‐1 is given as an adjuvant therapy for patients with stages II–IV gastric cancer after R0 (no residual tumor) or R1 (microscopic residual tumor) operation. Eastern Cooperative Oncology Group performance status ≤ 1 was required. Patients were required to have adequate bone marrow function: hemoglobin ≥ 9.0 g/dL, white blood cells between 4000 and 12 000/µL, neutrophils ≥ 2000/µL and platelets ≥ 100 000/µL. Other eligibility requirements included normal range of hepatic and renal functions (total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, blood urea nitrogen and serum creatinine ≤ the upper normal level). Informed consent was obtained from each patient before enrolment. The institutional review board for human experimentation in our hospital approved the protocol of this study.
Sample collection. The pharmacokinetics of 5‐FU, FT, CDHP, OXO, FUH2 and FBAL in plasma were measured within 2 weeks prior to the operation and 2 weeks or more after the operation, under the conditions that the patient had had a meal uneventfully and had no major operative complication such as intra‐abdominal bleeding, gastrointestinal bleeding, anastomotic leakage, intra‐abdominal abscess, liver dysfunction or renal dysfunction. On each of the two sampling days, a 5‐mL blood sample was collected prior to oral administration of S‐1 and again at 1, 2, 4, 6 and 10 h after administration. A standard clinical dose (40 mg/m2) as a single administration was used according to body surface area (BSA): BSA < 1.25 m2, 40 mg; 1.25 m2 ≤ BSA < 1.5 m2, 50 mg; 1.5 m2 ≤ BSA, 60 mg. To collect plasma, the blood samples in heparinized tubes were centrifuged immediately at 2000g for 5 min. The plasma was stored at –20°C until used for the assay.
On each of the two sampling days, urine was collected for 24 h after S‐1 administration. The urine volume during that 24‐h period was measured, and 10 mL of the collected urine was provided for the assays of 5‐FU, FUH2 and FBAL. For the measurement of DPD activity, blood samples of 25 mL each were taken in heparinized tubes prior to S‐1 administration. The sample was promptly and carefully layered on Ficoll and sodium diatrizoate. After centrifugation at 400g for 30 min, the mononuclear cells were removed and washed three times with phosphate‐buffered saline. The precipitate was stored at –80°C until used for the DPD assay.
Assay. The FT concentrations in plasma and FBAL in plasma and urine were measured by high‐performance liquid chromatography according to methods described elsewhere.( 10 , 11 ) Previously described gas chromatography–mass spectrometry methods were used to determine CDHP and OXO in plasma, as well as 5‐FU and FUH2 in plasma and urine.( 10 ) DPD activity was determined by a catalytic assay according to a method described elsewhere.( 12 )
Statistics. The area under the curve until 10 h after S‐1 administration (AUC1–10h) was calculated according to the trapezoidal rule using a WinNonlin program (Ver. 3.1, Pharsight Co., Mountain View, CA, USA). The paired Student's t‐test on log‐transformed plasma concentration data was used to compare preoperative and postoperative maximum concentration (Cmax) and AUC1–10h. The paired Student's t‐test was conducted in half‐life (T1/2), time required to reach Cmax (Tmax) and urine excretion amount to compare between preoperative and postoperative data. A P‐value of <0.05 was considered to be statistically significant.
Results
Clinical outcome. The final stagings of the six enrolled patients were: stage IA, 1; stage IB, 1; stage IIIB, 2; and stage IV, 2. R0 or R1 operations were done for all patients. The two patients with stages IA and IB were not given any anticancer drugs. We had planned to treat one of the stage IV patients with S‐1 after leaving our hospital; however, he was not available for follow up. Oral 5‐FU was administrated to one of the stage IIIB patients whose creatinine clearance was 43 mL/min and serum albumin was 3.2 g/dL. The other stage IIIB patient, whose creatinine clearance was 76% and serum albumin was 4.0 g/dL, was treated with a standard dose of S‐1. The patient suffered grade 1 diarrhea and grade 1 leukopenia; however, we have continued S‐1 administration without dose decrement for 14 months up to now. The second stage IV patient, whose creatinine clearance was 90% and serum albumin was 3.4 g/dL, experienced grade 3 leukopenia. For that patient, S‐1 administration was diminished to 75% of the standard dose and has been continued for 10 months up to now.
Dihydropyrimidine dehydrogenase activity. None of the six patients had DPD deficiency. The mean ± SD DPD activity in peripheral‐blood mononuclear cells of the patients was 355.8 ± 222.6 pmol/mg/min.
Pharmacokinetics of FT in plasma. Preoperative blood samples were obtained at 7.2 ± 3.9 days (range, 3–12 days) before operation. Postoperative blood samples were obtained at 22.7 ± 10.4 days (range, 14–37 days) after operation. There were no differences between the preoperative and postoperative T1/2, Cmax, Tmax or AUC1–10h of FT in plasma (Fig. 2; Table 1).
Figure 2.
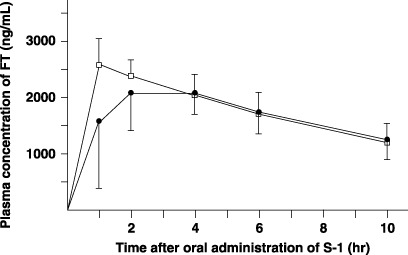
Plasma concentrations of tegafur (FT) after oral administration of 40 mg/m2 S‐1 in six patients. Closed circle, preoperative value; open column, value after total gastrectomy.
Table 1.
Comparison between preoperative and postoperative (total gastrectomy) pharmacokinetic parameters in the plasma of six patients receiving 40 mg/m2 S‐1 orally
| Parameter | Time of test | Tegafur | 5‐Fluorouracil | CDHP | OXO | FUH2 | FBAL |
|---|---|---|---|---|---|---|---|
| T1/2 (h) | Preoperative | 8.7 ± 4.2 | 2.2 ± 0.4 | 2.6 ± 0.6 | 2.8 ± 1.7 | NC | NC |
| Postoperative | 8.1 ± 1.8 | 2.3 ± 0.3 | 3.0 ± 0.6 | 3.5 ± 1.7 | NC | NC | |
| Cmax (ng/mL) | Preoperative | 2520 ± 794 | 125 ± 57 | 282 ± 69 | 55 ± 20 | 148 ± 77 | 62 ± 20 |
| Postoperative | 2715 ± 332 | 166 ± 77** | 453 ± 224 | 210 ± 184* | 156 ± 62 | 72 ± 23 | |
| Tmax (h) | Preoperative | 2.5 ± 1.2 | 4.0 ± 0.0 | 3.0 ± 1.1 | 3.0 ± 1.1 | 7.3 ± 2.1 | 8.7 ± 2.1 |
| Postoperative | 1.2 ± 0.4 | 3.3 ± 1.0 | 1.8 ± 0.4 | 3.0 ± 1.1 | 6.0 ± 0.0 | 8.0 ± 2.2 | |
| AUC1–10h (ng·h/mL) | Preoperative | 16 726 ± 3844 | 680 ± 298 | 1389 ± 398 | 276 ± 113 | 1055 ± 553 | 405 ± 156 |
| Postoperative | 17 726 ± 3368 | 1030 ± 501** | 2163 ± 933** | 1004 ± 904* | 1153 ± 454 | 546 ± 184* |
P < 0.05 and
P < 0.01 compared to preoperative values tested using the paired Student's t‐test. AUC1–10h, the area under the curve until 10 h after S‐1 administration; CDHP, 5‐chloro‐2,4‐dihydroxypyridine; Cmax, the maximum concentration; FBAL, α‐fluoro‐β‐alanine; FUH2, dihydrofluorouracil; NC, not calculated; OXO, potassium oxonate; T1/2, the half‐life; Tmax, the time required to reach Cmax.
Pharmacokinetics of 5‐FU in plasma and amount excreted in urine. The postoperative Cmax and AUC1–10h of 5‐FU in plasma were significantly higher than the preoperative values (P = 0.0075 and P = 0.0018, respectively) (Fig. 3; Table 1). The amount of 5‐FU excreted in urine did not differ between the pre‐ and postoperative samples (Table 2).
Figure 3.
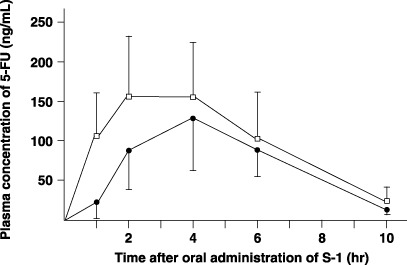
Plasma concentrations of 5‐fluorouracil (5‐FU) after oral administration of 40 mg/m2 S‐1 in six patients. Closed circle, preoperative value; open column, value after total gastrectomy.
Table 2.
Comparison between preoperative and postoperative (total gastrectomy) urine excretion amount in six patients in the first 24 h after oral administration of 40 mg/m2 S‐1
| Time of test | 5‐Fluorouracil | FUH2 | FBAL |
|---|---|---|---|
| Preoperative (ng) | 1379 ± 1015 | 824 ± 820 | 6149 ± 4489 |
| Postoperative (ng) | 1849 ± 846 | 1117 ± 595 | 13 715 ± 8861 |
| P‐value | 0.216 | 0.420 | 0.053 |
P‐value was calculated using the paired Student's t‐test comparing preoperative and postoperative levels. FBAL, α‐fluoro‐β‐alanine; FUH2, dihydrofluorouracil.
Pharmacokinetics of CDHP in plasma. The postoperative plasma AUC1–10h of CDHP was significantly higher than the preoperative value (P = 0.0048) (Fig. 4; Table 1).
Figure 4.
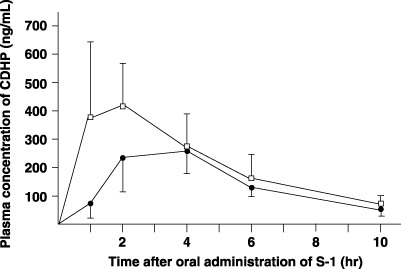
Plasma concentrations of 5‐chloro‐2,4‐dihydroxypyridine (CDHP) after oral administration of 40 mg/m2 S‐1 in six patients. Closed circle, preoperative value; open column, value after total gastrectomy.
Pharmacokinetics of OXO in plasma. The postoperative Cmax and AUC1–10h of OXO in plasma were significantly higher than the preoperative values (P = 0.0495 and P = 0.0449, respectively) (Fig. 5; Table 1).
Figure 5.
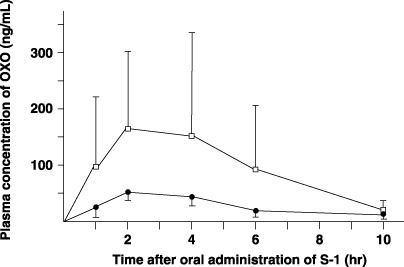
Plasma concentrations of potassium oxonate (OXO) after oral administration of 40 mg/m2 S‐1 in six patients. Closed circle, preoperative value; open column, value after total gastrectomy.
Pharmacokinetics of FUH2 in plasma and amount excreted in urine. There were no differences between the preoperative and postoperative Cmax, Tmax or AUC1–10h of FUH2 in plasma (Fig. 6; Table 1). The plasma T1/2 of FUH2 could not be calculated because FUH2 remained at high levels in the plasma at 10 h after S‐1 administration, the last examination time in the study setting (Fig. 6). The amount of FUH2 excreted in the urine did not differ under preoperative and postoperative conditions (Table 2).
Figure 6.
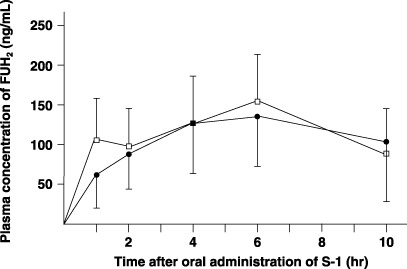
Plasma concentrations of dihydrofluorouracil (FUH2) after oral administration of 40 mg/m2 S‐1 in six patients. Closed circle, preoperative value; open column, value after total gastrectomy.
Pharmacokinetics of FBAL in plasma and amount excreted in urine. The postoperative AUC1–10h of FBAL in plasma was significantly higher than the preoperative value (P = 0.0295) (Fig. 7; Table 1). The plasma T1/2 of FBAL could not be calculated because FBAL remained at high plasma concentrations at 10 h after S‐1 administration (Fig. 7). The mean amount of FBAL excreted in the urine after total gastrectomy was more than twice the preoperative amount. A trend toward incremental increases in FBAL excreted from the urine postoperatively was recognized, compared to preoperative amounts (Table 2).
Figure 7.
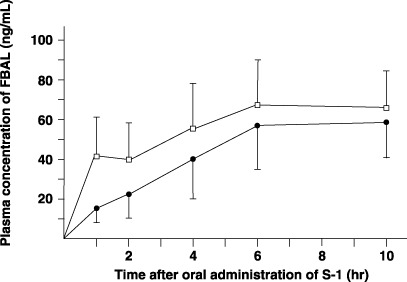
Plasma concentrations of α‐F‐β‐alanine (FBAL) after oral administration of 40 mg/m2 S‐1 in six patients. Closed circle, preoperative value; open column, value after total gastrectomy.
Discussion
Among patients with gastric cancer who undergo gastrectomy in Japan or the USA, approximately 30% receive a total gastrectomy.( 13 , 14 ) In the present study, patients receiving total gastrectomy were investigated because the condition after total gastrectomy was thought to be a better model of the lost stomach function of retention resulting in alternative pharmacokinetics of substances taken orally.
Hirata et al. showed that 5‐FU pharmacokinetic parameters, including Cmax and AUC (139.5 ± 43.0 ng/mL and 822.8 ± 246 ng·h/mL, respectively), in three patients receiving total gastrectomy were slightly, not significantly, higher than in nine non‐gastrectomy patients (124.9 ± 43.0 ng/mL and 691.0 ± 286.8 ng·h/mL, respectively), consisting of two gastric cancer, three breast cancer and four colorectal cancer patients.( 15 ) Tsuruoka et al. compared the plasma pharmacokinetics of 5‐FU and CDHP between postoperative and preoperative status in nine patients receiving distal gastrectomy and three patients receiving total gastrectomy.( 16 ) They concluded that gastrectomy had hardly any effect on the pharmacokinetic of S‐1 because the AUC1–8h (AUC until 8 h after S‐1 administration) of 5‐FU and CDHP were almost the same between pre‐ and postgastrectomy status at approximately 14 days after operation. However, our study demonstrated that the plasma Cmax and AUC1–10h of 5‐FU after total gastrectomy were significantly higher than before the operation. The discrepancy in the results between the previous reports and ours may be explained by the large number of total gastrectomy patients in our study compared with the others. Another explanation may be that we limited our study exclusively to patients undergoing total gastrectomy.
The plasma concentration and AUC of 5‐FU have been associated with the response to, and the toxicity treated by, a 5‐FU‐containing regimen.( 17 , 18 ) Thus far, no reports have compared the clinical efficacy of S‐1 between patients with a stomach and those without. Our data, which showed higher Cmax and AUC1–10h of 5‐FU after total gastrectomy compared with before, suggested that the anticancer effect of S‐1 might be enhanced by removal of the stomach.
Kinoshita et al. demonstrated an acceptable incidence of adverse effects in 41 patients (half of them receiving total gastrectomy and the others distal or proximal gastrectomy) treated by an adjuvant setting with S‐1; however, the incidence was slightly higher than that in unresectable or recurrent gastric cancer treated with S‐1.( 5 ) Moreover, statistically significant relationships were observed between S‐1‐induced diarrhea grade and AUC, Cmax and plasma concentration of 5‐FU.( 19 , 20 ) The pharmacokinetic data of plasma 5‐FU in our study suggested that the dose of S‐1 might be reduced when it is used in adjuvant chemotherapy for patients after total gastrectomy.
At high plasma concentrations of CDHP, catabolism of 5‐FU in the liver may be downregulated by the inhibited DPD activity, resulting in high plasma concentrations of 5‐FU. Consequently, the reason why plasma AUC1–10h of 5‐FU after total gastrectomy was higher than that before operation may be partly explained by the higher AUC1–10h of CDHP postoperatively compared to preoperatively. The higher AUC1–10h of postoperative CDHP compared to the preoperative value may be caused by rapid intestinal transit and absorption of this agent. Maehara et al. investigated the effects of gastrectomy on the pharmacokinetics of an oral fluoropyrimidine derivative, UFT (FT combined with uracil used as a DPD inhibitor).( 21 ) They demonstrated that total gastrectomy (n = 9) did not alter the AUC of FT or 5‐FU in plasma after oral administration of UFT. Regarding the pharmacokinetics of FT, their result was similar to ours, in that the plasma AUC of FT after S‐1 administration was almost the same following total gastrectomy as before the operation. However, their result that the AUC of 5‐FU after UFT administration did not increase after total gastrectomy is quite different from our pharmacokinetic results of 5‐FU using S‐1. The difference in results might be attributed to the different pharmacokinetics of individual DPD inhibitors after total gastrectomy. Contrary to our CDHP results, the plasma AUC of uracil after UFT administration following total gastrectomy did not differ from the preoperative value in their study.
In the present study, the postoperative Cmax and AUC1–10h of plasma OXO were significantly higher than postoperative values. OXO has been reported to be degraded rapidly in acidic solution.( 22 ) Therefore, it is reasonable that plasma OXO Cmax and AUC1–10h are higher after total gastrectomy without acidic gastric juice. Another report demonstrated that the 50% inhibitory concentration of OXO on the phosphorylation of 5‐FU was 3.7 µM (approximately 722 ng/mL).( 2 ) The Cmax level of OXO in the present study after operation was substantially lower compared with the 50% inhibitory concentration value for 5‐FU phosphorylation. Moreover, previous reports indicated that when OXO was administrated orally to rats, it was distributed at much higher concentrations in gastrointestinal tissues than in plasma but was not detected in tumor tissue.( 2 , 22 ) Consequently, such OXO concentrations after total gastrectomy may not suppress the anticancer effect of 5‐FU, and might even enhance the inhibition of gastrointestinal toxicity due to high OXO concentrations in gastrointestinal tissues.
The elevated 5‐FU plasma Cmax and AUC1–10h after total gastrectomy were thought to affect the plasma pharmacokinetics of its degradation products, such as FUH2 and FBAL, and to affect urine excretion of 5‐FU and its degradation. Among these substances, a significant change between pre‐ and postoperative status was found in the plasma AUC1–10h of FBAL. This suggested that the adverse effects induced by FBAL, including cardiotoxicity and neurotoxicity, might be increased by total gastrectomy.
The amount of FBAL excreted in urine tended to be higher after total gastrectomy than before, affected by the elevated AUC1–10h of FBAL in plasma. However, the amounts of 5‐FU and FUH2 excreted in urine did not differ significantly between pre‐ and postoperation, partly due to their large standard deviations.
In conclusion, a total gastrectomy may not negatively influence the anticancer effect of S‐1 treatment. However, adverse events must be monitored carefully due to the high plasma Cmax and AUC1–10h of 5‐FU and the high plasma AUC1–10h of FBAL after total gastrectomy when S‐1 is administered as adjuvant chemotherapy. Because the number of patients in the present study was small, further investigations of pharmacokinetics and clinical outcome comparing patients with and without a stomach are needed to elucidate the safety and effective dose of S‐1 after total gastrectomy. In the present study, the lack of pharmacokinetic data for patients who did not undergo total gastrectomy did not permit us to compare the pharmacokinetic changes of S‐1 after total gastrectomy with those after distal or proximal gastrectomy. Such a comparative study may also be required in the future.
References
- 1. Nagashima F, Ohtsu A, Yoshida S, Ito K. Japanese nationwide post‐marketing survey of S‐1 in patients with advanced gastric cancer. Gastric Cancer 2005; 8: 6–11. [DOI] [PubMed] [Google Scholar]
- 2. Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5‐fluorouracil without loss of its antitumor activity in rats. Cancer Res 1993; 53: 4004–9. [PubMed] [Google Scholar]
- 3. Okeda R, Shibutani M, Matsuo T, Kuroiwa T, Shimokawa R, Tajima T. Experimental neurotoxicity of 5‐fluorouracil and its derivatives is due to poisoning by the monofluorinated organic metabolites, monofluoroacetic acid and α‐fluoro‐β‐alanine. Acta Neuropathol 1990; 81: 66–73. [DOI] [PubMed] [Google Scholar]
- 4. Muneoka K, Shirai Y, Yokoyama N, Wakai T, Hatakeyama K. 5‐Fluorouracil cardiotoxicity induced by α‐fluoro‐β‐alanine. Int J Clin Oncol 2005; 10: 441–3. [DOI] [PubMed] [Google Scholar]
- 5. Kinoshita T, Nashimoto A, Yamamura Y et al . Feasibility study of adjuvant chemotherapy with S‐1 (TS‐1; FT, gimeracil, oteracil potassium) for gastric cancer. Gastric Cancer 2004; 7: 104–9. [DOI] [PubMed] [Google Scholar]
- 6. Nakata B, Mitachi Y, Tsuji A et al . Combination therapy of a novel oral fluorouracil derivative S‐1 with low‐dose cisplatin for unresectable and recurrent gastric cancer: a multicenter phase I study. Clin Cancer Res 2004; 10: 1664–9. [DOI] [PubMed] [Google Scholar]
- 7. Yoshida K, Ninomiya M, Takakura N et al . Phase II study of docetaxel and S‐1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 2006; 12: 3402–7. [DOI] [PubMed] [Google Scholar]
- 8. Ueda Y, Yamagishi H, Ichikawa D et al . Phase I study of a combination of S‐1 and weekly paclitaxel in patients with advanced or recurrent gastric cancer. Oncology 2005; 69: 261–8. [DOI] [PubMed] [Google Scholar]
- 9. Inokuchi M, Yamashita T, Yamada H et al . Phase I/II study of S‐1 combined with irinotecan for metastatic advanced gastric cancer. Br J Cancer 2006; 94: 1130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsushima E, Yoshida K, Kitamura R, Yoshida K. Determination of S‐1 (combined drug of FT, 5‐chloro‐2,4‐dihydroxypyridine and potassium oxonate) and 5‐fluorouracil in human plasma and urine using high‐performance liquid chromatography and gas chromatography‐negative ion chemical ionization mass spectrometry. J Chromatogr B 1997; 691: 95–104. [DOI] [PubMed] [Google Scholar]
- 11. Yamada Y, Hamaguchi T, Goto M et al . Plasma concentration of 5‐fluorouracil and F‐β‐alanine following oral administration of S‐1, a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5‐fluorouracil. Br J Cancer 2003; 89: 816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takechi T, Uchida J, Fujioka A, Fukushima M. Enhancing 5‐fluorouracil cytotoxicity by inhibiting dihydropyrimidine dehydrogenase activity with uracil in human tumor cells. Int J Oncol 1997; 11: 1041–4. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi K, Isobe Y, Honda I et al . Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer 2006; 9: 51–66. [DOI] [PubMed] [Google Scholar]
- 14. Hundahl SA, Menck HR, Mansour EG, Winchester DP. The national cancer data base report on gastric carcinoma. Cancer 1997; 80: 2333–41. [DOI] [PubMed] [Google Scholar]
- 15. Hirata K, Horikoshi N, Aiba K et al . Pharmacokinetics study of S‐1, a novel oral fluorouracil antitumor drug. Clin Cancer Res 1999; 5: 2000–5. [PubMed] [Google Scholar]
- 16. Tsuruoka Y, Kamano T, Kitajima M et al . Effect of gastrectomy on the pharmacokinetics of 5‐fluorouracil and gimeracil after oral administration of S‐1. Anti-Cancer Drug 2006; 17: 393–9. [DOI] [PubMed] [Google Scholar]
- 17. Fety R, Rolland F, Barberi‐Heyob M et al . Clinical impact of pharmacokinetically guided dose adaptation of 5‐fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res 1998; 4: 2039–45. [PubMed] [Google Scholar]
- 18. Ajani JA, Faust J, Ikeda K et al . Phase I pharmacokinetics study of S‐1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005; 23: 6957–65. [DOI] [PubMed] [Google Scholar]
- 19. Van Groeningen CJ, Peters GJ, Schornagel JH et al . Phase I clinical and pharmacokinetic study of oral S‐1 in patients with advanced solid tumors. J Clin Oncol 2000; 18: 2772–9. [DOI] [PubMed] [Google Scholar]
- 20. Cohen SJ, Leichman CG, Yeslow G et al . Phase I and pharmacokinetic study of once daily oral administration of S‐1 in patients with advanced cancer. Clin Cancer Res 2002; 8: 2116–22. [PubMed] [Google Scholar]
- 21. Maehara Y, Takeuchi H, Oshiro T et al . Effect of gastrectomy on the pharmacokinetics of FT, uracil, and 5‐fluorouracil after oral administration of a 1 : 4 FT and uracil combination. Cancer Chemother Pharmacol 1994; 33: 445–9. [DOI] [PubMed] [Google Scholar]
- 22. Yoshisue K, Masuda H, Matsushima E et al . Tissue distribution and biotransformation of potassium oxonate after oral administration of a novel antitumor agent (drug combination of FT, 5‐chloro‐2,4‐dihydroxypyridine, and potassium oxonate) to rats. Drug Metab Dispos 2000; 28: 1162–7. [PubMed] [Google Scholar]


