Abstract
The significance of lymphadenectomy in surgery for various kinds of cancer has been widely debated, particularly in the gynecological field. The cell wall skeleton of Mycobacterium bovis Bacillus Calmette‐Guérin (BCG‐CWS) has been used as an effective adjuvant for immunotherapy of a variety of cancer patients. Here we tested the immunological importance of lymph nodes in treatment of ovarian cancer patients with BCG‐CWS. After surgical removal of tumors, 73 ovarian cancer patients were intracutaneously inoculated with BCG‐CWS in the antigen‐unloaded state in the upper arm at 4‐week intervals at a dosage of 2–200 µg. Significant correlation of lymphadenectomy and reduced survival of patients was observed (stages I, II, III, IV; hazard ratio 2.38, 95% confidence interval 1.02–5.12, Cox regression model). Lymphadenectomy also compromised with induction of interferon‐γ. In view of the importance of the role of lymph nodes in stimulation of Toll‐like receptors by BCG‐CWS, it is suggested that lymph nodes should be kept as much as possible to preserve the patient's immunity against cancer. Application of these results to surgery for other cancers should be considered. (Cancer Sci 2009; 100: 1991–1995)
The efficacy of lymphadenectomy (LN‐ad) in surgery for ovarian cancer has become a focus of debate among gynecological oncologists.( 1 , 2 ) A paucity of pathological, immunological, and clinical data, however, has meant that the role of the lymph nodes (LN) in this cancer remains unclear. Reflecting this, the guidelines of the International Federation of Gynecology and Obstetrics for LN‐ad are based primarily on hypothesis.( 1 ) Nevertheless, various novel findings on LN and the role of the immune system in other cancers have progressed. For example, Hayashi et al. reported effective treatment of more than 235 cancer patients with the cell wall skeleton of Mycobacterium bovis Bacillus Calmette‐Guérin (BCG‐CWS) alone, after the removal of as many tumor cells as possible.( 3 , 4 ) Although BCG‐CWS was initially implicated as a simple adjuvant,( 5 , 6 ) it was noticed during trials that inoculated BCG‐CWS induced interferon (IFN)‐γ and stimulated the skin Langerhans cells and converted them to dendritic cells (DC), which finally clustered into the regional LN.( 7 , 8 , 9 ) DC are the most potent antigen‐presenting cells that induce specific cytotoxic T lymphocytes. Recently it was confirmed that Toll‐like receptor (TLR)‐2, and possibly TLR‐4, recognizes BCG‐CWS and induces innate immune responses.( 10 ) Therefore the anticancer effect of BCG‐CWS is based on activation of DC, and is an emerging therapeutic strategy in practice for cancer and infection.( 3 )
The number of patients treated with BCG‐CWS without administration of chemotherapy or radiation therapy has since increased, reaching more than 1000 of various cancer patients by 2007. Here we report the cases of 73 ovarian cancer patients treated with BCG‐CWS, and analyzed whether LN‐ad affects its efficacy. The prognosis of patients after surgery without LN‐ad was substantially better than that of those with LN‐ad. This finding highlights the importance of LN in eliciting immune reactions against ovarian cancer in response to BCG‐CWS.
Materials and Methods
Patients. From September 1993 to December 2008, a total of 73 patients with ovarian cancer were treated with BCG‐CWS at three institutions (Supporting Information Table 1). The three institutions were the Osaka Medical Center for Maternal and Child Health, Osaka Medical Center for Cancer and Cardiovascular Diseases, and CI Hayashi Immunotherapy Clinic and Research Institute. The premise and outline of the treatment was explained to the patients before the start of immunotherapy and written consent was obtained. The study was approved by the institutional review boards of all three institutions.
In most cases, immunotherapy was started after either or both cytoreductive surgery or chemotherapy, but in some cases after relapse and restaging. Survival was calculated from the beginning of immunotherapy. In three cases, immunotherapy was started within 2 weeks before surgery. LN‐ad here implies dissection of LN in the pelvic cavity and was carried out in a variety of degrees, ranging from none to complete LN‐ad. Incomplete cases often included those in which several LN were removed for factor N determination in the TNM system or for suspected LN metastasis only. We therefore classified patients as follows: –, no LN‐ad; +, partial LN‐ad; and ++, complete LN‐ad.
Inoculation of BCG‐CWS. BCG‐CWS used in the present study was identical to that first reported by Yamamura et al. in 1979,( 5 ) and was kindly supplied by Dr I. Azuma (Hokkaido Pharmaceutical University, Otaru, Hokkaido, Japan). The inoculation procedure has been described in detail elsewhere.( 7 ) Briefly, oil‐attached BCG‐CWS suspension at a final concentration of 1 mg/mL was intracutaneously inoculated into the upper arm in patients in an antigen‐unloaded state. In the sensitization phase, 200 µg was inoculated four times at 1‐week intervals. In the therapeutic phase, inoculation was continued at 4‐week intervals at 10–200 µg, depending on patient response monitored by IFN‐γ induction and physical condition (fever, skin reaction, general malaise).
IFN‐γ induction test. The effect of BCG‐CWS was evaluated at the time of the fourth sensitizing inoculation (S4 phase), or at the first or later inoculation in the therapeutic phase (T1 or more) in cases with chemotherapy or radiation therapy. IFN‐γ levels in the peripheral blood were determined before and at 18 h after inoculation.( 7 ) Measurement was done by BML (Tokyo, Japan). An IFN‐γ value of greater than 500 pg/mL was considered positive, leading to their generally good prognosis even after repeated chemotherapy.
Statistical analysis. The effect of LN‐ad on survival in patients who had surgery followed by immunotherapy was analyzed using a Cox proportional hazard model (Cox model), where the model included stage (I–IV) at the beginning of immunotherapy, LN‐ad (no, partial, or complete), and interaction between stage at surgery and LN‐ad as prognostic variables. A stepwise variable selection procedure was used to identify the variables retained in the Cox model. Furthermore, in order to simplify the model and to clarify interpretation of results, categories in ordered categorical variables such as stage and LN‐ad were reduced to two categories without violating the order, using Akaike Information Criterion.( 11 ) Survival curves were then estimated using the Cox model. All reported P‐values are two‐sided, and in all comparisons, P‐values of less than 0.05 were considered to indicate statistical significance. In addition, all analyses were carried out using SAS software version 9.1 (SAS Institute, Cary, NC, USA).
Results
Effect of BCG‐CWS on two ovarian cancers after surgery without LN‐ad. We first describe two notable cases of patients treated with BCG‐CWS after surgery without LN‐ad.
In the first case (case 4 in Supporting Information Table 1, clear cell adenocarcinoma, T1aN0M0, stage 1a) the patient, first diagnosed as myoma uteri in May 1996, underwent simple total hysterectomy on 7 June 1996. As the swelling of the right ovary was found at surgery, both ovaries were removed. Histological examination confirmed clear cell adenocarcinoma in the right ovary (Fig. 1b). The patient, refusing the suggested total LN‐ad followed by chemotherapy, opted for BCG‐CWS treatment, which started 20 days after surgery. Prior to the surgery the value of the tumor marker CA125 was 120 U/mL (normal range, <35 U/mL) and CA19‐9 was 199 U/mL (normal range, <37 U/mL). Those values recovered to near‐normal levels after surgery. This patient, keeping the normal levels of tumor markers, has remained alive more than 12 years in a disease‐free state (Fig. 1a).
Figure 1.
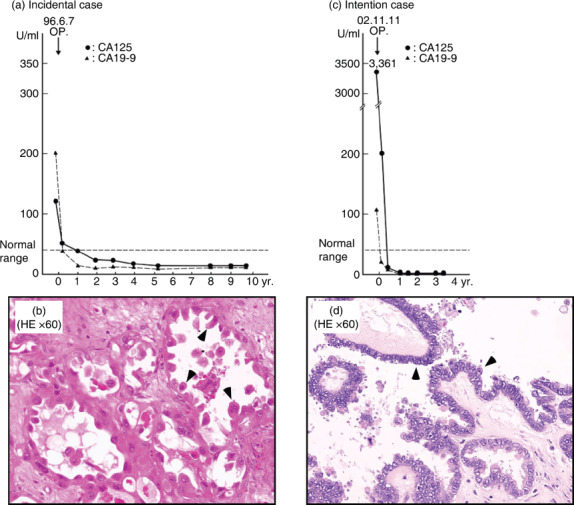
Two cases of patients treated with cell wall skeleton of Mycobacterium bovis Bacillus Calmette‐Guérin (BCG‐CWS) after surgery without lymphadenectomy. (a,b) An incidental case (case 4 in Supporting Information Table 1, clear cell adenocarcinoma, T1aN0M0, stage 1a). (a) The tumor markers CA125 and CA19‐9 were reduced after surgery and remained within normal levels while the patient was alive and free of disease. (b) HE, ×60. Clear cell adenocarcinoma showing a typical hob‐nail appearance (arrowheads), which has the worst prognosis among ovarian cancers. (c,d) An intentional case (case 27 in Supporting Information Table 1, serous papillary adenocarcinoma, T2cN1M0, stage IIc). This ovarian cancer patient had surgery and was subsequently treated with BCG‐CWS alone without chemotherapy. She is alive in a disease‐free state 6 years later. (c) The levels of tumor markers remained within the normal range after surgery. (d) HE, ×60. Serous papillary adenocarcinoma of moderate atypia (arrowheads).
In the case of intentional surgery without LN‐ad (case 27, serous papillary adenocarcinoma, T2cN1M0, stage 2c), the patient was associated with ascites and a high level of tumor marker: CA125 more than 3000 U/mL (Fig. 1c). Based on the previous incidental case 4, the author recommended her the surgery without LN‐ad in combination with BCG‐CWS treatment alone. After the immunotherapy started on 29 October 2002, she had simple total hysterectomy without LN‐ad in Hekinan Municipal Hospital, Aichi, Japan, on 11 November 2002. During the surgery, serous papillary adenocarcinoma with small amount of ascites and intra‐abdominal dissemination was confirmed (Fig. 1d). After the surgery the elevated CA125 marker immediately dropped to approximately 30 U/mL and remained within the normal range for more than 3 years (Fig. 1c). The patient is also alive in a disease‐free state for more than 6 years (as of March 2009).
Patient characteristics. Encouraged by the preliminary results, we treated more patients with BCG‐CWS without LN‐ad. Records of 73 ovarian cancer patients are shown in Supporting Information Table 1, and patient characteristics are shown in Table 1. The median age of the 73 patients was 54 years (interquartile range, 45–61 years). Median survival time from the immune therapy was 18.0 months (interquartile range, 7.8–40.2 months). A total of 40 patients (54.8%) received complete LN‐ad, 23 patients (31.5%) received partial LN‐ad, and 10 patients (13.7%) were without LN‐ad. The age distribution did not change by the status of LN‐ad, but in the group of patients with no or partial LN‐ad, median survivals from surgery were longer and the percentage of deaths decreased.
Table 1.
Patient characteristics
| Overall | LN‐ad | |||
|---|---|---|---|---|
| No | Partial | Complete | ||
| Number of patients (%) | 73 | 10 (13.7) | 23 (31.5) | 40 (54.8) |
| Median age at the baseline (IQR) in years | 54.0 (45.0–61.0) | 54.0 (45.0–60.0) | 55.0 (47.0–61.0) | 55.0 (43.0–63.5) |
| Median survival time (IQR) in months † | 41.8 (34.0–62.0) | 80.0 (39.2–149.8) | 39.6 (27.0–103.4) | 41.2 (25.5–53.6) |
| Median survival time (IQR) in months ‡ | 18.0 (7.8–40.2) | 64.1 (37.9–149.1) | 18.5 (7.8–83.0) | 15.2 (6.0–25.7) |
| Number of patients with IFN‐γ (+) (%) | 23 (31.5) | 6 (60.0) | 9 (39.1) | 8 (20.0) |
| Number of deaths (%) | 38 (52.1) | 2 (20.0) | 7 (30.4) | 29 (72.5) |
| Stage at immune therapy | ||||
| I | II | III | IV | |
| Number of patients (%) | 6 (8.2) | 6 (8.2) | 18 (24.7) | 43 (58.9) |
| Median age at the baseline (IQR) in years | 54.5 (51.0–60.0) | 56.0 (54.0–62.0) | 60.0 (45.0–70.0) | 54.0 (43.0–60.0) |
| Median survival time (IQR) in months ‡ | 80.3 (8.0–156.5) | 64.1 (25.9–122.5) | 29.6 (21.1–50.8) | 51.1 (6.3–22.0) |
| Number of patients with complete LN‐ad (%) | 1 (16.7) | 2 (33.3) | 10 (55.6) | 27 (62.8) |
| Number of patients with IFN‐γ (+) (%) | 2 (33.3) | 4 (66.7) | 5 (27.8) | 12 (27.9) |
| Number of deaths (%) | 0 (0.0) | 1 (16.7) | 10 (55.6) | 27 (62.8) |
Numbers of patients, survival time, and death in each category are summarized from Supporting Information Table 1.
From previous therapy (date indicated in column ‘Previous therapy’ in Supporting Information Table 1).
From immune therapy (date indicated in column ‘IT’ in Supporting Information Table 1).
Survival of patients correlates with IFN‐g induction and preservation of LN. In order to evaluate the contribution of various factors on patient's survival, we carried out Cox proportional hazard analysis on 73 patients described in Supporting Information Table 1, where the model included stage (I–IV) at the beginning of immunotherapy, LN‐ad (no, partial, or complete), and induction of IFN‐γ as prognostic variables. The age of the patients at the beginning of immunotherapy had no effect on survival. The best model selected after a stepwise variable selection and category reduction is shown in Table 2. The result indicates that LN‐ad (after regrouping into no or partial, and complete) had significant contribution to increased hazard ratio (2.38; 95% confidence interval, 1.02–5.12). Estimated survival‐free probability predicted from the Cox analysis is shown in Figure 2(a,b), which suggests that preservation of LN (no or partial LN‐ad) prolonged survival of patients after immunotherapy.
Table 2.
Cox regression model
| Variable | Coefficient | SE | χ2 statistics | P‐value | Hazard ratio | ||
|---|---|---|---|---|---|---|---|
| Estimate | 95% confidence interval | ||||||
| Lower limit | Upper limit | ||||||
| LN‐ad † | 0.83 | 0.41 | 4.02 | 0.0450 | 2.28 | 1.02 | 5.12 |
| Stage | 0.81 | 0.29 | 7.61 | 0.0058 | 2.25 | 1.27 | 4.00 |
| IFN‐γ | –1.25 | 0.46 | 7.31 | 0.0068 | 0.29 | 0.12 | 0.71 |
Three sets of parameters on patient survival were estimated by the Cox regression model. The result of the model that gave minimum Akaike's Information Criterion (AIC) is shown here.
The minimum value of AIC suggested that the most appropriate model was with lymphadenectomy (LN‐ad) being reduced to two categories, i.e. ‘complete LN‐ad’ and ‘LN‐ad or partial LN‐ad’, although stages were not reduced.
Figure 2.
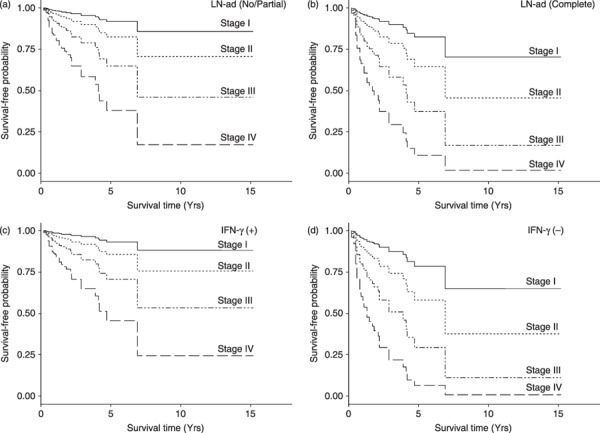
Estimated survival‐free probability of patients based on the Cox model. (a,b) Survival‐free probability of patients with (a) no or partial lymphadenectomy (LN‐ad) and (b) complete LN‐ad. (c,d) Survival‐free probability of patients (c) with and (d) without induction of IFN‐γ.
Further analysis revealed that IFN‐γ induction correlated with reduced hazard ratio (0.29; 95% confidence interval, 0.12–0.71), and contributed to survival of the patients (Fig. 2c,d). This result is consistent with our previous findings that IFN‐γ induction correlated with patient's response to BCG‐CWS and the general role of LN in immunity.( 7 , 12 )
Discussion
Our findings suggest that full or partial omission of LN‐ad in surgery for ovarian cancer during treatment with BCG‐CWS correlates well with prolonged survival of patients. Of particular note, our protocol was effective for patients with clear cell carcinoma, which is considered to be highly resistant to conventional chemotherapy. Given the role of LN as the maturation center for DC, this finding may be applicable to cancer immunotherapy in general.( 3 , 4 )
The first attempt in using BCG‐CWS or related Nocardia‐CWS in cancer immunotherapy was undertaken by Yamamura et al. in the late 1960s but the effects on lung cancer were insufficient to justify broad adoption.( 5 , 8 ) We consider that those poor results were deeply influenced by the immunosuppressive effects of chemotherapy and/or radiation therapy in patients during those trials. Partly in response to this experience, one of us (Hayashi) proposed that immunotherapy should be carried out priority to the protection and maintenance of the patient's immunity.( 3 ) The importance of preserving LN in IFN‐γ induction and BCG‐CWS treatment reported in this work further support this idea.
Murata recently reported that a highly purified preparation of BCG‐CWS, SMP‐105, activates TLR‐2 as the sole target of its anticancer activity in the mouse model system.( 13 ) Although some preparations of BCG‐CWS have been shown to activate both TLR‐2 and TLR‐4 in in vitro systems,( 10 ) it is unlikely that activation of TLR‐4 contributed to our clinical cases because we did not observe any significant reaction typical of TLR‐4 activation by lipopolysaccharide (LPS) during the clinical trial with our preparation of BCG‐CWS.
We speculate that inoculated BCG‐CWS directly stimulated skin Langerhans cells by binding to their TLR‐2 and converted them to activated DC,( 7 , 9 ) which migrate to regional LN and activate circulating naive T cells for production of IFN‐γ( 7 ) and propagation of CD28+ lymphocytes.( 9 ) It is also possible that activated DC migrate to LN in the pelvic cavity where they phagocytose tumor cells and present tumor antigens to naive T cells for stimulation of tumor‐draining T‐cell activity. BCG‐CWS is expected to be a practical and multipurpose immunotherapy, provided that the LN system is intact.
LN‐ad is an established procedure in current surgical practice because the survival of patients with LN‐ad is somewhat longer than that of those without it if chemotherapy is used after surgery. However, complete cure is not expected in these cases and most patients will die shortly after relapse. As no other effective therapy has been available, LN‐ad is used widely in surgery in spite of sequelae such as edema. Given the role of the LN as an important bridge between innate and adaptive immunity,( 11 ) we suggest that in many cases LN‐ad might impair immunity and reduce long‐term survival of patients. Our results introduce a new aspect in clinical care: surgery without LN‐ad.
We should mention that a randomized double‐blind trial has not been attempted in these cases due to patient demand and the difficulty in finding an appropriate placebo that causes a skin response comparable to BCG‐CWS. Nevertheless, this work provides the first case of a quantitative estimate of the effect of LN‐ad on cancer immunotherapy using BCG‐CWS, and warrants further large‐scale clinical investigation. We propose that BCG‐CWS should be considered as a cost‐effective alternative for conventional antitumor treatment. Whereas chemotherapy and radiation therapy have generally failed to offer broad cure of cancers, but rather serve to extend survival only, BCG‐CWS should be considered as a practical immunotherapy for facilitating a higher quality of life for cancer patients.
Supporting information
Table S1. Ovarian cancers treated with immunotherapy using Bacillus Calmette‐Guérin cell wall skeleton
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
We thank Dr Shigeo Hayashi for support in manuscript preparation. The authors are grateful to Dr S. Ishida (Department of Gynecology and Obstetrics, Tousei Hospital, Aichi, Japan) for his kind supply of the incidental case's data. This work was partly supported by research grants from the Princess Takamatsu Cancer Research Fund, the Organization for Pharmaceutical Safety and Research, and the Osaka Society for Prevention of Cancer and Cardiovascular Diseases.
References
- 1. Panici PB, Angioli R. Role of lymphadenectomy in ovarian cancer. Clin Obstet Gynecol 2002; 16: 529–51. [DOI] [PubMed] [Google Scholar]
- 2. Re F, Di, Baiocchi G. Value of lymph node assessment in ovarian cancer: status of the art at the end of the second millennium. Int J Gynecol Cancer 2000; 10: 435–42. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi A, Doi O, Azuma I, Toyoshima K. Immuno‐friendly use of BCG‐cell wall skeleton remarkably improves the survival rate of various cancer patients. Proc Japan Acad 1998; 74 (Ser B): 50–55. [Google Scholar]
- 4. Hayashi A, Nakamura H, Sugihara T, Azuma I. BCG‐cell wall skeleton completely cures the immunologically eligible acute leukemia patients. Proc Japan Acad 1999; 75 (Ser B): 295–300. [Google Scholar]
- 5. Yamamura Y, Sakatani M, Ogura T, Azuma I. Adjuvant immunotherapy of lung cancer with BCG cell wall skeleton (BCG‐CWS). Cancer 1979; 43: 1314–19. [DOI] [PubMed] [Google Scholar]
- 6. Yasumoto K, Manabe H, Yanagawa E et al . Nonspecific adjuvant immunotherapy of lung cancer with cell wall skeleton of Mycobacterium bovis Bacillus Calmette‐Guérin. Cancer Res 1979; 39: 3262–7. [PubMed] [Google Scholar]
- 7. Hayashi A. Interferon‐γ as a marker for the effective cancer immunotherapy with BCG‐cell wall skeleton. Proc Japan Acad 1994; 70 (Ser B): 205–9. [Google Scholar]
- 8. Tsuji S, Matsumoto M, Takeuchi O et al . Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette‐Guérin: involvement of toll‐like receptors. Infec Immun 2000; 68: 6883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayashi A. BCG‐cell wall skeleton can induce the practical and effective dendritic cell‐based immunotherapy for cancer and infection. Proceeding of 12th International Congress of Immunology and 4th Annual Conference of FOCIS 2004; E718C6062: 365–68. [Google Scholar]
- 10. Uehori J, Matsumoto M, Tsuji S et al . Simultaneous blocking of human toll‐like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette‐Guérin peptidoglycan. Infect Immun 2003; 71: 4238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, eds. Proceedings of the Second International Symposium on Information Theory. Budapest: Akademi Kido, 1972; 267–81. [Google Scholar]
- 12. Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science 1996; 272: 50–3. [DOI] [PubMed] [Google Scholar]
- 13. Murata M. Activation of Toll‐like receptor 2 by a novel preparation of cell wall skeleton from Mycobacterium bovis BCG Tokyo (SMP105) sufficiently enhances immune response against tumors. Cancer Sci 2008; 99: 435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Ovarian cancers treated with immunotherapy using Bacillus Calmette‐Guérin cell wall skeleton
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


