Abstract
A transcriptional factor, CCAAT/enhancer binding protein‐β (C/EBP‐β), regulates a variety of cell functions in normal and neoplastic cells. Although the involvement of C/EBP‐β in metastasis has been demonstrated clinicopathologically in several types of human cancer, the mechanism by which it functions during the multistep process of metastasis remains largely unknown. We investigated the role of C/EBP‐β in the intravascular step of hematogenous metastasis in a rat pancreatic tumor cell line, AR42J‐B13, as this step profoundly affects metastatic efficiency. C/EBP‐β‐transfected AR42J‐B13 (βB13) cells acquired considerable resistance against serum toxicity, which was primarily mediated by apoptosis in vitro. Upregulated expression of Bcl‐2 and Bcl‐xL was seen in βB13 cells. Enhanced early survival of intraportally injected βB13 cells in the BALB/c nu/nu male mice liver, detected by the mRNA of a vector‐specific gene, was observed. Nick‐end labeling analysis of the tumor‐injected liver revealed significantly lower rates of apoptosis among intravascular βB13 tumor cells than among empty vector‐transfected AR42J‐B13 (mB13) cells. Finally, intrasplenically injected βB13 cells established a larger number of colonies in the liver than did the mB13 cells. These findings suggest that C/EBP‐β may enhance hematogenous metastasis and its antiapoptotic effects may promote the survival of intravascular tumor cells. (Cancer Sci 2007; 98: 1706–1713)
Abbreviations:
- AFP
α‐fetoprotein
- βB13
C/EBP‐β‐transfected AR42J‐B13
- C/EBP‐β
CCAAT/enhancer binding protein‐β
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HRP
horseradish peroxidase
- LAP
liver‐activating protein
- LIP
liver inhibitory protein
- mB13
mock‐transfected AR42J‐B13
- mTOR
mammalian target of rapamycin
- PBS
phosphate‐buffered saline
- PCR
polymerase chain reaction
- RT
reverse transcription
- SDS
sodium dodecylsulfate
- TBS‐T
100 mM Tris (pH 7.5)
150 mM NaCl and 0.05% Tween‐20
Metastatic inefficiency has been observed in a variety of metastatic experiments in animals.( 1 ) The vast majority of intravascular tumor cells are killed, and only a very small fraction of such cells successfully establish metastasis in a target organ.( 1 , 2 ) Harmful intravascular factors include inflammatory and immune cells such as natural killer cells,( 3 ) blood flow stress,( 4 ) antibody binding followed by cytotoxicity,( 5 ) and serum constituents such as lipoproteins.( 6 ) In addition, anoikis, a form of apoptosis resulting from the loss of anchorage growth, is thought to be among the important reasons for intravascular tumor cell death.( 7 ) Recent studies have indicated that intravascular growth is crucial in the establishment of organ‐destructive metastases of clinical importance; thus, the results of such studies have indicated intravascular tumor cells in the target organ as important therapeutic targets.( 8 , 9 ) The mechanism by which intravascular tumor cells survive or die remains largely unknown, although the apoptosis of intravascular tumor cells is expected to be crucial for achieving metastatic inefficiency.( 10 )
CCAAT/enhancer binding protein‐β is a transcription factor with three domains: DNA‐binding, basic leucine zipper and activation domains.( 11 ) Leaky ribosomal scanning of C/EBP‐β mRNA generates isoforms, that is, the activating form (LAP) and the inhibitory form (LIP).( 12 ) LAP contains both activation and basic leucine zipper domains, whereas only the latter is present in LIP. LIP can therefore act as a dominant negative inhibitor of C/EBP function by forming non‐functional heterodimers with other members of this protein family.( 12 )
CCAAT/enhancer binding protein‐β regulates the expression of a variety of genes, including the genes involved in the differentiation of adipocytes,( 13 , 14 ) immune function,( 15 , 16 ) female reproduction,( 17 ) cell survival,( 18 ) and tumor invasiveness and progression.( 19 , 20 ) In addition, C/EBP‐β is highly expressed in hepatocytes during hepatogenesis and liver regeneration. The maintenance of adult liver cell function by processes such as the synthesis of serum proteins requires C/EBP‐β, and therefore C/EBP‐β is a member of the liver‐enriched transcription factors.( 21 , 22 ) In this context, it is of interest that C/EBP‐β has been reported to lead to the transdifferentiation of a rat pancreatic tumor cell line, AR42J‐B13, into a hepatocellular direction.( 23 ) Thus, models using AR42J‐B13 cells are useful for understanding the molecular and cellular events that occur during hepatic transdifferentiation.( 24 ) Clinically, adenocarcinomas with hepatic differentiation are highly malignant due to frequent vascular invasion and highly metastatic potency.( 25 , 26 ) However, it remains unknown whether or not the enhancement of metastatic potency takes place in the hepatic‐differentiated AR42J‐B13 cells.
Recent studies have suggested that the expression of C/EBP‐β in breast cancer cells correlates with the clinicopathology of this disease.( 27 ) Data indicative of the importance of C/EBP‐β have also been obtained from studies of renal tumors( 19 ) and colorectal tumors.( 28 ) Another recent study demonstrated that the upregulation of an antiapoptotic protein, Bcl‐2, was mediated by C/EBP‐β in t(14;18) lymphoma cells.( 18 ) Taken together, these findings led us to hypothesize that C/EBP‐β could induce a survival phenotype in intravascular tumor cells, possibly via its antiapoptotic activity. We therefore investigated C/EBP‐β‐induced tumor cell survival or death with exposure to pure serum, as well as in an intravascular microenvironment in vivo using AR42J‐B13 cells.
Materials and Methods
Cell culture and transfection of C/EBP‐β. A rat pancreatic tumor cell line, AR42J‐B13 (kindly provided by Professor Itaru Kojima, Gunma University, Japan), was maintained in DMEM (Sigma Chemical Co., St Louis, MO, USA) supplemented with 10% FBS (Sigma Chemical Co.) under 5% CO2 at 37°C. The expression vector for the C/EBP‐β gene, pcDNA3‐C/EBP‐β (kindly provided by Professor David Tosh, University of Bath, UK), was transfected into the AR42J‐B13 cells using lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. After transfection, we carried out a selective culture using G418 (800 µg/mL) (Invitrogen) to establish a βB13 clone that stably expressed C/EBP‐β. The empty vector pcDNA3 was also transfected into the AR42J‐B13 cells, and we obtained a clone of a mock transfectant, mB13.
Cell proliferation assay. βB13 and mB13 cells (3 × 104 cells/well) were cultured in 96‐well microtiter plates. Two culture media with different concentrations of FBS (10 or 100%) were applied. We added the standard amount of DMEM powder to 100% FBS to create the 100% FBS medium. The number of viable cells was measured based on the absorption of WST‐1 (2‐[4‐iodophenyl]‐3‐[4‐nitrophenyl]‐5‐[2,4‐disulfophenyl]‐2H‐tetrazolium; monosodium salt) using Cell Counting Kits (Dojindo, Kumamoto, Japan).
Flow cytometric detection of apoptosis. βB13 and mB13 cells were cultured in medium with either 10 or 100% FBS for 24 h, and flow cytometric detection of early apoptosis in these cells was carried out. Early apoptotic cells were defined as annexin V‐positive and propidium iodide‐negative cells. FITC‐conjugated annexin V and propidium iodide were purchased from BD Biosciences (Heidelberg, Germany).
Western blot analysis. The cells were homogenized in cell lysis buffer with protease inhibitors (PBS with 1% Nonidet P‐40, 0.5% sodium deoxycholate, 0.1% SDS, 0.5 mM phenylmethyl sulfonyl fluoride, 3% aprotinin, 0.1 mM leupeptin, 0.1 mM pepstatin A and 0.1 mM chymostatin), and the samples were then stored on ice for 2 h. The extracted proteins (50 µg) were mixed with sample buffer (0.5 M Tris‐HCl [pH 6.8], 20% SDS, 1% bromophenol blue, 20% glycerol, 10%β‐mercaptoethanol), and electrophoresis was carried out on 15% SDS–polyacrylamide gels (SPU‐15, ATTO, Tokyo, Japan). The proteins were then transferred to nitrocellulose membranes (Nihon Eido, Tokyo, Japan). After the non‐specific binding sites were blocked, the blots were incubated with primary antibodies (1.5 µg/mL) in TBS‐T containing 2% non‐fat skim milk for 4 h at 4°C. The membranes were washed with three successive solutions of TBS‐T containing 2% skim milk at room temperature for 30 min, and were then incubated with HRP‐conjugated anti‐immunoglobulin (1:2000 dilution) for 2 h at 4°C. The membranes were then washed with three successive TBS‐T solutions for 30 min, and the signals were detected by enhanced chemiluminescence using a Hybond ECL protocol (Amersham Pharmacia Biotech, Buckinghamshire, UK). The antibodies used were as follows. Mouse monoclonal anti‐C/EBP‐β, goat polyclonal anti‐AFP, antialbumin, antiamylase and the HRP‐conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal anti‐Bcl‐2 antibody was purchased from R&D Systems (Minneapolis, MN, USA). Monoclonal anti‐Bcl‐xL, anti‐Bax and anti‐Bad antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Mouse monoclonal antirat β‐actin antibody was obtained from Sigma Chemical Co.
Animals. Male BALB/c nu/nu mice, 6–8 weeks old, were obtained from Japan SLC (Hamamatsu, Japan). All mice were maintained under specific pathogen‐free conditions at the Center for Animal Experimentation, Chiba University Graduate School of Medicine. Regular laboratory food and tap water for drinking were made available ad libitum. All animal experiments were carried out under the guidelines of the National Research Council and Chiba University.
Subcutaneous xenografts. Viable βB13 and control mB13 cells (1 × 107/100 µL PBS) were injected subcutaneously into the dorsal surface of nude mice under anesthetization of the animals with diethylether. Mice were killed when the tumor size reached a volume of approximately 1 cm3. The tumor tissues were used for immunohistochemistry and western blot analysis.
Immunohistochemical staining. Formalin‐fixed, paraffin‐embedded sections were stained with hematoxylin–eosin, and these sections were also used for immunohistochemical analysis. Immunostaining was carried out using labeled streptavidin–biotin–peroxidase (Dako Cytomation Co., Kyoto, Japan) and microwave antigen retrieval techniques. Mouse monoclonal anti‐C/EBP‐β (1:100; Santa Cruz Biotechnology) and mouse monoclonal anti‐Bcl‐2 (1:100; R&D Systems) were used as the primary antibodies. Diaminobenzidine tetrahydrochloride substrate was used to visualize positively stained cells.
Detection of immediate entrapment of intraportally injected B13 cells and nick‐end labeling for detection of apoptosis in tissue sections. Male nude mice, 6–8 weeks old, were used. Either βB13 or mB13 cells (1 × 107/100 µL PBS) were injected intraportally using 27G needles under anesthetization of the animals with diethylether. The livers were then removed 2 h after the injection. The livers were cut into two slices, and each slice was formalin fixed and paraffin embedded. Four mice were injected with βB13 cells, and four were injected with mB13 cells. The immediate entrapment of intraportally injected βB13 or mB13 cells was evaluated by counting the number of cells in five random 1 × 1 cm2 fields in the histological slides. The nick‐end labeling detection of apoptosis in the histological sections was carried out using an Apoptosis In situ Detection Kit (Wako, Tokyo, Japan) according to the manufacturer's instructions. Based on the staining pattern, tumor cells within the blood vessels in the liver were determined to be either apoptotic or non‐apoptotic.
Detection of viable βB13 or mB13 cells in the mouse liver. An incision was made in the abdominal wall, and the portal vein was exposed under anesthetization of the animals with diethylether. βB13 or mB13 cells (1 × 104/100 µL PBS) were injected into the portal vein with 27G needles. After 24 h, the mice were killed and the livers were removed (n = 4 in each group). Total RNA was prepared from the liver tissues. The RT‐PCR detection of Neor mRNA was then carried out.
RNA isolation and RT‐PCR. Total RNA was obtained using an RNAeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Total RNA (1 µg) was reverse transcribed by random priming using the SuperScript First‐Strand Synthesis System for RT‐PCR (Invitrogen). PCR was carried out in a volume of 25 µL containing 1 µL first‐strand cDNA, forward and reverse primer (0.4 µM each), dNTP (0.2 mM), MgCl2 (1.5 mM), PCR buffer and Taq polymerase (Amersham Pharmacia Biotech). Samples were amplified through 35 consecutive cycles, or through other numbers of cycles in order to evaluate sample quantity. Each cycle consisted of denaturation at 95°C for 60 s, annealing at 54°C for 30 s, and extension for 60 s, with a final extension for 5 min at 72°C. A 6‐µL volume of the PCR mixture was electrophoresed in a 2.0% agarose gel and stained with ethidium bromide. The following PCR primers for β‐actin and the vector‐derived neomycin resistance gene (Neor ) were used: for rat or mouse β‐actin, 5′‐CTC TTT GAT GTC ACG CAC GAT TTC C‐3′ and 5′‐ATC CTG ACC CTG AAG TAC CCC ATT G‐3′, amplifying a 430‐bp fragment; and for Neor, 5′‐GCT TGG GTG GAG AGG CTA TTC GG‐3′ and 5′‐GCC AGT CCC TTC CCG CTT CAG TG‐3′, amplifying a 235‐bp fragment.
Blood‐borne metastasis to the liver. An incision was made in the left abdominal wall after the animals were anesthetized with diethlyether. Either βB13 or mB13 cells (1 × 106/100 µL PBS) were injected into the spleen. At 6 weeks after the injection, the mice were killed and the livers were removed (number of mice in each group: βB13, 14; mB13, 15). The entire liver from each mouse was cut evenly into three slices, and a tissue section from each slice was stained with hematoxylin–eosin. Microcolonies of tumor cells were counted under a microscope. The sum of the counts from the three slices was considered as the number of micrometastatic colonies in the liver.
Statistical analysis. Statistical analysis of the results was carried out using Student's t‐test. StatView J‐5.0 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses. All P‐values below 0.05 were considered statistically significant.
Results
Generation of βB13 cells and their properties. Either empty (pcDNA3) or C/EBP‐β expression vectors (pC/EBP‐β) were stably transfected into AR42J‐B13 cells, and the clones obtained were tested for C/EBP‐β expression by western blot analysis. The C/EBP‐β‐transfected clone (βB13) was found to produce activating isoforms of C/EBP‐β proteins, namely, full‐length C/EBP‐β and LAP. No LIP was detected. The empty vector‐transfected clone (mB13) did not produce any detectable C/EBP‐β protein (Fig. 1a). To examine the localization of C/EBP‐β in the cellular compartments, immunohistochemical detection was used. Intense nuclear localization was observed in the subcutaneous βB13‐cell tumors, but not in the mB13‐cell tumors (Fig. 1b).
Figure 1.
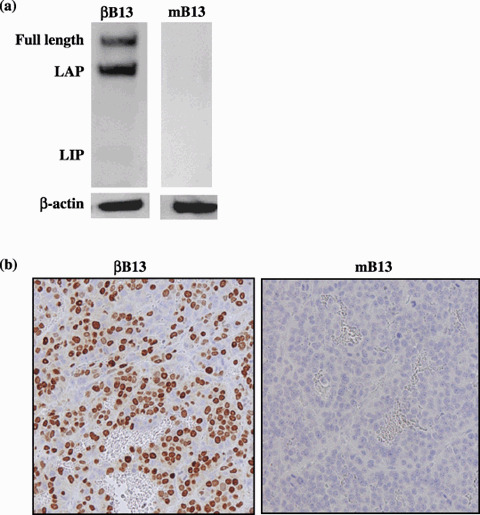
Transfection of C/EBP‐β to rat pancreatic tumor cells. (a) The C/EBP‐β‐transfected AR42J‐B13 (βB13) cells expressed the full‐length and activated isoform liver‐activating protein (LAP), whereas the inhibitory isoform liver inhibitory protein (LIP) was not detectable by western blot analysis. Negligible C/EBP‐β protein was detected in mock‐transfected AR42J‐B13 (mB13) cells. (b) Xenografted βB13 cells (left) expressed high levels of C/EBP‐β, whereas xenografted mB13 cells (right) did not.
As it had previously been reported that C/EBP‐β‐transfected AR42J‐B13 cells transdifferentiate toward hepatocytes,( 23 ) the expression of AFP, albumin and amylase was investigated by western blot analysis. AFP expression was induced, and a slight upregulation of albumin protein was also seen, which indicated the presence of C/EBP‐β‐modified, cell lineage‐specific proteins in the AR42J‐B13 pancreatic tumor cells. However hepatic transdifferentiation was not complete in the case of the βB13 cells, as indicated by a slight upregulation of exocrine pancreatic cell‐specific protein amylase (Fig. 2).
Figure 2.
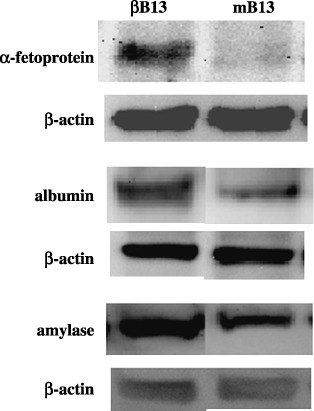
Regulation of cell type‐specific proteins by C/EBP‐β. α‐Fetoprotein expression was induced in C/EBP‐β‐transfected AR42J‐B13 (βB13) cells. An equivalent upregulation of both albumin and amylase was seen in βB13 cells.
Viability, proliferation and apoptosis in 100% FBS in βB13 cells. Tumor cells are directly, without interposition of the basement membrane, exposed to serum when they enter blood vessels. Here, we evaluated the viability and proliferation of AR42J‐B13 cells in 100% FBS in vitro. When incubated in 10% FBS, no differences between βB13 and mB13 cells were observed in terms of either viability or proliferation (Fig. 3a). The βB13 cells were able to proliferate in 100% FBS, whereas no proliferation of mB13 cells was observed in a 100% FBS environment (P < 0.0001) (Fig. 3b). Early apoptotic cells, recognized as annexin V‐positive and simultaneously propidium iodide‐negative cells in 100% FBS, were present in much greater numbers among the mB13 cells than among the βB13 cells, whereas in 10% FBS, apoptosis appeared to occur at a similar rate among mB13 and βB13 cells (Fig. 3c).
Figure 3.
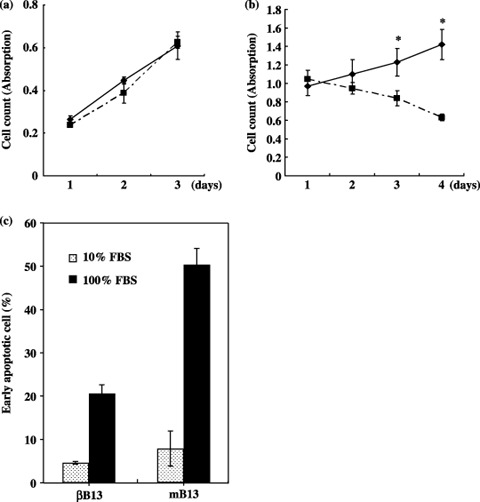
Anti‐apoptotic properties in C/EBP‐β‐transfected AR42J‐B13 (βB13) cells. (a) Proliferation of βB13 (◆) and mB13 (▪) cells in ordinary culture medium (10% fetal bovine serum [FBS]). (b) Proliferation of βB13 (◆) and mB13 (▪) cells in 100% FBS (*P < 0.0001). (c) Early apoptotic cells in 24‐h culture in 100% FBS. Early apoptotic cells were defined as the annexin V‐positive, propidium iodide‐negative cell population, as determined by flow cytometric examination. Early apoptotic cells in 100% FBS were more numerous in βB13 than in mB13 cell cultures.
Augmented early survival of intraportally inoculated βB13 cells in the liver. As the product of the neomycin‐resistance gene Neor has the potential to be used as a marker of surviving cells in mouse tissues, the detection of Neor mRNA was carried out by RT‐PCR. Semiquantitative RT‐PCR was done in order to verify that Neor mRNA was produced in identical amounts by mB13 and βB13 cells. Almost identical amplification of the housekeeper gene rat β‐actin was observed in mB13 and βB13 cells both treated for 20 as well as 25 cycles. In addition, the signals for the products amplified for 25 cycles were more intense than those amplified for 20 cycles; these results indicated the exponential stage of amplification and provided support for the notion that the total cDNA amount produced from mB13 and βB13 cells was identical. No difference in Neor mRNA expression was observed between mB13 and βB13 cells with respect to cDNA with exponential amplifications of 30, 35 and 40 cycles (Fig. 4a).
Figure 4.
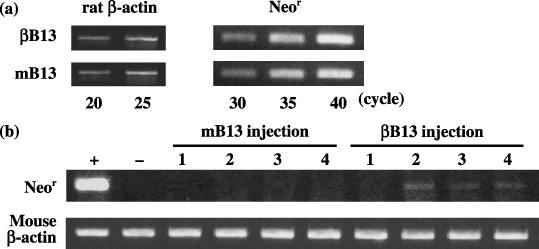
Early survival of intraportally injected C/EBP‐β‐transfected AR42J‐B13 (βB13) cells in liver tissue. (a) Expression of Neor mRNA in βB13 and mB13 cells. Semiquantitative reverse transcription–polymerase chain reaction analysis revealed that Neor mRNA expression was almost identical among cultured βB13 and mB13 cells. (b) Early survival of rat tumor cells (B13) 24 h after intraportal injection. Neor mRNA expression was detected in three out of four livers in βB13‐injected animals, and in none of four livers in mB13‐injected animals. +, βB13 cells as a positive control; –, liver tissue from a phosphate‐buffered saline‐injected mouse as a negative control.
The results of this preliminary investigation suggest that the amount of amplified Neor mRNA product in mouse tissues could serve as a marker of the number of viable (surviving) B13 cells. The amplified products of Neor mRNA were detected 24 h after intraportal injection in three out of four mouse liver tissue samples that had been inoculated with βB13 cells, whereas the products of Neor mRNA were detected in none of four liver samples previously inoculated with mB13 cells (Fig. 4b). These observations suggest that the βB13 cells acquired an advantage that allowed them to maintain viability in the liver after the intraportal injection of the cells.
Anti‐apoptotic property of intraportally inoculated βB13 cells. The amplified products of Neor mRNA were only detected in βB13 cell‐injected mouse liver tissue samples. However, in this experiment, the results of RT‐PCR may have been affected by a difference in the number of entrapped cells. Thus, we microscopically investigated livers in which tumor cells had been intraportally injected. The inoculated B13 cells were located within small branches of the portal veins and sinusoidal vasculature in the liver. The inoculated B13 cells could be distinguished from the intravascular leukocytes based on cellular size and shape, the presence of nuclear pleomorphisms, and the chromatin pattern. The immediate entrapment of intravascularly injected B13 cells was examined as the sum of apparent viable and degenerated B13 cells in the liver; this sum did not differ in the case of either βB13 (377.6 ± 253.7/cm2) or mB13 cells (442.2 ± 424.8/cm2).
Next, the nick‐end labeling method of detecting fragmented DNA was carried out in order to visualize apoptotic B13 cells. Significantly more mB13 cells than βB13 cells were positive, thus indicating that the βB13 cells were antiapoptotic (Fig. 5) in the vasculature.
Figure 5.
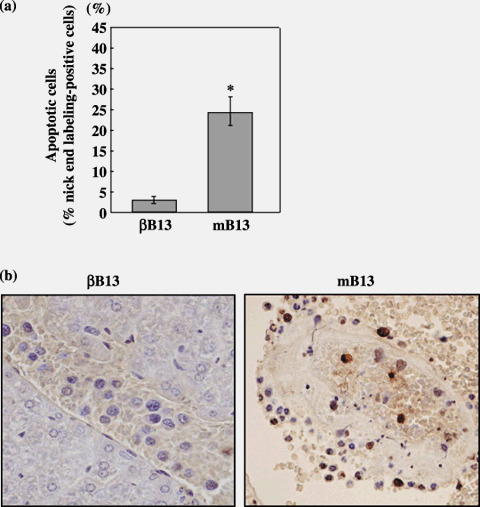
Apoptosis among C/EBP‐β‐transfected AR42J‐B13 (βB13) and mB13 cells intraportally injected into the mouse liver. Liver tissues were removed from cell‐injected mice, and intravascular tumor cells were examined for apoptosis by the nick‐end labeling method. (a) Nick‐end labeling‐positive cells in intravascular βB13 and mB13 cells in the liver (*P = 0.001). Cells with fragmented DNA were more numerous among mB13 cells than among βB13 cells. (b) Nick‐end labeling‐positive cells in intravascular βB13 (left) and mB13 (right) samples.
Induction of Bcl‐2 in AR42J‐B13 cells by C/EBP‐β. We examined the expression of Bcl‐2 and its family members, Bcl‐xL, Bax and Bad, because antiapoptotic properties were observed in βB13 cells, both in vitro and in vivo. Western blot analysis revealed that expression of the antiapoptotic proteins Bcl‐2 and Bcl‐xL was upregulated in the βB13 cells under normal culture conditions, whereas no remarkable change was observed in expression of the apoptosis‐inducing proteins Bax and Bad (Fig. 6a). Immunohistochemical analysis revealed that Bcl‐2 was more frequently positive among βB13 cells than among mB13 cells in the liver of tumor‐injected mice (Fig. 6b).
Figure 6.
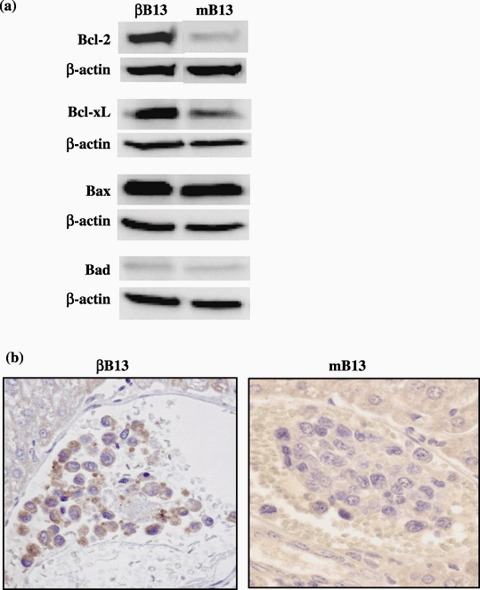
The expression of Bcl‐2 family proteins in C/EBP‐β‐transfected AR42J‐B13 (βB13) cells. (a) Augmented expression of Bcl‐2 and Bcl‐xL was observed in βB13 cells cultured in vitro by western blot analysis. (b) Representative result of the immunohistochemical study of Bcl‐2 expression in vivo. The intravascular βB13 cells stained positive for Bcl‐2.
Enhanced metastatic properties of β‐B13 cells in vivo. The number of established microcolonies and the maximal diameter of individual microcolonies were examined 6 weeks after intrasplenic inoculation of βB13 or mB13 cells. Both the number and maximal size of the colonies were greater in the βB13‐inoculated livers compared with the mB13‐inoculated livers (Fig. 7), thus indicating that C/EBP‐β enhanced the blood‐borne metastatic properties of AR42J‐B13 cells.
Figure 7.
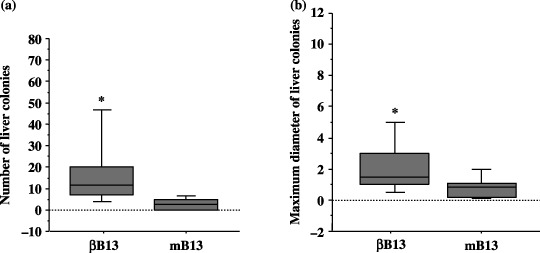
Metastatic capacity of C/EBP‐β‐transfected AR42J‐B13 (βB13) cells. (a) The number of established colonies detected 6 weeks after an intraportal injection of βB13 or mB13 cells (*P = 0.0104). (b) The maximal diameter of individual microcolonies 6 weeks after an intraportal inoculation with βB13 or mB13 cells (*P < 0.0001). The boxes show a range of 25–75%. The horizontal bars in each box indicate median values.
Discussion
The formation of microcolonies within blood vessels precedes the establishment of clinically relevant metastatic tumors;( 8 , 9 ) survival and proliferation in blood vessels increases the opportunity for extravasation and growth into large metastatic nodules. Here, inoculation with βB13 and mB13 cells both exhibited early intrahepatic entrapment 2 h after intraportal cell injection, and early intrahepatic entrapment was observed at the same rate in both groups. One striking observation was that the intravascular βB13 cells were more likely to escape apoptosis than were the control mB13 cells. The upregulation of Bcl‐2 and Bcl‐xL, a key regulator in cell survival, was seen in βB13 cells, as is also seen in t(14;18) lymphoma cells.( 18 ) These observations indicated that βB13 cells acquired the property of early survival (2 h) in the intravascular milieu. The detection of vector‐specific mRNA in the liver 24 h after injection also supported the notion of enhanced early intravascular survival via C/EBP‐β activity. Additionally, intrasplenically injected βB13 cells were found to establish more numerous colonies in the liver, which indicated an enhancement of the metastatic potential of βB13 cells. These results suggest the possibility that the promotion of metastatic ability was, in this case, at least partially mediated by the antiapoptotic properties of βB13 cells in the intravascular milieu.
However, the mechanisms underlying the enhanced metastatic potency of βB13 cells should be carefully assessed, because it is also possible that other C/EBP‐β functions, such as the upregulation of certain molecules known to be important for cancer metastasis,( 29 ) can also mediate metastatic enhancement. It has been demonstrated that the increased expression of apoptotic inhibitors results in resistance to anoikis among cancer cells in circulation.( 30 ) Thus, it is also possible that the antiapoptotic effects of C/EBP‐β could antagonize anoikis, in turn resulting in increased intravascular survival.( 30 , 31 ) However, no significant difference in proliferation was observed between βB13 and mB13 cells cultured in a non‐adherent dish, which suggested that anoikis was not suppressed in βB13 cells (Kishimoto et al., unpublished data).
Many tumor cells were observed in the liver 2 h after cell injection, but mB13 cells could not be detected by RT‐PCR analysis 24 h after cell injection. This indicated that most of the mB13 cells were eliminated during the 24 h following cell injection. Previous observations are not in conflict with our results; the majority of cell loss occurred within 24–48 h of the intravascular invasion of transformed rat embryo cells,( 10 ) and during the first 1.5 h to 3 days in an experiment using melanoma cells.( 32 )
Culture medium supplemented with 10–20% FBS is used optimally for in vitro cell expansion, but higher serum concentrations are often inappropriate for cultivating tumor cells. Common epithelial cells do not make direct contact with blood plasma; however, carcinoma cells are exposed to plasma when they enter vessels. In this context, we examined cell proliferation in culture medium supplemented with 100% FBS. It was observed that βB13 cells proliferated in 100% serum culture, whereas the mB13 cells did not, which indicated that C/EBP‐β conferred upon the AR42J‐B13 cells a tolerance to serum toxicity, even though the species difference should be considered. The observed tolerance appeared to be mediated to some degree by an antiapoptotic mechanism, because serum‐induced apoptosis was less frequently seen among βB13 than among mB13 cells in vitro. These findings appeared to correspond with the in vivo observations in this study, and also with those of a previous study showing enhanced metastasis in a colon cancer cell line that conferred tolerance to serum toxicity induced by dexamethasone treatment.( 33 ) However, the toxic factor in FBS is still unknown, and thus further study will be needed to establish that antiserum toxicity is involved in the mechanism of metastatic efficiency.
The results obtained with the present experimental system appear to suggest that a single transcription factor, C/EBP‐β, can simultaneously regulate both a metastatic property and the direction of differentiation in solid tumor cells. It has been shown that C/EBP‐β can force AR42J‐B13 cells to transdifferentiate in a hepatocellular direction, in a study that revealed the ability of C/EBP‐β‐expressing AR42J‐B13 cells to produce AFP, a protein that serves as a marker of embryonal hepatocytes.( 23 ) Similar AFP expression was reproduced in the present study. In addition, albumin expression was found to be slightly increased. Clinically, aberrant hepatic differentiation is well described (e.g. AFP production associated with highly malignant properties with frequent metastasis); however, the precise mechanism governing the emergence of hepatic differentiation within adenocarcinomas remains unclear. Interestingly, in AFP‐producing gastric adenocarcinoma cell lines, C/EBP‐β is expressed, and its isoforms are regulated by a predominance of LAP.( 34 )
The introduced C/EBP‐β cDNA generated both full‐length and LAP isoforms in AR42J‐B13 cells. In addition, a far less inhibitory isoform, LIP, was generated. Several lines of evidence have indicated that C/EBP‐β‐expressing cells exhibit a unique LAP/LIP ratio, depending on the cell type, thus suggesting that C/EBP‐β does not always function in a positive manner when the expression of LIP exceeds negligible levels. The results of the present study suggested that the activating isoforms of C/EBP‐β most likely mediate antiapoptosis in intravascular AR42J‐B13 cells. Thus, it can be hypothesized that the inhibitory isoform does not necessarily mediate this function, and this lack of mediation most likely results in a tendency toward unaltered or even augmented apoptosis. The LAP/LIP ratio is known to be regulated by several intracellular proteins, including the mTOR signal pathway proteins,( 35 ) RNA‐dependent kinase pathway proteins( 35 ) and triplet repeat‐binding proteins.( 36 , 37 ) For example, rapamycin, an inhibitor of mTOR, is known to modulate the LAP/LIP ratio, resulting in a relative LIP increase.( 35 ) These ratio regulators might alter C/EBP‐β‐induced modulation, thereby exerting an influence on intravascular survival.
To summarize, we will review the three important findings of this study, although the results were obtained from only one established cell line. First, the expression levels of Bcl‐2 and Bcl‐xL were upregulated in C/EBP‐β‐introduced AR42J‐B13 cells. Next, the viability of these cells was preserved by an escape from apoptosis in the liver vasculature during the early period following the in vivo intraportal injection of C/EBP‐β‐introduced AR42J‐B13 cells. Finally, more numerous metastatic colonies were generated in the livers of those mice that had been intrasplenically inoculated with C/EBP‐β‐introduced AR42J‐B13 cells. These results provide support for the hypothesis that the antiapoptotic activity of C/EBP‐β promotes the survival of tumor cells in an intravascular microenvironment, a step considered important for the establishment of metastasis.
Acknowledgments
The authors would like to thank Professor Hiroshi Ishikura (Chiba University Graduate School of Medicine), who passed away in 2006, for his support and valuable advice.
References
- 1. Weiss L. Cancer cell traffic from the lungs to the liver: an example of metastatic inefficiency. Int J Cancer 1980; 25: 385–92. [DOI] [PubMed] [Google Scholar]
- 2. Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer 1983; 48: 665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanna N, Burton RC. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo . J Immunol 1981; 127: 1754–8. [PubMed] [Google Scholar]
- 4. Weiss L, Dimitrov DS, Angelova M. The hemodynamic destruction of intravascular cancer cells in relation to myocardial metastasis. Proc Natl Acad Sci USA 1985; 82: 5737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gresser I, Carnaud C, Maury C et al . Host humoral and cellular immune mechanisms in the continued suppression of Friend erythroleukemia metastases after interferon alpha/beta treatment in mice. J Exp Med 1991; 173: 1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan SY, Pollard M. In vitro effects of lipoprotein‐associated cytotoxic factor on rat prostate adenocarcinoma cells. Cancer Res 1978; 38: 2956–61. [PubMed] [Google Scholar]
- 7. Liotta LA, Kohn E. Anoikis: cancer and the homeless cell. Nature 2004; 430: 973–4. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Mehdi AB, Tozawa K, Fisher AB et al . Intravascular origin of metastasis from the proliferation of endothelium‐attached tumor cells: a new model for metastasis. Nat Med 2000; 6: 100–2. [DOI] [PubMed] [Google Scholar]
- 9. Wong CW, Song C, Grimes MM et al . Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol 2002; 161: 749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong CW, Lee A, Shientag L et al . Apoptosis: an early event in metastatic inefficiency. Cancer Res 2001; 61: 333–8. [PubMed] [Google Scholar]
- 11. Akira S, Isshiki H, Sugita T et al . A nuclear factor for IL‐6 expression (NF‐IL6) is a member of a C/EBP family. EMBO J 1990; 9: 1897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Descombes P, Schibler U. A liver‐enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 1991; 67: 569–79. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka T, Yoshida N, Kishimoto T et al . Defective adipocyte differentiation in mice lacking the C/EBP‐β and/or C/EBP‐δ gene. EMBO J 1997; 16: 7432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeh WC, Cao Z, Classon M et al . Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 1995; 9: 168–81. [DOI] [PubMed] [Google Scholar]
- 15. Screpanti I, Romani L, Musiani P et al . Lymphoproliferative disorder and imbalanced T‐helper response in C/EBP‐β‐deficient mice. EMBO J 1995; 14: 1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka T, Akira S, Yoshida K et al . Targeted disruption of the NF‐IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell 1995; 80: 353–61. [DOI] [PubMed] [Google Scholar]
- 17. Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBP‐β in female reproduction. Genes Dev 1997; 11: 2153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heckman CA, Wheeler MA, Boxer LM. Regulation of Bcl‐2 expression by C/EBP in t(14;18) lymphoma cells. Oncogene 2003; 22: 7891–9. [DOI] [PubMed] [Google Scholar]
- 19. Oya M, Horiguchi A, Mizuno R et al . Increased activation of CCAAT/enhancer binding protein‐β correlates with the invasiveness of renal cell carcinoma. Clin Cancer Res 2003; 9: 1021–7. [PubMed] [Google Scholar]
- 20. Sundfeldt K, Ivarsson K, Carlsson M et al . The expression of CCAAT/enhancer binding protein (C/EBP) in the human ovary in vivo: specific increase in C/EBPβ during epithelial tumour progression. Br J Cancer 1999; 79: 1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greenbaum LE, Cressman DE, Haber BA et al . Coexistence of C/EBPα, β, growth‐induced proteins and DNA synthesis in hepatocytes during liver regeneration. J Clin Invest 1995; 96: 1351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takiguchi M. The C/EBP family of transcription factors in the liver and other organs. Int J Exp Pathol 1998; 79: 369–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen CN, Slack JMW, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol 2000; 2: 879–87. [DOI] [PubMed] [Google Scholar]
- 24. Burke ZD, Shen CN, Ralphs KL, Tosh D. Characterization of liver function in transdifferentiated hepatocytes. J Cell Physiol 2006; 206: 147–59. [DOI] [PubMed] [Google Scholar]
- 25. Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology 1997; 31: 47–54. [DOI] [PubMed] [Google Scholar]
- 26. Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer 1993; 72: 1827–35. [DOI] [PubMed] [Google Scholar]
- 27. Zahnow CA, Younes P, Laucirica R et al . Overexpression of C/EBPβ‐LIP, a naturally occurring, dominant‐negative transcription factor, in human breast cancer. J Natl Cancer Inst 1997; 89: 1887–91. [DOI] [PubMed] [Google Scholar]
- 28. Milde‐Langosch K, Loning T, Bamberger AM. Expression of the CCAAT/enhancer‐binding proteins C/EBPα, C/EBPβ, and C/EBPδ in breast cancer: correlations with clinicopathologic parameters and cell‐cycle regulatory proteins. Breast Cancer Res Treat 2003; 79: 175–85. [DOI] [PubMed] [Google Scholar]
- 29. Omori K, Naruishi K, Nishimura F et al . High glucose enhances interleukin‐6‐induced vascular endothelial growth factor 165 expression via activation of gp130‐mediated p44/42 MAPK‐CCAAT/enhancer binding protein signaling in gingival fibroblasts. J Biol Chem 2004; 279: 6643–9. [DOI] [PubMed] [Google Scholar]
- 30. Berezovskaya O, Schimmer AD, Glinskii AB et al . Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res 2005; 65: 2378–86. [DOI] [PubMed] [Google Scholar]
- 31. Douma S, Van Laar T, Zevenhoven J et al . Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004; 430: 1034–9. [DOI] [PubMed] [Google Scholar]
- 32. Luzzi KJ, MacDonald IC, Schmidt EE et al . Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998; 153: 865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshida Y, Kishimoto T, Ishiguro H et al . Dexamethasone modifies the susceptibility to serum cytotoxicity and increases the metastatic efficiency of a colon carcinoma cell line. Exp Mol Pathol 2006; 81: 77–84. [DOI] [PubMed] [Google Scholar]
- 34. Supriatna Y, Kishimoto T, Furuya M et al . Expression of liver‐enriched nuclear factors and their isoforms in α‐fetoprotein‐producing gastric carcinoma cell. Exp Mol Pathol 2007; 82: 316–21. [DOI] [PubMed] [Google Scholar]
- 35. Calkhoven CF, Müller C, Leutz A. Translational control of C/EBPα and C/EBPβ isoform expression. Genes Dev 2000; 14: 1920–32. [PMC free article] [PubMed] [Google Scholar]
- 36. Timchenko LT, Iakova P, Welm AL et al . Calreticulin interacts with C/EBPα and C/EBPβ mRNA and represses translation of C/EBP proteins. Mol Cell Biol 2002; 22: 7242–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Timchenko NA, Welm AL, Lu X et al . CUG repeat binding protein (CUGBP1) interacts with the 5′ region of C/EBPβ mRNA and regulates translation of C/EBPβ isoforms. Nucl Acids Res 1999; 27: 4517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


