Abstract
Mesothelioma is an aggressive tumor arising from the mesothelium, and is usually associated with previous exposure to asbestos. The incubation period of asbestos‐related mesothelioma is estimated to be approximately 30–40 years. Once mesothelioma has occurred, there is no effective treatment. So, identification of tumor markers and a method for early diagnosis using such markers are urgently needed. Recently, several enzyme‐linked immunosorbent assay systems for Erc/mesothelin have been developed, the reported usefulness of which has been assessed and demonstrated as a diagnostic tool. Asbestos‐related mesothelioma should be ascribed as a typical environmental carcinogen. In this review, we will present asbestos‐related mesothelioma for the study of problems in environmental carcinogenesis. (Cancer Sci 2007; 98: 1147–1151)
Environment and heredity both play a role in the origin of human cancer. These environmental and genetic determinants of cancer can be classified into four groups, designated ‘oncodems’ by Knudson in 1986.( 1 ) Oncodem 1 is the irreducible ‘background’ level of cancer due to spontaneous mutagenesis. Oncodem 2 is ‘environmentally induced’ cancer, the causative agents of which are chemical carcinogens, radiation, or viruses. Oncodem 3 is basically ‘environmentally induced’ cancer, but there are genetically determined differences among persons, for example, regarding the activation or inactivation of carcinogens. Most human cancers are believed to belong to oncodem 2 and/or 3 (approximately 80%), for which the probability of the occurrence of carcinogenic steps is increased, although the number of steps is not decreased. Oncodem 1 would contain the 20% that would remain if ‘environmental induced’ cancers (oncodem 2 and/or 3) were prevented. Oncodem 4 is ‘hereditary’ cancer. Hereditary cancers have been important in the understanding of carcinogenesis, and have pointed to the first ‘tumor suppressor gene’, from the work of Knudson (‘two‐hit’) on human retinoblastoma in 1971.( 2 ) In the present review, the authors will present asbestos‐related mesothelioma for the study of problems in environmental carcinogenesis.
Environmental carcinogens
‘Environmental carcinogens’ came to light in 1775 when the English surgeon Percival Pott reported scrotal cancer associated with chimney‐sweeps in ‘cancer stimulated by chimney soot.’ The industrialization following the Industrial Revolution of the later half of the 18th century witnessed the introduction of environmental pollutants. This introduction created the tragic reality of environmental carcinogens, to which the public is forcefully exposed without their knowledge. Asbestos‐related mesothelioma should be ascribed as a typical environmental carcinogen.
Recently, the media in Japan has taken up and reported on a wide scale on ‘mesothelioma caused by asbestos.’ This is truly ironic because Japan was a pioneering nation in chemical‐induced carcinogenesis. How, then, should we approach such a critical problem? What lessons must we learn from these past mistakes in order that we do not make the same kind of mistakes in the future?
Concerning asbestos, the preventative measures in Japan, including practical responses, are decades behind other nations, despite the fact that there has been pathological and epidemiological evidence pointing to ‘risk.’‘Carcinogenic’ research for the future must include risk evaluation along with risk management, as well as open communication.
Federal headship of carcinogenesis
Cancer is a heritable disorder of somatic cells. The authors have proposed a diagrammatic similarity between carcinogenesis and an opened Japanese fan, because initiated cells grow in several directions and knowledge of clinical tumors suggests that the ‘edge of the fan’ has many gene abnormalities: genetic instability might also play a role( 3 ) (Fig. 1).
Figure 1.
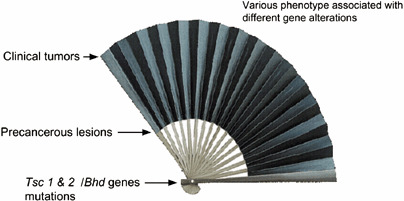
Fan model of renal carcinogenesis. Carcinogenesis looks like an opened Japanese fan. Primal force is ‘two hits’ of the predisposing gene. Initiated cells grow in many directions and culminate in diverse clinical cancers at the periphery. The edge of the fan corresponds to clinical tumors.
The Eker (Tsc 2gene mutant) rat model of hereditary renal carcinoma (RC) is an example of Mendelian dominantly inherited predisposition to a specific cancer in an experimental animal.( 4 ) Recently, the authors discovered a new hereditary renal carcinoma in the rat in Japan, and the rat was named the ‘Nihon’ rat and its predisposing gene (Bhd) could be a novel renal tumor suppressor gene.( 5 )
The germline mutation is like an ‘initial gene’ (Tsc2, Bhd genes) of the abnormal networks of gene expression that are involved in tumor formation (federal headship of carcinogenesis). The authors’ opened carcinogenesis fan model can be an analogy not only for the ‘two‐hit’ model, but also for the ‘multi‐hit’ mutational model, because it incorporates the phenomena of genetic instability and of factors that have altered gene expression.
To search for such alterations, the authors identified the highly expressed genes in Eker RC as the C3 gene encoding the third component of complement, the annaxin II gene encoding the calpactine 1 heavy‐chain, the Erc (expressed in renal carcinoma) gene, and the fra‐1 gene encoding a transcriptional factor activator protein 1 (AP‐1).( 6 )
Erc/MPF/mesothelin
After the authors determined the complete primary structure of rat Erc cDNA, it was showed that the putative rat Erc product has an identity with human megakaryocyte potentiating factor, megakaryocyte potentiating factor (MPF)/mesothelin.( 7 ) A database search revealed that this predicted amino acid from Erc cDNA had 87.4% and 56.1% identity, respectively, to the mouse and human mesothelin gene. Two hydrophobic regions, a putative signal peptide near the amino‐terminus and a putative glycosylphosphatidylinositol anchorage sequence near the carboxy‐terminus, have been identified in human MPF/mesothelin. A putative furin cleavage sequence was also conserved in rat Erc. Rat Erc and human mesothelin were localized in chromosomes 10q12–21 and 16p13.3, respectively, both of which coincided with the locus of the tumor suppressor gene, the Tsc2/TSC gene. Therefore, rat Erc and human mesothelin are functional orthologues and the authors shall refer to this protein as Erc/mesothelin.
The authors also found that Erc was expressed at higher levels in RC compared with the normal kidney of the Eker rat. Erc may be related to carcinogenesis in the Eker rat model.
Asbestos‐related mesothelioma
Mesothelioma is an aggressive tumor arising from the mesothelium, a membrane lining several body cavities, including the pleura, peritoneum, pericardium and tunica vaginalis, and is usually associated with asbestos exposure.
Asbestos is the commercial name for a group of hydrated magnesium silicate fibrous minerals. Three types are mainly recognized: chrysotile (white asbestos), crocidolite (blue asbestos), and amosite (brown asbestos). Asbestos is valued in industry for its resistance to heat and combustion, and has been used in the production of cement, ceiling and pool tiles, automobile brake linings, and in shipbuilding. In ancient times, it was used as the cloth for the binding of the mummies of ancient Egypt. Several types of asbestos‐related illnesses other than mesothelioma are known: pleural plaques, pleural thickening, asbestosis, and lung cancer.
Mesothelioma was rare until the second half of the twentieth century. In 1960, Carbone reported a mesothelioma epidemic among crocidolite asbestos miners in South Africa.( 8 ) In 1973, the World Health Organization's International Agency for Research on Cancer (IARC) pointed out the higher risk of lung cancer and mesothelioma for laborers in asbestos mines. Since then, the use of asbestos, particularly blue and brown asbestos, has been banned or restricted in Europe, the USA and other countries.
In Japan, the amount of asbestos imports increased between 1960 and 1974, and reached a peak in 1974.( 9 ) At the time, airborne asbestos on building sites was banned. Moreover, in the 1980s, the use of asbestos for public school buildings and labware (e.g. metal mesh with asbestos) became controversial and labware disappeared. In 1995, the production and use of the most carcinogenic blue asbestos only were banned. The problems of airborne asbestos in the dismantling of old buildings with asbestos components still remain. The safety concerns about asbestos have not been cast aside.
Erc/mesothelin as a diagnostic marker of mesothelioma. The incub‐ation period of asbestos‐induced mesothelioma is estimated to be approximately 30–40 years. Once mesothelioma has occurred, there is no effective treatment.
Recently, pemetrexed disodium, a new antifolate with multiple targets, has been developed and granted marketing approval for use in combination with cisplatin for the treatment of chemotherapy‐naïve patients with non‐resectable malignant pleural mesothelioma and is expected to be an effective treatment.( 10 , 11 , 12 )
The most important modalities for the diagnosis of mesothelioma are chest radiography, chest computed tomography (CT), and magnetic resonance imaging (MRI) of the chest. However, it may be difficult to make an early diagnosis of mesothelioma using the currently available diagnostic imaging techniques, and identification of tumor markers and a method for early diagnosis using such markers are urgently needed.
As an aside, the authors have found the Erc (expressed in renal carcinoma) gene during research into genetic kidney cancer in rats, and it was suggested that rat Erc and human mesothelin are functional orthologues, as described above.
The Erc/mesothelin protein is present on the normal mesothelium, a membrane lining several body cavities including the pleura, peritoneum, pericardium and tunica vaginalis. It could be speculated that mesothelioma derived from the mesothelium should demonstrate overexpression of Erc/mesothelin. Yamashita et al. implied that Erc/mesothelin is associated with cell‐to‐cell adhesion.( 7 ) The overexpression of Erc/mesothelin in the mesothelioma is thought to be involved in invasion, as it is expressed at high levels in some tumors showing aggressive peritoneal spreading and/or local invasion such as mesothelioma and ovarian carcinoma (cited in Yamashita et al.( 7 )).
The authors have thus succeeded in establishing specific antibodies against Erc/mesothelin, and after validation by immunohistochemical studies on diseased tissue of mesothelioma patients, an enzyme‐linked immunosorbent assay (ELISA) system has been developed, the usefulness of which has been assessed and demonstrated as a diagnostic tool.( 13 , 14 ) Indeed, this is a real‐life example of translational research.
Measurements of serum Erc/mesothelin in mesothelioma patients. The full‐length of human mesothelin gene codes the primary product being a 71‐kDa precursor protein. It can be physiologically cleaved by some furin‐like proteases into a 40‐kDa C‐terminal fragment that remains membrane‐bound, and a 31‐kDa N‐terminal fragment, which is secreted into the blood.( 15 ) The C‐terminal 40‐kDa fragment is referred to as mesothelin. In contrast, the N‐terminal 31‐kDa fragment is a secreted protein identified as MPF (Fig. 2).
Figure 2.
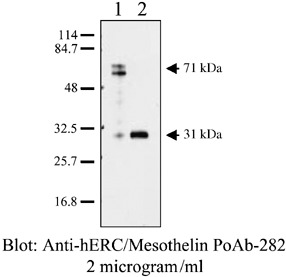
Characterization of anti‐Erc/mesothelin antibodies using western blot analysis. Lane 1, lane 2: lysate and culture supernatant of COS‐1 cells transfected with Erc/mesothelin cDNA, respectively. Soluble N‐Erc/mesothelin was detected in the culture supernatant.
Henceforth, the gene product of mesothelin has been referred to by each name. In order to avoid confusion arising from the use of the term mesothelin, in the present paper the term N‐Erc/mesothelin shall be used for the N‐terminal 31‐kDa fragment and C‐Erc/mesothelin for the C‐terminal 40‐kDa fragment.
The authors have developed a novel ELISA system for the detection of N‐Erc/mesothelin in the serum of mesothelioma patients, and have begun to examine its clinical usefulness.( 16 ) Mouse monoclonal antibodies (clone 7E7) and rabbit polyclonal antibodies (PoAb‐282) were raised against human N‐Erc/mesothelin and used for the establishment of the ELISA system (Fig. 3). Serum samples from seven patients with mesothelioma, four patients with carcinomatous pleuritis pleural metastasis (lung cancer), three with benign asbestos pleuritis, and 13 healthy volunteers were evaluated. The diagnosis in patients with mesothelioma was confirmed immunohistochemically (all epithelial types).
Figure 3.
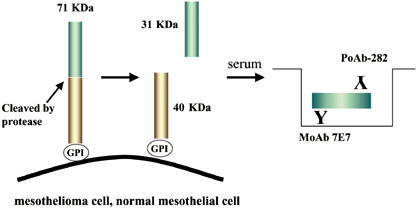
Products of the Erc/mesothelin gene and the enzyme‐linked immunosorbent assay (ELISA) system. The primary product of the Erc/mesothelin gene, a 71‐kDa precursor protein. This protein is physiologically cleaved, releasing its 31‐kDa N‐terminal fragment into the blood. The authors’ system detects 31‐kDa N‐terminal fragments using a sandwich ELISA containing two antibodies against N‐terminal fragments.
The median serum concentrations in the seven mesothelioma patients, 13 healthy controls, four pleural metastasis (lung cancer) patients and three benign asbestos pleuritis patients were 25.12 ng/mL (range: 10.32–53.13 ng/mL), 1.4 ng/mL (range: 0.67–7.60 ng/mL), 1.31 (range: 0.68–4.33 ng/mL), and 1.10 ng/mL (range: 0.3–1.19 ng/mL), respectively (Fig. 4). Until now, the authors’ ELISA system has detected much higher serum levels of N‐Erc/mesothelin in mesothelioma patients than in healthy controls or patients with other lung/pleural diseases.
Figure 4.
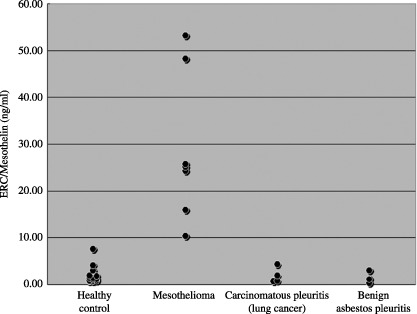
Scatter plot of serum N‐Erc/mesothelin. The serum concentrations of N‐Erc/mesothelin were much higher in mesothelioma patients than in the healthy controls, carcinomatous pleuritis (lung cancer) patients and benign asbestos pleuritis patients.( 16 )
Recently, Onda et al. also reported the generation of anti‐MPF monoclonal antibodies and the development of ELISA to MPF (N‐Erc/mesothelin).( 17 ) Their ELISA data showed that MPF levels were elevated in 91% of patients (51 of 56) with mesothelioma compared with healthy controls. Furthermore, MPF levels decreased in patients after tumor debulking surgery. Thus, N‐Erc/mesothelin may represent a promising tumor marker for mesothelioma.
So far, two groups have reported on ELISA systems for the detection of C‐Erc/mesothelin. Robinson et al. have established an ELISA system for the detection of SMRP. In their first paper they assayed the serum concentrations of SMRP in serum samples obtained from 44 patients with histologically proven mesothelioma, 68 matched healthy controls, 40 of whom had a history of exposure to asbestos, and 160 patients with other inflammatory or malignant lung or pleural diseases.( 18 ) They showed that 84% of the 44 mesothelioma patients had elevated serum concentrations of SMRP, compared with 2% of the 160 with other lung/pleural diseases. In their second paper they reported that measurement of the concentrations of SMRP in the serum as a marker of mesothelioma had a sensitivity of 83% and specificity of 95% for the diagnosis of mesothelioma in the first 48 mesothelioma patients examined.( 19 )
Scherpereel et al. reported their data obtained using this ELISA kit (Mesomark; Fujirebio Diagnostic, Malvern, PA, USA).( 20 ) The mean serum SMRP level was higher in patients with mesothelioma (2.05 ± 2.57 nmol/L, n = 28) than in those with pleural metastasis (1.02 ± 1.79 nmol/L, n = 35) or benign lesions of the pleura (0.55 ± 0.59 nmol/L, n = 28). The pleural fluid SMRP concentrations were higher than the serum SMRP concentrations in all the patient groups (mesothelioma, 46.1 ± 83.2 nmol/L; benign lesions, 6.4 ± 11.1 nmol/L; metastasis, 6.36 ± 21.73 nmol/L).
Beyer et al. reported the analytical and preliminary clinical studies of MESOMARKTM .(21) They measured SMRP using this assay in 409 apparently healthy individuals, 177 patients with non‐malignant conditions, and 500 cancer patients, including 88 with malignant pleural mesothelioma (MPM). As a result, the mean SMRP was significantly higher in sera from patients with MPM (7.5 nmol/L; 95% confidence interval [CI] = 2.8–12.1 nmol/L; n = 88). SMRP was increased in 52% and 5% of MPM patients and asbestos‐exposed individuals, respectively.( 21 )
Hassan et al. have pursued the development of mAb against Erc/mesothelin. Some of these antibodies have been applied for the treatment of mesothelioma using immunotoxin technology.( 22 ) Recently, a new mAb against C‐Erc/mesothelin( 23 ) and an ELISA system for the detection of C‐Erc/mesothelin using these mAb have been produced, and they reported elevated serum levels of mesothelin in 40 of 56 (71%) mesothelioma patients and 14 of 21 (67%) ovarian cancer patients.( 24 ) The serum levels of mesothelin were increased in 80% of patients who had positive tumor immunohistochemistry for mesothelin. They also observed a rapid decrease in the serum mesothelin levels after surgery in patients with peritoneal mesothelioma.
These studies showed the possibility of diagnosis of mesothelioma. Several ELISA systems for Erc/mesothelin have been developed and must be evaluated in parallel or systematic studies. Retrospective and prospective studies using these ELISA systems should be conducted in collaboration with immunohistochemical and imaging diagnosis.
Mechanisms of asbestos‐related carcinogenesis
In 1987 the IARC classified asbestos as a group I carcinogen, defined as ‘exposure circumstance entails exposures that are carcinogenic’ based on literature that found an increased risk of lung cancer for laborers working in factories producing asbestos products.
However, the basic roles or physiological functions of, and relationship between Erc/mesothelin‐ and asbestos exposure‐mediated carcinogenesis remain to be resolved. The precise mechanism has not yet been fully elucidated.
Carbone et al. summarized the possible mechanisms of carcinogenesis by mineral fibers, include asbestos.( 8 ) Crocidolite exposure can induce the expression of both tumor‐necrosis factor‐κ (TNF‐κ) and its receptor (TNF‐R1) in mesothelial cells and in macrophages that phagocytose asbestos. Indeed, Tnfr1 knockout mice do not develop fibroproliferative lesions after asbestos exposure. Crocidolite induces cell lysis and apoptosis; however, asbestos‐induced TNF‐κ secretion activates nuclear factor‐κB (NF‐κB) that protects mesothelial and other cells from crocidolite‐induced cell lysis. Therefore, asbestos‐damaged mesothelial cells divide rather than die and can propagate the genetic damage that is induced by mutagenic oxygen radicals released by mononuclear phagocytes that have ingested asbestos. Moreover, asbestos induces the phosphorylation of extracellular signal‐regulated kinases 1 and 2 (ERK1 and ERK2), which leads to the activation of the transcription factor AP1 and stimulates cell division. Activation of this pathway can also promote cell invasion by causing the induction and release of cellular metalloproteinases (cited in Carbone( 6 )).
In contrast, it has been reported that mesothelioma is highly likely to be caused by asbestos exposure in genetically predisposed individuals in a certain limited area.( 25 ) There is the possibility of the existence of mesothelioma susceptibility genes.
Recently, Nakaishi et al. have developed antibodies against the amino‐terminal portion of the rat Erc, and demonstrated the existence of a 30‐kDa secretory form in the supernatant of cultured cells derived from rat renal carcinoma.( 26 ) The ELISA system using these antibodies detected a high concentration of this form in the sera of Eker rats bearing renal carcinomas, and in the sera of rats transplanted with mesothelioma cells. There are several rat model systems of mesothelioma that are promising tools in the development of antimesothelioma treatment. The authors hope their ELISA to detect the soluble form of rat Erc/Mesothelin is useful in the rat model system to elucidate the mechanism of asbestos‐induced carcinogenesis, extrapolation from which research could develop an antimesothelioma therapy to be used in human patients.
Asbestos‐related mesothelioma based on carcinogenic research. Asbestos‐related health damage is spreading to neighborhood residents as well as factory employees, and the problem is expected to become even more serious. The cause of public unrest can be attributed to the confusion regarding the relation between the length and amount of asbestos exposure and the development of cancer. The time is now right to start a project focused on the environmental carcinogenesis, concentrating on asbestos‐related mesothelioma, with the aim of obtaining relief for victims. To this end, the authors have started a large‐scale prospective study on building construction workers, who run the risk of asbestos exposure, using their ELISA system.
Taking the long incubation period of mesothelioma into con‐sideration, the development of a system is required to maintain follow‐up investigation for asbestos‐exposed individuals. Moreover, the formation of a network system of hospitals that take care of diagnosis and treatment in a comprehensive manner is necessary.
At the same time, it is absolutely essential through basic research to resolve the mechanism of mesothelioma occurrence associated with asbestos. The specific mechanism should throw new light on a definitive therapy for mesothelioma.
Conclusion
‘Ominous clouds are in the sky’, and unless we seize this opportunity and seriously face the problem of ‘environmental carcinogens’ as a society, we will certainly face the second and third iteration of the ‘asbestos problem’. Will the next issue we face be nanoparticles?
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Cancer Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labor and Welfare of Japan.
References
- 1. Knudson AG. Hereditary cancer, oncogenes and antioncogenes. Cancer Res 1985; 45: 1437–43. [PubMed] [Google Scholar]
- 2. Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971; 68: 820–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hino O, Majima S, Kobayashi T et al . Multistep renal carcinogenesis as gene expression disease in tumor suppressor TSC2 gene mutant model – genotype, phenotype and environment. Mutat Res 2001; 477: 155–64. [DOI] [PubMed] [Google Scholar]
- 4. Eker R, Mossige JA. A dominant gene for renal adenomas in the rat. Nature 1961; 189: 858–9. 13780066 [Google Scholar]
- 5. Kouchi M, Okimoto K, Matsumoto I, Tanaka K, Yasuba M, Hino O. Natural history of the Nihon (Bhd gene mutant) rat, a novel model for human Birt‐Hogg‐Dube syndrome. Virchows Arch 2006; 448: 463–71. [DOI] [PubMed] [Google Scholar]
- 6. Hino O, Kobayashi E, Nishizawa M et al . Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol 1995; 121: 602–5. [DOI] [PubMed] [Google Scholar]
- 7. Yamashita Y, Yokoyama M, Kobayashi E, Takai S, Hino O. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun 2000; 275: 134–40. [DOI] [PubMed] [Google Scholar]
- 8. Carbone M, Emri S, Dogan AU et al . A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer 2007; 7: 147–54. [DOI] [PubMed] [Google Scholar]
- 9. Morinaga K, Kishimoto T, Sakatani M, Akira M, Yokoyama K, Sera Y. Asbestos‐related lung cancer and mesothelioma in Japan. Ind Health 2001; 39: 65–74. [DOI] [PubMed] [Google Scholar]
- 10. Rowinsky EK, Beeram M, Hammond LA et al . A phase I and pharmacokinetic study of pemetrexed plus irinotecan in patients with advanced solid malignancies. Clin Cancer Res 2007; 13: 532–9. [DOI] [PubMed] [Google Scholar]
- 11. Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 2007; 6: 404–17. [DOI] [PubMed] [Google Scholar]
- 12. Green J, Dundar Y, Dodd S, Dickson R, Walley T. Pemetrexed disodium in combination with cisplatin versus other cytotoxic agents or supportive care for the treatment of malignant pleural mesothelioma. Cochrane Database of Systematic Reviews 2007. (Jan 24); Issue 1 : CD005574. [DOI] [PMC free article] [PubMed]
- 13. Maeda M, Hino O. Molecular tumor markers for asbestos‐related mesothelioma: serum diagnostic markers. Pathol Int 2006; 56: 649–54. [DOI] [PubMed] [Google Scholar]
- 14. Maeda M, Hino O. Blood test for asbestos‐related mesothelioma. Oncology 2006; 71: 26–31. [Epub 2007 Mar 5.] [DOI] [PubMed] [Google Scholar]
- 15. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 1996; 93: 136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiomi K, Miyamoto H, Segawa T et al . A novel ELISA system for detection of a ‘N‐ERC/Mesothelin’ in the sera of mesothelioma patients. Cancer Sci 2006; 97: 928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onda M, Nagata S, Ho M et al . Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res 2006; 12: 4225–31. [DOI] [PubMed] [Google Scholar]
- 18. Robinson BW, Creaney J, Lake R et al . Mesothelin‐family proteins and diagnosis of mesothelioma. Lancet 2003; 362: 1612–16. [DOI] [PubMed] [Google Scholar]
- 19. Robinson BW, Creaney J, Lake R et al . Soluble mesothelin related protein: a blood test for mesothelioma. Lung Cancer 2005; 49: S109–11. [DOI] [PubMed] [Google Scholar]
- 20. Scherpereel A, Grigoriu B, Conti M et al . Soluble mesothelin related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006; 173: 1155–60. [DOI] [PubMed] [Google Scholar]
- 21. Beyer HL, Geschwindt RD, Glover CL et al . MESOMARKTM: a potential test for malignant pleural mesothelioma. Clin Chem 2007; 53: 666–72. [Epub 2007 Feb 8.] [DOI] [PubMed] [Google Scholar]
- 22. Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004; 10: 3937–42. [DOI] [PubMed] [Google Scholar]
- 23. Onda M, Willingham M, Nagata S et al . New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence‐activated cell sorting, Western blotting, and ELISA. Clin Cancer Res 2005; 11: 5840–6. [DOI] [PubMed] [Google Scholar]
- 24. Hassan R, Remaley AT, Sampson ML et al . Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006; 12: 447–53. [DOI] [PubMed] [Google Scholar]
- 25. Dogan AU, Baris YI, Emri S, Steele I, Elmishad AG, Carbone M. Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Res 2006; 66: 5063–8. [DOI] [PubMed] [Google Scholar]
- 26. Nakaishi M, Kajino K, Ikesue M et al . Establishment of the enzyme‐linked immunosorbent assay system to detect the amino terminal secretory form of rat Erc/Mesothelin. Cancer Sci 2007; 98: 659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


